-
Medical journals
- Career
Esophageal dysplasia and adenocarcinoma: a study with double immunostaining for intestinal and gastric markers
Authors: Alena Chlumská 1,2; Petr Mukenšnabl 1,2; Tomáš Waloschek 1,2; Michal Zámečník 3,4
Authors‘ workplace: Šikl`s Department of Pathology, Medical Faculty of Charles University, Pilsen, Czech Republic 1; Laboratory of Surgical Pathology, Pilsen, Czech Republic 2; AGEL Laboratories a. s., Department of Pathology, Nový Jičín, Czech Republic 3; Medirex Group Academy n. o., Bratislava, Slovak Republic 4
Published in: Čes.-slov. Patol., 54, 2018, No. 2, p. 81-85
Category: Original Article
Overview
Columnar lined esophagus is a complication of long term gastroesophageal reflux disease and the main precursor of esophageal adenocarcinoma. Incomplete intestinal metaplasia in reflux esophagitis represents one of the most important risk factors for neoplastic transformation through the metaplasia–dysplasia–adenocarcinoma sequence. However, recent studies suggest that cardiac type mucosa also shows molecular abnormalities which are similar to those of incomplete intestinal metaplasia. Immunohistochemically, three types of esophageal dysplasia and adenocarcinoma are recognized: adenomatous-intestinal, hybrid/mixed and foveolar gastric types. We are interested in the phenotypes of these dysplasias and adenocarcinomas, especially in the possible relationship between them. For this reason, we evaluated the immunohistochemical expression of intestinal and gastric markers in a series of 30 cases of esophageal high-grade dysplasia (high-grade intraepithelial neoplasia) and of 70 adenocarcinomas. For immunohistochemical classification, we used double immunohistochemical reactions CDX2/MUC5AC and CDX2/MUC6, respectively. In cases of incomplete intestinal metaplasia, hybrid/mixed high-grade dysplasia and hybrid/mixed adenocarcinoma, we found the expression of gastric mucins MUC5AC and MUC6 only in cells with intestinal differentiation (with nuclear positivity for CDX2). The double immunostaining excluded the presence of the cells with “pure” foveolar gastric phenotype in hybrid lesions. Thus, the hybrid category actually represents the intestinal type dysplasia/adenocarcinoma (which is known to have a better prognosis than the foveolar gastric type).
Keywords:
immunohistochemistry – double immunostaining – reflux esophagitis – Barrett esophagus – esophageal dysplasia – esophageal adenocarcinoma
Reflux esophagitis with columnar lined esophagus is a complication of long-term gastroesophageal reflux disease and represents the main precursor of esophageal adenocarcinoma (1-4). Three epithelial types, cardiac, oxyntocardiac and intestinal are recognized to constitute gastroesophageal reflux disease often in a specific direction from distal to proximal in the esophagus (2,3,5,6). The presence of intestinal metaplasia (IM) has a greater impact on prognosis (2,3). On the basis of morphology and mucin production, the following immunohistochemical classification of IM was proposed: 1. the mixed intestinal and gastric type, i.e. incomplete IM with intestinal epithelium, producing gastric mucins and generally lacking an absorptive epithelium, and 2. the purely intestinal type, i.e. complete IM with the presence of goblet cells, absorptive cells, neuroendocrine cells and Paneth cells (7-11). Recently, it has been suggested that incomplete IM which produces gastric mucins represents an initial, immature, and genetically and chromosomally unstable stage (11) with increased proliferation activity (9). This incomplete IM may either undergo maturation into complete non proliferative IM or it persists and progresses to dysplasia and adenocarcinoma (10-18). The esophageal dysplasia and adenocarcinoma are currently classified into the following three types: 1. adenomatous/intestinal, 2. hybrid/mixed, 3. foveolar gastric (19). This classification is based on the presence of intestinal and/or gastric foveolar differentiation. It is suggested that intestinal differentiation means a better prognosis in comparison with foveolar gastric differentiation (8,15,16), and therefore an examination of the phenotype of the lesion is important. To further characterize an immunophenotype of dysplasias and adenocarcinomas occurring in Barrett’s esophagus and to evaluate possible relationships between types of dysplasia, we immunohistochemically studied a series of 30 cases of high grade dysplasia and 70 adenocarcinomas (all in patients with long-term reflux disease), using double immunostaining for both intestinal and gastric markers. Our results indicate that the hybrid/mixed phenotype in fact represents an intestinal phenotype, and that it should be regarded as such.
MATERIALS AND METHODS
Our study includes 30 patients with esophageal high grade dysplasia (26 males and 4 females, with a mean age 60 years, ranging in the males from 33 to 83 years and in the females from 69 to 88 years), and 70 cases of esophageal adenocarcinoma (61 males and 9 females, with a mean age of 71 years, ranging in males from 42 to 88 years and in females from 52 to 93 years). Endoscopic biopsies (specimens as a single or multiple pieces 2-3 mm in diameter) were processed routinely and stained with hematoxylin-eosin, AB-PAS (alcian blue at pH 2.5, periodic acid-Schiff reagent). Immunohistochemistry was performed using standard avidin-biotin complex peroxidase technique, and included markers of intestinal differentiation (CDX2, clone EPR2764), gastric differentiation (MUC5AC, clone MRQ-19, Cell Marque, MUC6, clone MRQ-20, Cell Marque), and Ki 67 (clone MIB1, Ventana). We evaluated intestinal metaplasia, high-grade dysplasia, and adenocarcinoma in dual-color immunohistochemical staining, performed on a single slide by sequentially applying the antibodies CDX2 and MUC5AC, or CDX2 and MUC6, respectively.
High-grade dysplasia was classified histologically as the adenomatous, hybrid/mixed, or foveolar gastric type, respectively. Adenomatous and hybrid high-grade dysplasia resembled colonic adenoma with columnar cells and penicillate nuclei. Foveolar gastric high-grade dysplasia was composed of cuboidal to columnar cells with a pale clear cytoplasm and hyperchromatic round nuclei. In accordance with the WHO classification (19), the carcinomas were classified into following histological types: well differentiated papillary and tubular adenocarcinoma, moderate and poorly differentiated tubular adenocarcinoma, mucinous adenocarcinoma, signet ring cell adenocarcinoma, and diffuse or poorly cohesive adenocarcinoma. Immunohistochemically, they were classified as follows: the intestinal type, hybrid type, or foveolar gastric type. The immunophenotype was classified as intestinal if the lesion stained positively for CDX2 and negatively for both MUC5AC and MUC6; as foveolar gastric if positive for MUC5AC and MUC6 and negative for CDX2; and as hybrid/mixed if positive for combination of CDX2 and gastric markers. A diagnosis of hybrid high-grade dysplasia/adenocarcinoma was done only in cases which showed CDX2 positivity and positivity for MUC5AC and MUC6 in a minimum of 5% of the cells (12). The background mucosa was classified as cardiac-type, cardiac-type with focal incomplete IM, or as oxyntocardiac-type. Squamous islands, squamous epithelium overlying glands (buried columnar epithelium), multilayered epithelium and gland ducts were identified for confirmation of the esophageal location.
RESULTS
All cases of esophageal high-grade dysplasia and adenocarcinoma (Fig. 1) showed variable positivity for the immunohistochemical markers. Immunohistochemically, 6 of 30 cases (20 %) of high grade esophageal dysplasia were of the adenomatous type, with positivity for CDX2. 23 of 30 cases (77 %) of the high-grade dysplasia were of the hybrid/mixed type. Hybrid/mixed dysplasia showed expression of both CDX2 and MUC5AC (Fig. 2A). Only one case of high grade dysplasia (3 %) was of the foveolar gastric type, with immunohistochemical negativity of CDX2 and with expression of MUC5AC.
1. A. High-grade dysplasia. B. Esophageal adenocarcinoma of intestinal type. C. Esophageal adenocarcinoma of foveolar gastric type, with formation of solid nests. D. Esophageal adenocarcinoma of hybrid type (HE, original magnifications 200x in A, 100x in B-D, respectively). 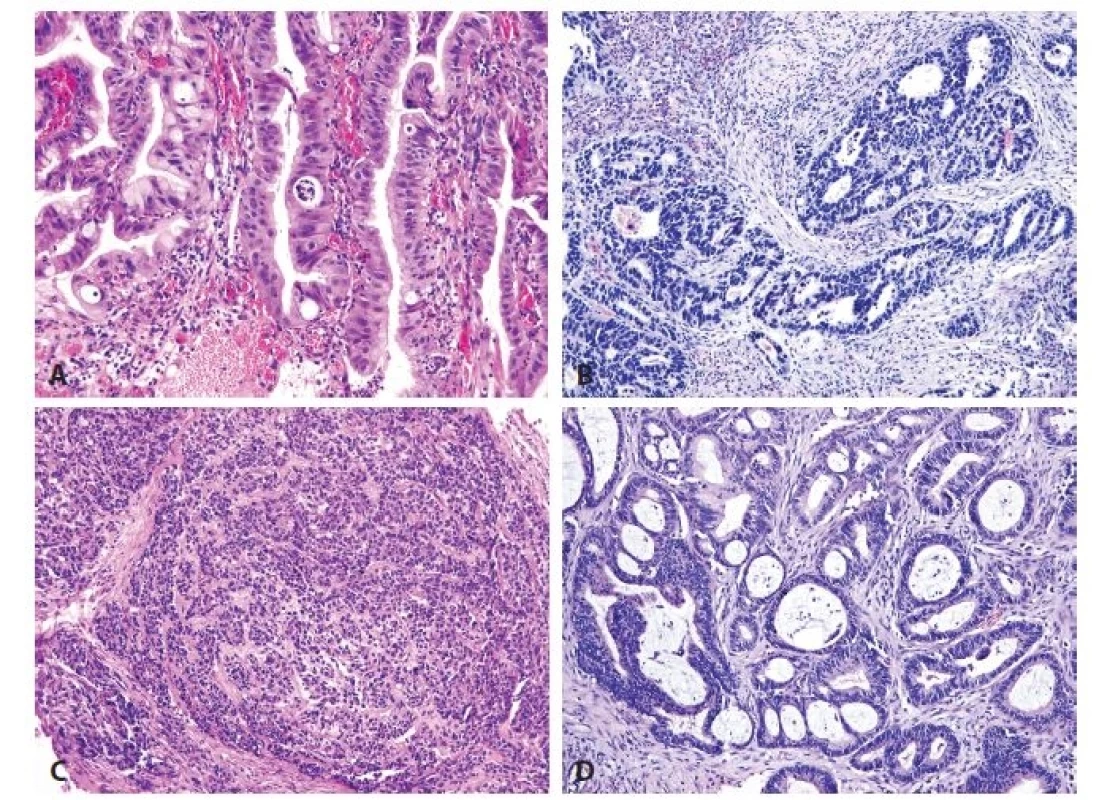
2. Immunohistochemical findings. A. High-grade hybrid dysplasia with nuclear CDX2 positivity (red) and cytoplasmic MUC5AC expression (brown) in cells. No cell with negativity of CDX2 and positivity of MUC5AC is seen. B. Esophageal adenocarcinoma of intestinal type shows CDX2 nuclear positivity. C. Esophageal adenocarcinoma of foveolar gastric type is positive for MUC5AC. D. Esophageal adenocarcinoma of hybrid type. The nuclei are positive for CDX2 (red) and cytoplasm for MUC5AC (brown). No cell with negativity of CDX2 and positivity of MUC5AC is seen (original magnifications 400x in A, 100x in B-D, respectively). 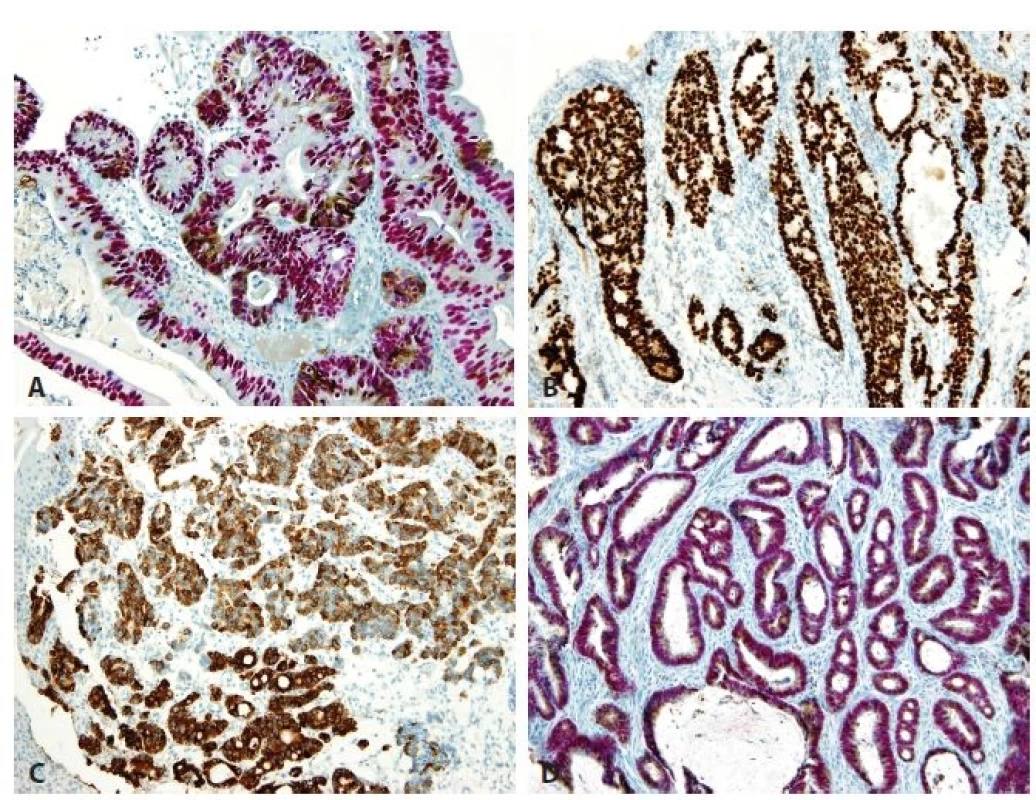
In the series of 70 adenocarcinomas, 16 cases (23 %) were well differentiated, 19 cases (27 %) were moderately differentiated, and 35 cases (50 %) were poorly differentiated. Immunohistochemically, intestinal type adenocarcinomas positive only for CDX2 (Fig. 2B) accounted for 14 of 70 adenocarcinoma cases (20 %). They were more numerous than the group of 5 foveolar gastric type adenocarcinomas (7 %) which were all positive for MUC5AC and MUC6 and negative for CDX2 (Fig. 2C). Fifty hybrid/mixed cases of adenocarcinoma (73%) showed diffuse positivity for CDX2 (Fig. 2D). Simultaneously, the CDX2 positive cells were often positive for MUC5AC (Fig. 2D), but none of the cells showed isolated expression of gastric mucins without positivity for CDX2. MUC6 was positive in 26 (52 %) hybrid adenocarcinomas and in 6 cases (20 %) of hybrid high-grade dysplasia (in addition to MUC5AC positivity). None of our cases of high-grade dysplasia and adenocarcinoma was of the null phenotype that is characterized by loss of intestinal and gastric markers.
The grade of differentiation of adenocarcinomas varied (Tab. 1). Among well and moderately differentiated adenocarcinomas, the intestinal type was seen in 8 cases (11%) and the hybrid type in 26 cases (43 %). Only a single case of the foveolar gastric type adenocarcinoma was well differentiated. The microscopic assessment of lateral margin status and depth of carcinoma invasion could not be determined.
1. Grading and immunohistochemical types of adenocarcinomas (numbers of cases). 
Previous biopsies performed several month beforehand, with findings of high-grade dysplasia, were available in only 7 cases of adenocarcinoma. In all of these previous biopsies, the type of high-grade dysplasia correlated with the type of subsequent adenocarcinoma (3 cases of the intestinal type, 3 cases of the hybrid type, and one case of the foveolar gastric type).
Regarding findings in non-dysplastic background mucosa, 30 biopsies with high-grade dysplasia contained cardiac type mucosa in 16 cases, oxyntocardiac type mucosa in 2 cases, incomplete intestinal metaplasia in 12 cases (Fig. 3A), and squamous cell islands in 14 cases. Among 70 cases of adenocarcinoma, the adjacent mucosa showed the following morphology: cardiac type in 23 cases, oxyntocardiac type in 5 cases, incomplete IM in 15 cases, and squamous epithelium in 13 cases (the squamous epithelium covered partially cardiac mucosa or adenocarcinoma). The background mucosa of the cardiac and oxyntocardiac types was positive for gastric mucins (MUC5AC and MUC6) a negative for CDX2. Incomplete IM showed typical nuclear positivity for CDX2 and cytoplasmic positivity for gastric mucins MUC5AC/MUC6 (Fig. 3B).
3. Esophageal incomplete intestinal metaplasia. A. Cylindrical cells with mucin production and without brush border. B. Double immunohistochemical reaction shows nuclear positivity for CDX2 (red) and cytoplasmic expression for MUC5AC (brown). No cell with negativity of CDX2 and positivity of MUC5AC is seen (original magnifications 200x). 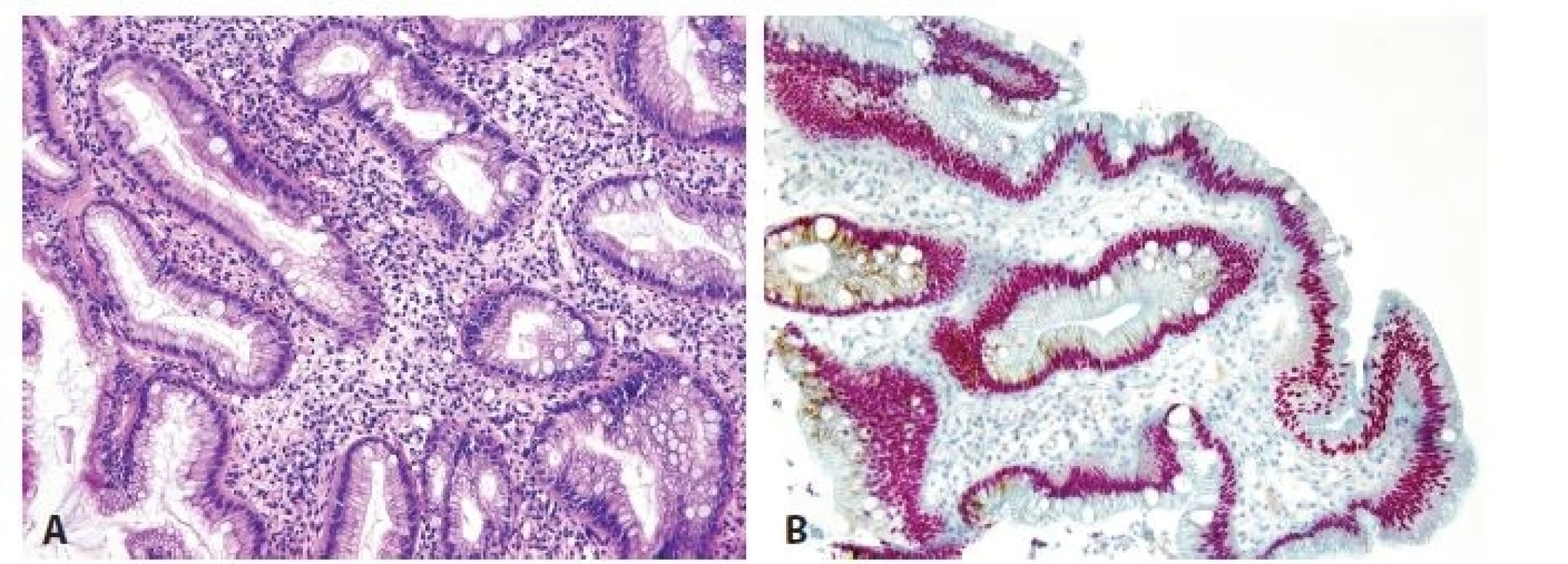
DISCUSSION
Barrett’s esophagus is the predisposing condition of esophageal adenocarcinoma (1,4,7,9,10,16,17,20). IM arises in cardiac-type mucosa (1,2,9,12,15) and is almost always incomplete (8,11-14). Incomplete IM represents an unstable epithelium with high proliferation activity and precancerous potential (1,7,10). It differs from complete IM that is regarded as a terminally differentiated mucosa which is composed of non-proliferative cells (9,11,13). There is evidence that gastric epithelium in cardiac-type mucosa undergoes intestinal differentiation in which CDX2 expression is an early event, whereas goblet cells and MUC2 positivity occur late in the intestinalization process (4,8,9,21,22). Due to its high sensitivity, we used CDX2 as a marker for intestinal differentiation. In addition, we did take advantage of double immunostaining for CDX2 and gastric markers, because this technique can demonstrate a relationship between cells with intestinal and gastric differentiation.
Using this method we have seen that all cells of incomplete IM (4,8) exhibited nuclear positivity of CDX2. A part of these cells, including goblet cells, were also positive for gastric mucins, whereas some cells were only of the intestinal type (CDX2+/MUC5AC-/MUC6-).
We also found that high-grade dysplasia and adenocarcinoma showed often, in double immunostaining, a simultaneous expression of both CDX2 and gastric mucins as is seen in incomplete IM, but importantly, epithelial cells with gastric mucin-only phenotype were not present in any case. The lesions with simultaneous positivity for CDX2 and gastric mucins represent a category of hybrid/mixed dysplasia and hybrid/mixed adenocarcinoma in current classification. Our results indicate, however, that these lesions are of the adenomatous/intestinal type and that they do not represent an admixture of both adenomatous and gastric cell types (8,14,15). For this reason, the separation of a hybrid category from intestinal dysplasia and adenocarcinoma could be abandoned (15).
It is evident that intestinal metaplasia may not be a prerequisite to all cases of dysplasia and adenocarcinoma arising in columnar lined esophagus. Recent studies suggest that cardiac foveolar epithelium also shows a loss of heterozygosity and chromosomal abnormalities which are similar to those of incomplete IM (1,4-7,9,15-18,23). It is commonly believed that dysplasia and adenocarcinoma in columnar lined esophagus take two main forms: the adenomatous-intestinal/hybrid type and the foveolar gastric type (7,8,14,15,17).
Regarding the frequency of the two main types of dysplasia, i.e. the adenomatous/hybrid type and the foveolar gastric type, the existing studies have brought conflicting results. Brown et al. (15) found foveolar gastric high-grade dysplasia in 46 % of cases, and Khor et al. found it even in 73 % of cases (8). However, a majority of studies found a high predominance of the adenomatous/hybrid type (7,9,19,20,23,24). Similarly, we have seen the adenomatous/hybrid type in 97 % of high-grade dysplasia.
Among adenocarcinomas, the reported frequency of both types is also various. Agoston et al. (24) found an approximately similar frequency of both types. Bosman (19) and Smith et al. (3) described a predominance of the intestinal type, whereas Aida et al. and Khor et al. found a predominance of the foveolar gastric type (5,8). In our study, we have seen in the double color method the intestinal type in 20 % and the hybrid type in 73 %, i.e. intestinal and hybrid types together represent 93 % of adenocarcinomas, whereas the foveolar gastric type was found in 7 % of cases, only. We think that it is difficult to explain these conflicting results. They are probably related to differences in immunohistochemical techniques. The advance of our study is in the use of dual-color immunohistochemical staining, which has not been performed in previous studies.
Regarding the correlation between the type of high-grade dysplasia and the type of subsequent carcinoma, our 7 cases of adenocarcinoma showed high-grade dysplasia in previous endoscopic biopsies. In the remaining cases, such a biopsy was not performed. Although our number of cases is low, it shows 100% correlation between the type of adenocarcinoma and the type of previously diagnosed dysplasia. Previous studies by Agoston et al. (24) and Brown et al. (15) also reported positive correlation between dysplasia and subsequent adenocarcinoma, whereas Khor et al. (8) found a small discordance between immunophenotype of dysplasia and intramucosal adenocarcinoma (in three of 23 adenocarcinomas).
In the studies of Takubo et al. (6) and Watanabe et al. (12), the majority of all tumors were of the mixed immunophenotype, but the foveolar gastric type was more common among minute carcinomas. Khor et al. (8) suggest a phenotype shift from gastric to mixed phenotypic expression during tumor growth. Our results as well as those of others do not confirm this opinion on the phenotype shift.
We did not find a decline in CDX2 expression from incomplete IM to dysplasia to adenocarcinoma, as was reported by Khor et al. (8). In some of our cases, an intensity of CDX2 expression fluctuated irregularly, irrespective of the type of the lesion. This small variability was probably related to fixation and tissue processing.
The behavior of esophageal carcinoma is related to its phenotype and differentiation. Intestinal type esophageal adenocarcinomas have a better prognosis when compared with foveolar gastric type tumors (8,15,16,21). It is explained by a possible tumor suppressor function of CDX2 (8,16,21). Also, at the level of dysplasia, the foveolar gastric type shows a higher rate of DNA abnormalities and is more frequently high-grade in comparison with the adenomatous/hybrid type (8,11,23), reflecting aforementioned differences in behavior between both types. In addition, intestinal type adenocarcinoma shows abnormal nuclear beta-catenin expression more frequently, whereas EGFR amplification and poor survival were more often associated with foveolar gastric type tumors (16).
In conclusion, our findings indicate that the two following types of dysplasia and adenocarcinoma develop in columnar lined esophagus: adenomatous-intestinal and foveolar gastric. Using double immunostaining for CDX2 and gastric mucins, we excluded the dual phenotype of the cells in hybrid type dysplasia/adenocarcinoma. Although some cells of these so-called hybrid type lesions express gastric mucins, they all are always positive for the intestinal marker CDX2. For this reason, we concur with the opinion that the present hybrid type of dysplasia/adenocarcinoma should be regarded as an intestinal type. Differentiation between the intestinal and foveolar gastric types of dysplasia and adenocarcinoma has a prognostic and potentially therapeutic importance.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of this paper.
Correspondence address:
Alena Chlumska, MD
Biopticka laboratoř, s.r.o.
Mikulasske nam. 4, 32600 Plzen
phone: +420-737-220-403
e-mail: chlumska@medima.cz
Labels
Anatomical pathology Forensic medical examiner Toxicology
Article was published inCzecho-Slovak Pathology

2018 Issue 2-
All articles in this issue
- Frozen section: history, indications, contraindications and quality assurance
- Frozen section examination of pancreas, gallbladder, extrahepatic biliary tree, liver, and gastrointestinal tract
- Intraoperative diagnosis of the head and neck lesions, thyroid and parathyroid gland, bone and soft tissue, and genitourinary tract
- Esophageal dysplasia and adenocarcinoma: a study with double immunostaining for intestinal and gastric markers
- Results of morphological screening for Lynch syndrome during the period 2013-2016
- Czecho-Slovak Pathology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Frozen section: history, indications, contraindications and quality assurance
- Frozen section examination of pancreas, gallbladder, extrahepatic biliary tree, liver, and gastrointestinal tract
- Intraoperative diagnosis of the head and neck lesions, thyroid and parathyroid gland, bone and soft tissue, and genitourinary tract
- Results of morphological screening for Lynch syndrome during the period 2013-2016
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

