-
Medical journals
- Career
Angiotensin converting enzyme inhibitors in paediatric cardiac patients – current experience and practice
Authors: L. Muhammad Najih; Z. Slavík
Authors‘ workplace: Department of Paediatrics, Royal Brompton and Harefield NHS Foundation Trust, Sydney Street, London, SW3 6NP, United Kingdom
Published in: Čes-slov Pediat 2020; 75 (5): 292-302.
Category: Review
Overview
Paediatric heart failure is a relatively common and potentially life-threatening complication of various diseases, including congenital heart defects and myocardial diseases (e.g. cardiomyopathy and myocarditis). Angiotensin converting enzyme (ACE) inhibitors are currently accepted part of treatment in paediatric heart failure. Captopril, Enalapril, Lisinopril, and Ramipril are the most frequently used ACE inhibitors in paediatric cardiac patients.
We reviewed published data and our institutional practice related to the use of ACE inhibitors in children with heart failure to assess their indications, dose range, safety profile, and outcome data where available.
Although paediatric large scale randomized trails are rare, the published results of ACE inhibitor therapy in managing paediatric heart failure are encouraging. We recommend early initiation, appropriate choice of medication, adequate dosage, and close follow up for monitoring of potential adverse effects.
Keywords:
heart failure – paediatric – angiotensin-converting enzyme inhibitor
INTRODUCTION
Paediatric heart failure (HF) is most often associated with congenital heart disease, cardiomyopathy and acquired heart disease [1]. The incidence of congenital heart disease appeared relatively stable between 6–8/1000 live births world-wide before the impact of fetal cardiac diagnoses and termination of pregnancy in complex congenital heart defects was present [2, 3]. However, only a small percentage of these defects are severe enough to result in heart failure during childhood, while most of the lesions are diagnosed and corrected at an early age. The incidence of primary cardiomyopathy (CM) in developed countries is 0.8 and 1.3 cases per 100,000 children but the risk is up to 10 times higher among infants <1 year old [4, 5]. The majority (87%) of new-onset heart failure patients are diagnosed in a state of severe decompensation (Table 1) [5], and only less than 50% of these children will survive for 5 years without cardiac transplantation [6]. As a result, cardiomyopathy contributes significantly to the number of paediatric patients who present with the symptoms of cardiac failure.
1. NYHA and Modified Ross Heart Failure Classification for Children. 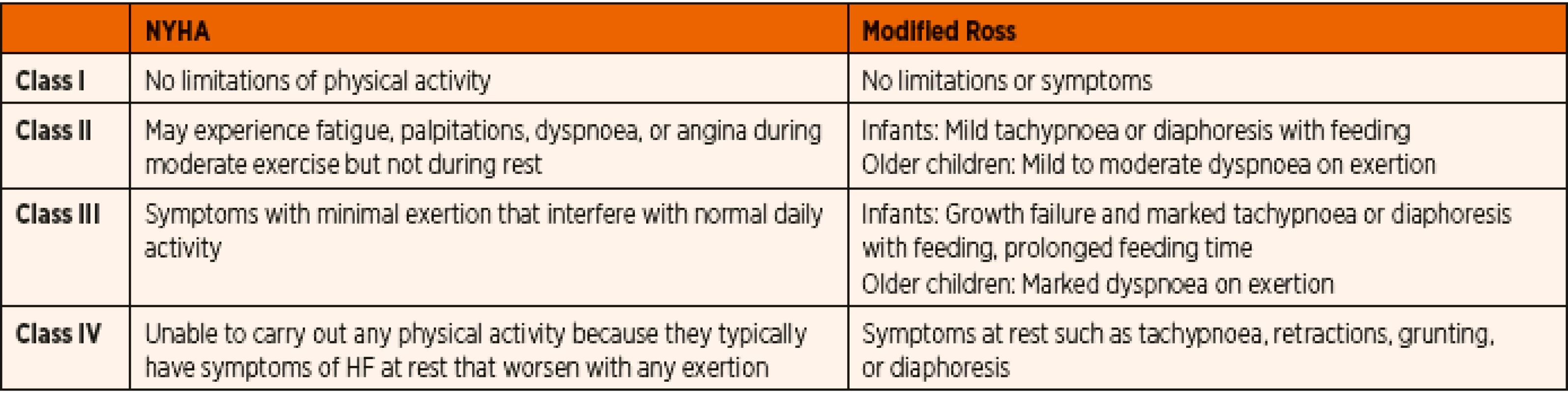
METHODS
Review of the available published evidence (until October 2019) for paediatric heart failure management was undertaken. Literature searches were done using a variety of search engines including PubMed, Medline, Cochrane Database of Systemic Reviews, BMJ Clinical Evidence, and Google scholar to identify recent literature. We also reviewed our institutional database in a tertiary paediatric cardiac center to assess the contemporary use of ACE inhibitors in children, their indications, dose range, safety profile, and outcome data where available.
RATIONALE FOR THE USE OF ACE INHIBITORS IN PAEDIATRIC HEART FAILURE
Paediatric heart failure relates to abnormal changes in cardiac preload, afterload, and/or myocardial function. It leads to impaired end organ perfusion and neuro-hormonal response [7]. One of the compensatory mechanisms is activation of the renin-angiotensin-aldosterone axis with increase of levels of angiotensin and aldosterone. These contribute to vasoconstriction to maintain end organ perfusion [8, 9]. Angiotensin II, due to its potent vasoconstrictor effect, causes release of norepinephrine and induces myocardial remodeling. This leads to myocardial hypertrophy, apoptosis as well as fibrosis [9–11]. There is a marked decrease in the plasma renin activity even within the initial few days of the ACE inhibitor therapy [12, 13]. The angiotensin II activity as well as the aldosterone level in the plasma decreases significantly with time [14, 15]. There is a linear correlation of the plasma ACE activity with the serum ACE inhibitor concentration [16]. Due to its effect of decreasing angiotensin II and aldosterone levels ACE inhibitors have vasodilatory effect on both arterial and venous beds [17]. Therefore, these drugs have a favourable effect on the systemic afterload by decreasing systemic vascular resistance thereby enhancing stroke volume with positive impact on acute and chronic heart failure [18–21].
ACE inhibitors have a proven direct effect on systemic vascular resistence and aortic pressure in paediatric population [22–24]. More importantly, the drop in systemic vascular resistance occurred only in patients where this resistance was elevated and not in those with low systemic vascular resistence [23]. ACE-inhibitors have somewhat less predictable effect on the pulmonary vascular resistance due to decrease in left atrial pressure. Beneficial effect in heart volume overload related heart failure due to large left-to-right shunt relates to alteration in the ratio between systemic and pulmonary vascular resistance with ensuing reduction of the volume of left-to-right shunt [13, 24, 25].
CHOICE OF ACE INHIBITOR IN THE TREATMENT OF HEART FAILURE
As large randomized trials are rare in paediatric heart failure patients, experience with the use of ACE inhibitors has been and still is mostly derived from the adult cardiac failure studies. The beneficial effects of ACE inhibitors in reducing the mortality in adults with heart failure have been long established by the CONSENSUS and SOLVD trials [26, 27]. The adult studies have shown significant decrease in the symptoms, mortality, and morbidity, mainly with the use of enalapril, lisinopril, and ramipril [28–30]. There is currently no data directly comparing the different ACE inhibitors and their impact on adult heart failure patients.
In the neonatal and paediatric population, majority of the studies have used captopril and enalapril, with modest data for cilazapril, ramipril, lisinopril. Predominantly, these studies have been retrospective and assessed surrogate markers for clinical outcomes. Ramipril and lisinopril were mainly studied for their effects on systemic hypertension. Captopril was proposed for addition to the WHO essential medicine list – in children, however only enalapril is included in the 2019 list for its use in systemic hypertension [31]. Newer generation drugs such as benazepril, fosinopril,moexipril, trandolapril and quinapril are predominantly used in adult population.
EVIDENCE FOR POSITIVE IMPACT OF ACE INHIBITORS ON PAEDIATRIC HEART FAILURE
There is evidence for acute and long term improvement of the left ventricular ejection fraction and stroke volume without a significant change in the heart rate [17, 32] more pronounced in dilated than restrictive cardiomyopathy [12, 33]. Variable effect was present in patients with left-to-right shunt [14, 32, 34]. Children with significant aortic or mitral valvar regurgitation, either native or post-operative, showed improvement in the left ventricular dimensions, regurgitation fraction, and left ventricular mass [15, 35, 36]. Improvement in the postoperative cardiac function with ACE inhibitor therapy has been demonstrated after surgical repair of congenital heart defects [16, 34].
a) Heart failure due to congenital heart defects with volume overload
Congenital heart defects (CHD) are the most common cause of paediatric heart failure and 20% of all patients with a CHD has HF [37, 38]. The worldwide estimate for live births with CHD was one million in the year 2001 [39]. The prevalence of CHDs are ever increasing owing to better diagnostic modalities, survival of preterm neonates and availability of corrective as well as palliative surgical treatment options. Consequently, the number of children with associated complications and morbidities requiring medical treatment will continue to increase [40]. These patients will include those awaiting cardiac surgical treatment or those with residual post-operative lesions in need of medical therapy.
Published evidence on ACE inhibitor use in paediatric population with structural congenital heart disease is summarized in Table 2. Early prospective and retrospective studies suggested clinical improvement with better weight gain, lower respiratory rate, and improved feeding. [14, 41–43]. Smaller studies evaluating the echocardiographic parameters show conflicting outcomes with regards to left ventricular dimensions and function. In the most recent review, Slipszuk et al. concluded that ACE inhibitors are an effective treatment in patients with mitral valve regurgitation without hypertrophic cardiomyopathy or mitral valve prolapse [44]. The International Society for Heart and Lung Transplantation (ISHLT) guidelines recommended routine incorporation of ACE inhibitor therapy for symptomatic chronic systolic heart failure with reduced ejection fraction, unless there is specific contraindication [1]. The advice is to start at a low dose and titrate to a maximum tolerated safe dose. For those remaining asymptomatic, routine use of ACE inhibitors was also advocated.
2. Published evidence on ACE inhibitor (ACEi) therapy in paediatric structural congenital heart disease. 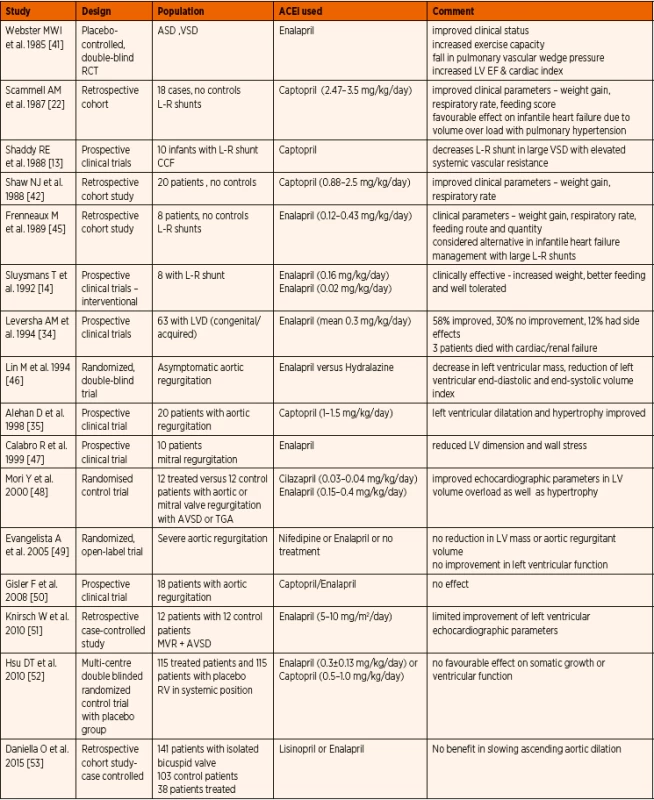
Abbreviations: ASD – atrial septal defect, AVSD – atrio-ventricular septal defect, L-R – left to right, LVD – left ventricular dilatation, MVR – mitral valve regurgitation, RV – right ventricle, TGA – transposition of great arteries, VSD – ventricular septal defect b) Heart failure due to primary cardiomyopathy
Published evidence on the use of ACE inhibitors in paediatric cardiomyopathy patients is listed in Table 3. Dilated cardiomyopathy (DCM) is the most common form of cardiomyopathy in children [6, 54] with primary myocardial systolic dysfunction. Pharmacotherapy of DCM includes positive inotropic agents, diuretics, and substances decreasing afterload. Most published evidence showed clinical improvement even beyond the first year of therapy and better left ventricular size and systolic function. Invasive quantification techniques provided evidence of improved cardiac index, stroke volume, and a considerable decrease in afterload.
3. Published data on ACE inhibitor therapy in paediatric primary cardiomyopathy. 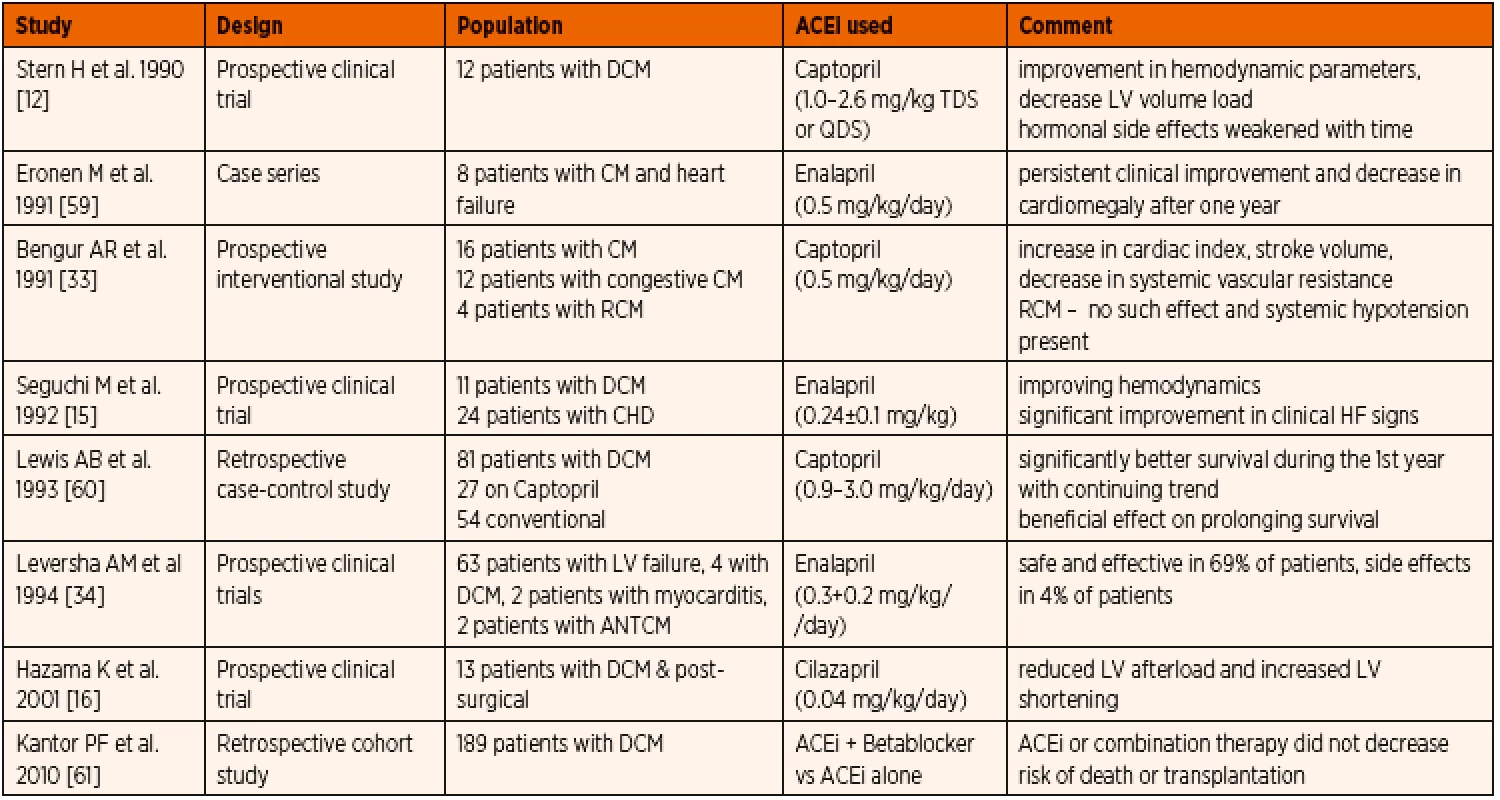
Abbreviations: ACEi – angiotensin convering enzyme inhibitor, ANT – anthracycline, CHD – congenital heart disease, CM – cardiomyopathy, DCM – dilated cardiomyopathy, HF – heart failure, LV – left ventricle In restrictive cardiomyopathy (RCM), ACE inhibitor treatment could be considered, however systemic vasodilation in the setting of reduced left ventricular diastolic function can have deleterious effect on end organ perfusion [33]. While inhibition of renin-angiotensin-aldosteron system can have a favourable impact on ventricular hypertrophy and diastolic function in secondary myocardial hypertrophy [55] when used in patients with dynamic left ventricular outflow obstruction as seen in hypertrophic cardiomyopathy (HOCM), a deleterious fall in cavity size and worsening outflow gradient along with impaired LV compliance could be observed [56]. Hence vasodilators including ACE inhibitors are usually avoided in severe form of HOCM or late stages of RCM [56, 57].
The ISHLT recommends initiation of ACE inhibitor therapy in individuals with Duchenne muscular dystrophy unless contraindicated [1]. The appropriate age of initiation of therapy is not specified. The Canadian guidelines on paediatric heart failure management strongly recommended ACE inhibitor therapy in children with primary left ventricular myocardial disease states, though the current level of evidence was moderate [58].
c) Heart failure due to chemotherapy induced cardiotoxicity
Anthracycline is a topoisomerase-interacting agent used in most malignancy treatment protocols. As most of the chemotherapeutic regimens depend on the agents like doxorubicin or daunorubicin with known affinity to cardiolipin, the long term cardiac effects cause considerable concern. The initial results from study of Lipshultz et al. were encouraging as to improved left ventricular shortening fraction and mass in the initial 6 years, followed by deterioration and associated mortality or need for heart transplantation [32]. This could be attributed to the inherent prognosis of the disease and not solely on the ACE inhibitor therapy. Silber et al. reported a lower left ventricular wall stress during the first 5 years of treatment with ACE inhibitors maintained throughout the study period, however there was no parallel improvement in cardiac index, stress-velocity index or fractional shortening [62]. In more recent studies, preservation of left ventricular function or its less significant deterioration were observed. The biomarkers of cardiac failure were also lower in the group receiving ACE inhibitors when compared to placebo [63–65]. When parameters with better sensitivity such as strain analysis with speckle tracking technique was employed, a significant improvement in the cardiac function was appreciated which continued follow up [63–65]. These studies are encouraging the routine use of ACE inhibitors to prevent cardiac deterioration after anthracycline exposure (Table 4).
4. Published evidence on the use of ACE inhibitors in paediatric anthracycline induced cardiomyopathy. 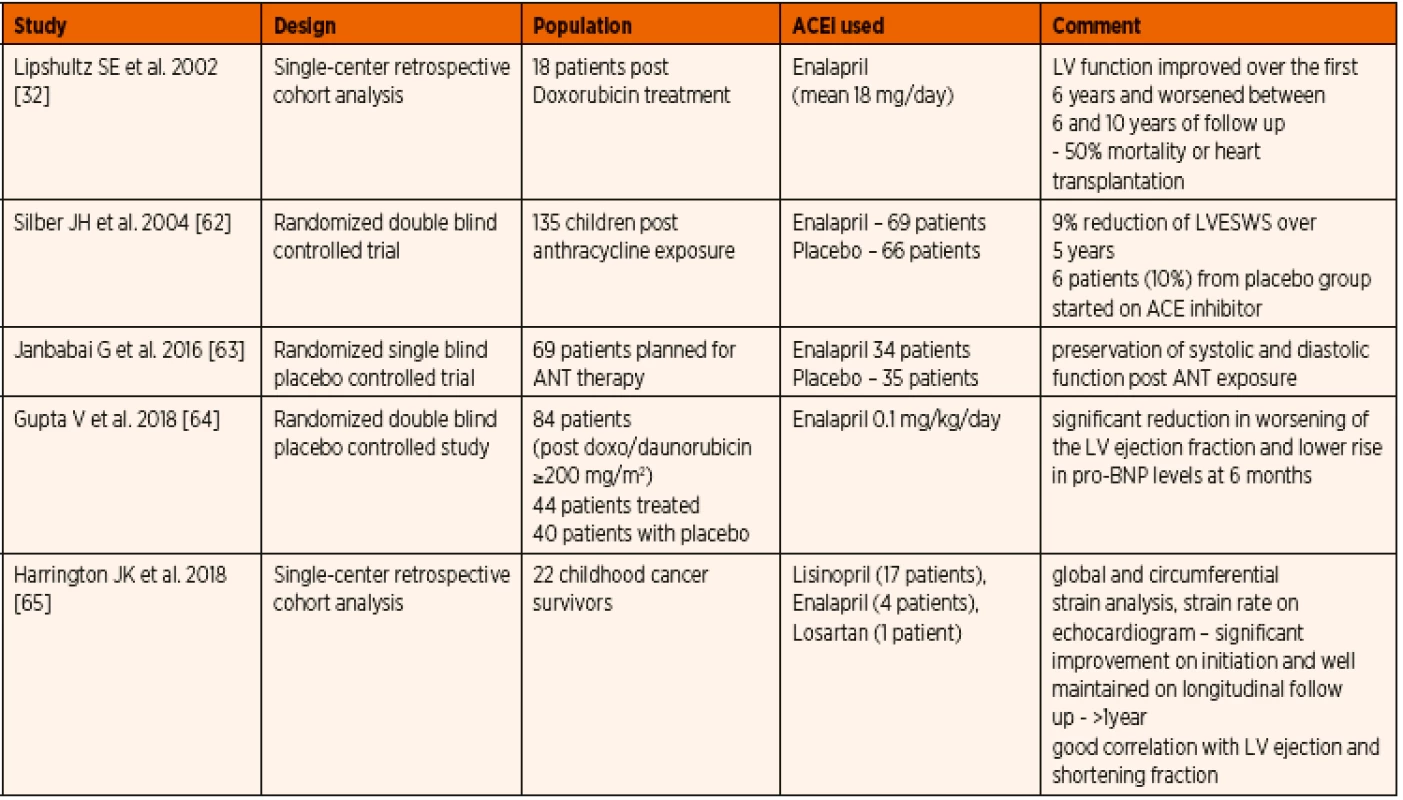
Abbreviations: ANT – anthracycline, LV – left ventricle, LVEWS – left ventricular end-systolic wall stress, pro-BNP – pro-brain natriuretic peptide d) Prophylactic use in single ventricle congenital heart defect physiology
Initial study focusing on the somatic growth during the inter-stage periods for completion of single ventricle palliation pathway did not show any significant advantage of ACE inhibitor therapy [52]. Also, when non-invasive ventricular functional assessment was done, there was no demonstrable effect with prophylactic ACE inhibitors administration [66]. At the time of invasive assessment of cardiac function during cardiac catheterization, the end diastolic pressure and pulmonary arterial and mean atrial pressures were lower among patients receiving ACE inhibitor therapy [67]. In a large multi-centre retrospective case controlled cohort study extracted from National Pediatric Cardiology Quality Improvement Collaborative database between 2008 and 2015, Doris et al. concluded that there has been no beneficial effect of prophylactic ACE inhibitor therapy to prevent inter-stage heart failure in infants with single ventricle physiology [68].
Prophylactic interstage ACE inhibitor therapy for children undergoing staged surgical palliation for single ventricular congenital heart defects has always shown discouraging evidence with no significant added benefits. The recommendation from the ISHLT guideline is that ACE inhibitor therapy should not be routinely instituted for all patients with single ventricle, however its use could be considered in specific cases such as valvar regurgitation or ventricular dysfunction [1] (Table 5).
5. Summary of published experience with ACE inhibitor therapy in paediatric single ventricle physiology. 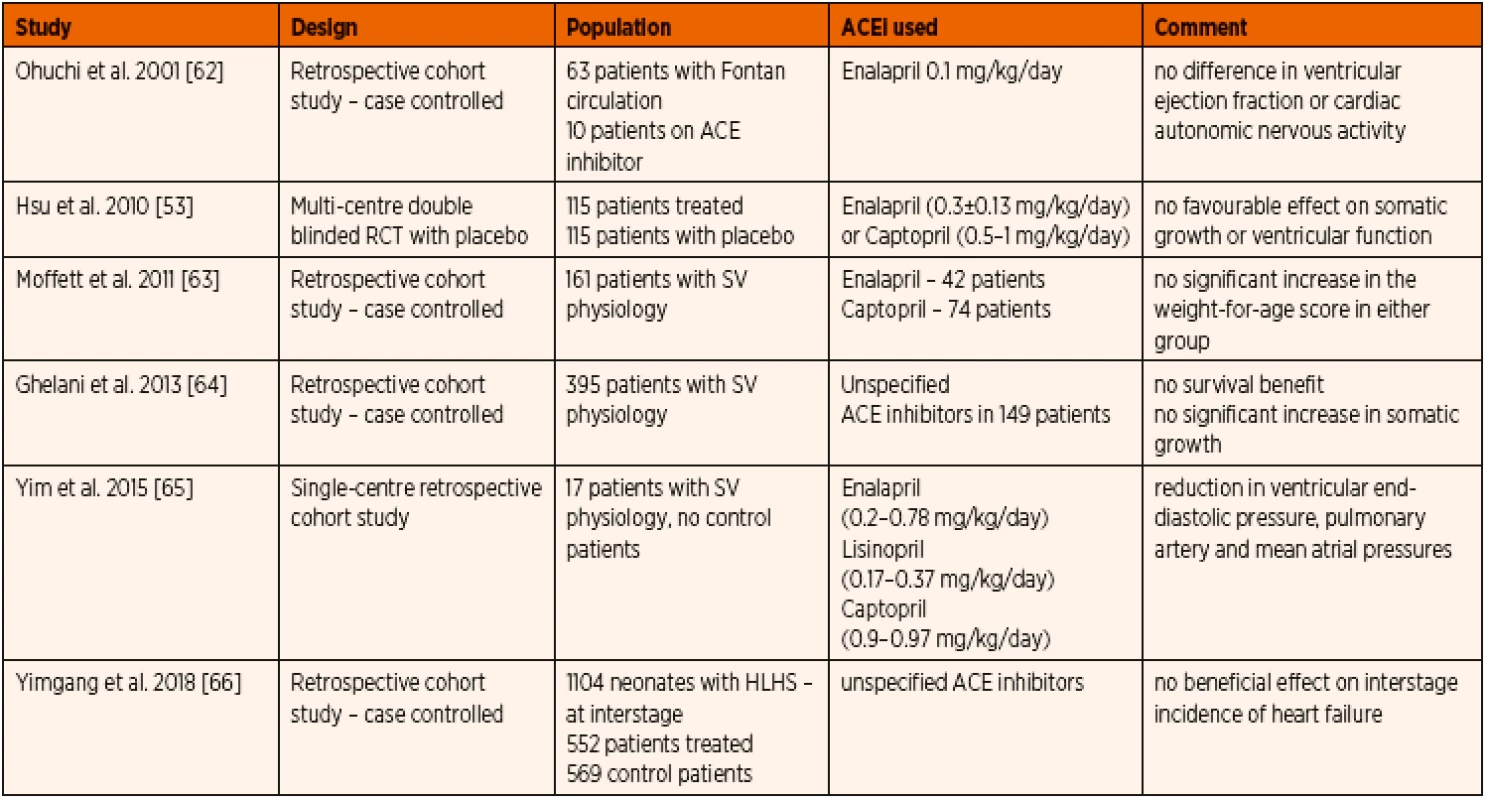
Abbreviations: HLHS – hypoplastic left heart syndrome, SV – single ventricle e) ACE inhibitor therapy in early paediatric post-operative heart failure
Heart failure, both due to cardiac or extra-cardiac cause is a common diagnosis in paediatric intensive care (PICU). In a cardiac PICU, it is frequently seen immediately following a cardiac surgical procedure. This leads to prolonged hospitalization, significant morbidity as well as mortality [69]. There have been reports of considerable improvement with enalapril in postoperative heart failure [34]. Though no large randomized controlled trails assessing their routine use in PICU have been reported, the consensus statement from the Paediatric Cardiac Intensive Care Society from 2014 has recommended the routine use of ACE inhibitors in these children [70]. This is in line with the Canadian Cardiovascular Society and ISHLT guidelines [1, 58].
PHARMACOKINETICS
Being a heterogeneous group of drugs, ACE inhibitors have varied pharmacokinetic profiles which enables the clinician to choose wisely for a given patient (Table 6). Lisinopril and captopril do not require hepatic activation and hence can be safely prescribed even in severe hepatic failure [71]. Enalapril is the only intravenous preparation available and it is also safe to use in liver disease. Lisinopril is almost exclusively excreted by kidneys. Fosinopril is unique as it does not require dose adjustment in renal disease (all other ACE inhibitors need dose modification) owing to its unique property of compensatory dual routes of elimination [71]. Captopril interacts with food and need spacing after meals.
6. Pharmacokinetics of commonly used ACE inhibitors used in paediatric patients [70]. ![Pharmacokinetics of commonly used ACE inhibitors used in paediatric patients [70].](https://www.prolekare.cz/media/cache/resolve/media_object_image_small/media/image_pdf/d3bcaca3c3789c8452a9a7a705985f54.png)
With limited number of pharmacokinetic studies in paediatric population, it has been shown that in infants the peak serum concentration of enalaprilat occurs at 8 hours and the serum half-life (T ½) ranged from 6 to 10 hours [72]. In infants with heart failure, it was concluded that the enalapril was less bioavailable with shorter duration of action. It was suggested that the dose of enalapril needs to be calculated based on body surface area rather than the body weight in paediatric heart failure patients. The area under the curve (AUC) was similar both in children and adults when a dose of 1 mg/m2 of enalapril was used instead of body weight directed dosing. However, this was not true for preterm neonates who showed much higher AUC with delayed peak and delayed decline [73]. For cilazapril, a dose of 0.04 mg/kg demonstrated sufficient inhibition of plasma ACE activity and hemodynamic effects [16].
DOSE INITIATION AND TITRATION
The general consensus on ACE inhibitor therapy is to begin with a smaller ‘test’ dose and follow it up with gradual increase of doses as tolerated in order to avoid potential hypotension [70]. As the neonatal age group seems to react significantly differently, dosage needs to be markedly reduced in the setting of heart failure. Patients developing acute renal failure due to prolonged persistent inhibition of renin-angiotensin-aldosterone may benefit from either a short acting ACE inhibitor or a lower dose of long acting alternative. Appropriate choice of drug as well as tailored dosage needs to be prescribed in patients with hepatic or renal impairment.
The Paediatric Cardiac Intensive Care consensus document suggests a well-structured drug dosage for initiation and continuation therapy [70]. Our hospital guidelines for paediatric patients are listed in Table 7.
7. Royal Brompton and Harefield NHS Trust Guidelines for the use of ACE inhibitors in paediatric patients. 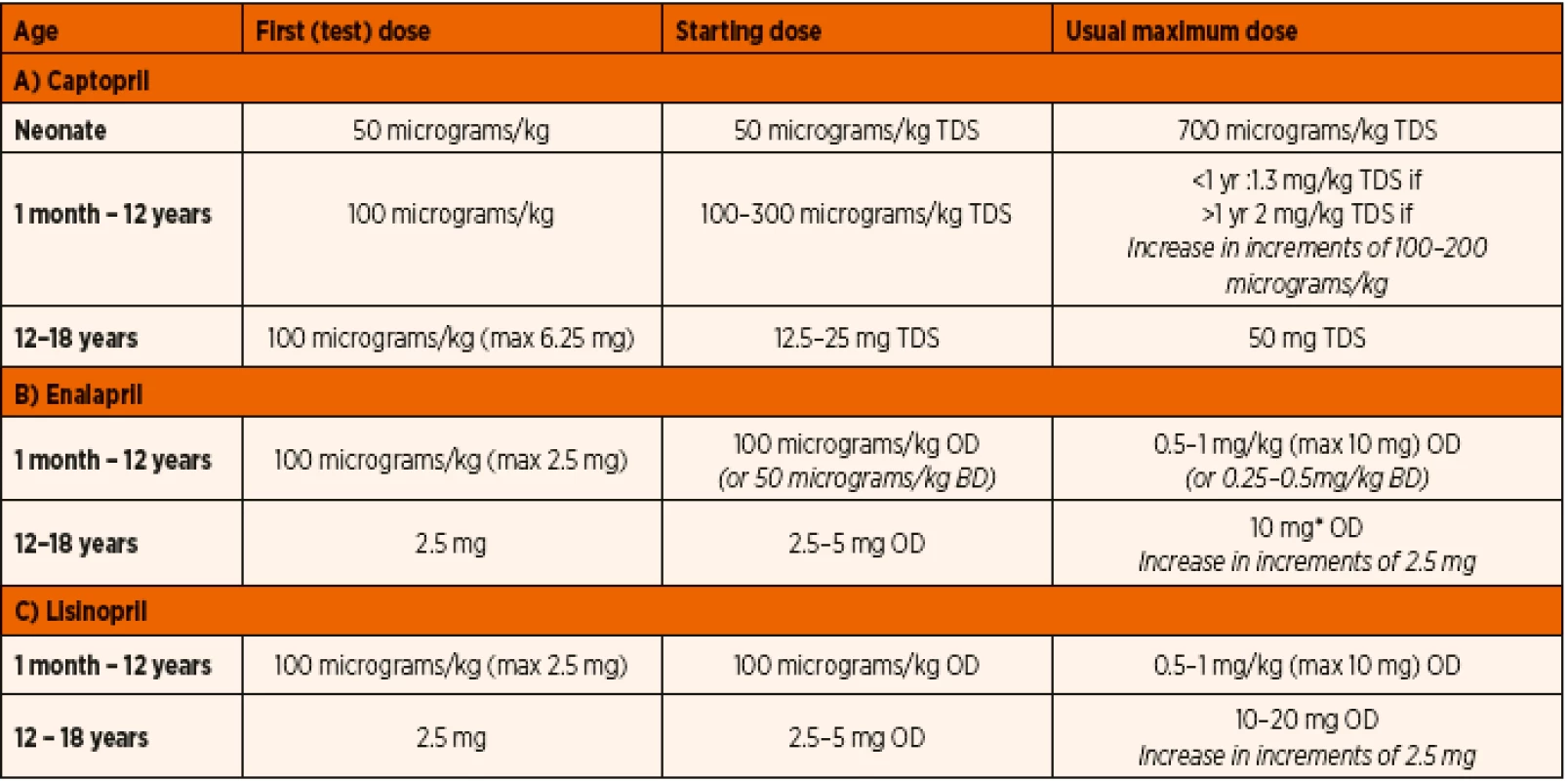
SAFETY PROFILE AND ADVERSE EFFECTS
Common adverse effects due to direct inhibition of renin-angiotensin-aldosterone axis include systemic hypotension, hyperkalemia, renal failure, and possible fetal anomalies (Table 9). As ACE inhibitors increase the bradykinin levels in the body, the resulting cough can be a significant problem [74]. As captopril contains a sulfhydryl group, it can lead to adverse effects like neutropenia and proteinuria. Systemic hypotension is reported in up to 19% of patients, necessitating dose modification, and rarely transient inotropic support [75]. Hyperkalemia has been reported but none resulting in cardiac arrhythmia.
Nephrotoxicity of ACE inhibitors was predominantly seen in younger age and lower weight patients [34]. Neonates had a greater risk of renal functional impairment associated with ACE inhibitor treatment than older children [79]. When compared to term neonates, preterm neonates are at a significantly higher risk of similar treatment complications. This is probably owing to the renal immaturity, reduced glomerular filtration rate with impaired renal autoregulation [78]. Renal failure has been described even with the initial doses of ACE inhibitors [34] with a dramatic rise in serum urea, creatinine as well as development of oliguria [15]. Fortunately these adverse effects were short-lived and resolved within a few days of treatment cessation [15]. Most importantly, the coexistent conditions leading to dehydration such as gastroenteritis, poor oral fluid intake and/ /or concomitant use of diuretics can pre-dispose to higher incidence of acute kidney injury [81]. Spironolactone and chlorothiazide administration have been described as an independent risk factor for nephrotoxicity with ACE inhibitor therapy [78, 79]. Mortality has been reported in two studies involving patients treated with ACE inhibitors, however, no direct association with the ACE inhibitor administration was proven [34, 75] (Table 8).
8. Summary of published data on paediatric safety profile and adverse effects related to ACE inhibitor administration. 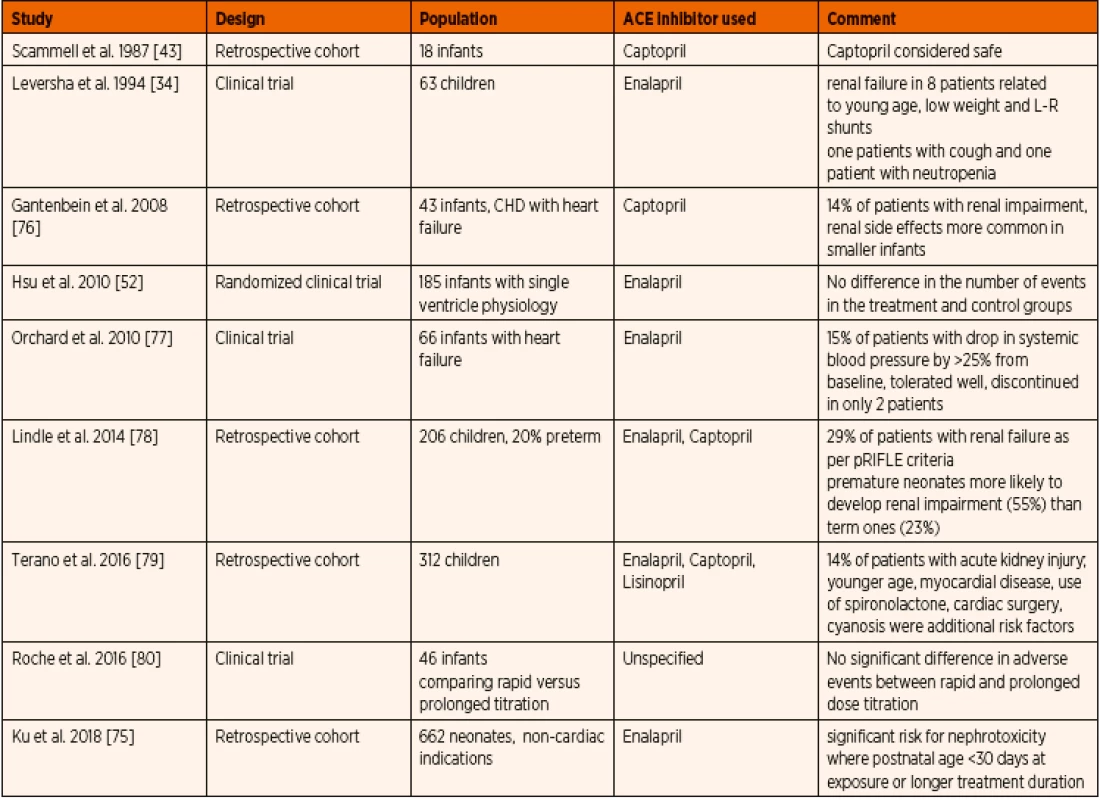
Our own institutional experience relates to audit of the use of ACE inhibitor in 124 paediatric patients between January and December 2018. The most common indications for this type of treatment was myocardial dysfunction in 38% of the patients. Apart from a mild and transient reduction in systemic blood pressure associated with the initial doses of ACE inhibitors, we have not encountered any significant side effects.
CONCLUSIONS
Although paediatric large scale randomized trails are rare, the published experience with ACE inhibitor therapy in managing paediatric heart failure is encouraging. We recommend their early initiation in carefully selected patients, appropriate choice of medication, adequate dosage, and close follow up for early diagnosis of potential adverse effects. Careful monitoring of blood pressure, serum electrolytes, and renal function is essential in paediatric patients treated with ACE inhibitors.
Došlo: 6. 3. 2020
Přijato: 13. 5. 2020
Corresponding author:
Zdeněk Slavik, MD, FRCPCH
Royal Brompton and Harefield
NHS Foundation Trust
Sydney Street
London, SW3 6NP
United Kingdom
e-mail: z.slavik@rbht.nhs.uk
Sources
1. The International Society for Heart and Lung Transplantation Guidelines for the management of pediatric heart failure: Executive summary – J Heart and Lung Transplant 2019. https://www.jhltonline.org/article/S1053-2498(14)01156-5/fulltext.
2. Marek J, Tomek V, Skovranek J, et al. Prenatal ultrasound screening of congenital heart disease in an unselected national population: a 21-year experience. Heart Br Card Soc 2011; 97 (2): 124–130.
3. Samanek M, Slavik Z, Zborilova B, et al. Prevalence, treatment, and outcome of heart disease in live-born children: a prospective analysis of 91,823 live-born children. Pediatr Cardiol 1989; 10 (4): 205–211.
4. Lipshultz SE, Sleeper LA, Towbin JA, et al. The incidence of pediatric cardiomyopathy in two regions of the United States. N Engl J Med 2003; 348 (17): 1647–1655.
5. Andrews RE, Fenton MJ, Ridout DA, et al. British Congenital Cardiac Association. New-onset heart failure due to heart muscle disease in childhood: a prospective study in the United kingdom and Ireland. Circulation 2008; 117 (1):79–84.
6. Towbin JA, Lowe AM, Colan SD, et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA 2006; 296 (15): 1867–1876.
7. Timberlake K, Kantor PF. Pharmacologic therapy of heart failure in children. Pharmacol Res 2011; 64 (5): 427–430.
8. Baylen BG, Johnson G, Tsang R, et al. The occurrence of hyperaldosteronism in infants with congestive heart failure. Am J Cardiol 1980; 45 (2): 305–310.
9. Hein S, Arnon E, Kostin S, Schönburg M, et al. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation 2003; 107 (7): 984–991.
10. Sadoshima J, Izumo S. Molecular characterization of angiotensin II induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ Res 1993; 73 (3): 413–423.
11. Kromer EP, Riegger GA. Effects of long-term angiotensin converting enzyme inhibition on myocardial hypertrophy in experimental aortic stenosis in the rat. Am J Cardiol 1988; 62 (1): 161–163.
12. Stern H, Weil J, Genz T, Vogt W, et al. Captopril in children with dilated cardiomyopathy: Acute and long-term effects in a prospective study of hemodynamic and hormonal effects. Pediatr Cardiol 1990; 11 (1): 22–28.
13. Shaddy RE, Teitel DF, Brett C. Short-term hemodynamic effects of captopril in infants with congestive heart failure. Am J Dis Child 1988; 142 (1): 100–105.
14. Sluysmans T, Styns-Cailteux M, Tremouroux-Wattiez M, et al. Intravenous enalaprilat and oral enalapril in congestive heart failure secondary to ventricular septal defect in infancy. Am J Cardiol 1992; 70 (9): 959–962.
15. Seguchi M, Nakazawa M, Momma K. Effect of enalapril on infants and children with congestive heart failure. Cardiol Young 1992; 2 (1): 14–19.
16. Hazama K, Nakazawa M, Momma K. Effective dose and cardiovascular effects of cilazapril in children with heart failure. Am J Cardiol 2001; 88 (7): 801–805.
17. Schneeweiss A. Cardiovascular drugs in children: Angiotensin--converting enzyme inhibitors. Pediatr Cardiol 1988; 9 (2): 109–115.
18. Levine TB, Olivari MT, Cohn JN. Hemodynamic and regional blood flow response to captopril in congestive heart failure. Am J Med 1984; 76 (5): 38–42.
19. Fagard R, Amery A, Reybrouck T, et al. Response of the systemic and pulmonary circulation to alpha - and beta-receptor blockade (labetalol) at rest and during exercise in hypertensive patients. Circulation 1979; 60 (6): 1214–1217.
20. Treatment of Chronic Congestive Heart Failure with Captopril, an Oral Inhibitor of Angiotensin-Converting Enzyme. NEJM 2020. https://www.nejm.org/doi/full/10.1056/NEJM197907193010301.
21. Kramer BL, Massie BM, Topic N. Controlled trial of captopril in chronic heart failure: a rest and exercise hemodynamic study. Circulation 1983; 67 (4): 807–816.
22. Scammell AM, Arnold R. The effect of the first dose of captopril on blood pressure in infants in heart failure. Int J Cardiol 1989; 22 (3): 377–379.
23. Rheuban KS, Carpenter MA, Ayers CA, et al. Acute hemodynamic effects of converting enzyme inhibition in infants with congestive heart failure. J Pediatr 1990; 117 (4): 668–670.
24. Webster MW, Neutze JM, Calder AL. Acute hemodynamic effects of converting enzyme inhibition in children with intracardiac shunts. Pediatr Cardiol 1992; 13 (3): 129–135.
25. Montigny M, Davignon A, Fouron JC, et al. Captopril in infants for congestive heart failure secondary to a large ventricular left-to-right shunt. Am J Cardiol 1989; 63 (9): 631–633.
26. Consensus Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (Consensus). N Engl J Med 1987; 316 (23): 1429–1435.
27. Yusuf S, Pitt B, Davis CE, et al. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325 (5): 293–302.
28. Packer M, Poole-Wilson PA, Armstrong PW, et al. Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. ATLAS Study Group. Circulation 1999; 100 (23): 2312–2318.
29. Cohn JN, Archibald DG, Ziesche S, et al. Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration Cooperative Study. N Engl J Med 1986; 314 (24): 1547–1552.
30. Swedberg K, Eneroth P, Kjekshus J, et al. Effects of enalapril and neuroendocrine activation on prognosis in severe congestive heart failure (follow-up of the Consensus trial). Consensus Trial Study Group. Am J Cardiol 1990; 66 (11): 40D–45D.
31. WHO|WHO Model Lists of Essential Medicines 2020. http://www.who.int/medicines/publications/essentialmedicines/en/.
32. Lipshultz SE, Lipsitz SR, Sallan SE, et al. Long-term enalapril therapy for left ventricular dysfunction in doxorubicin-treated survivors of childhood cancer. J Clin Oncol Off J Am Soc Clin Oncol 2002; 20 (23): 4517–4522.
33. Bengur AR, Beekman RH, Rocchini AP, et al. Acute hemodynamic effects of captopril in children with a congestive or restrictive cardiomyopathy. Circulation 1991; 83 (2): 523–527.
34. Leversha AM, Wilson NJ, Clarkson PM, et al. Efficacy and dosage of enalapril in congenital and acquired heart disease. Arch Dis Child 1994; 70 (1): 35–39.
35. Alehan D, Ozkutlu S. Beneficial effects of 1-year captopril therapy in children with chronic aortic regurgitation who have no symptoms. Am Heart J 1998; 135 (4): 598–603.
36. Beekman RH, Rocchini AP, Dick M, et al. Vasodilator therapy in children: acute and chronic effects in children with left ventricular dysfunction or mitral regurgitation. Pediatrics 1984; 73 (1): 43–51.
37. Kay JD, Colan SD, Graham TP. Congestive heart failure in pediatric patients. Am Heart J 2001; 142 (5): 923–928.
38. Kantor PF, Mertens LL. Clinical practice: Heart failure in children. Part I: Clinical evaluation, diagnostic testing, and initial medical management. Eur J Pediatr 2010; 169 (3): 269–279.
39. Moorthie S, Blencowe H, Darlison MW, et al. Estimating the birth prevalence and pregnancy outcomes of congenital malformations worldwide. J Community Genetics 2018; 9 (4): 387–396.
40. van der Linde D, Konings EEM, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol 2011; 58 (21): 2241–2247.
41. Webster MWI, Fitzpatrick MA, Hamilton EJ, et al. Effects of enalapril on clinical status, biochemistry, exercise performance and haemodynamics in heart failure. Drugs 1985; 30 (1): 74–81.
42. Shaw NJ, Wilson N, Dickinson DF. Captopril in heart failure secondary to a left to right shunt. Arch Dis Child 1988; 63 (4): 360–363.
43. Captopril in treatment of infant heart failure: a preliminary report – Int J Cardiol 2020. https://www.internationaljournalofcardiology.com/article/0167-5273(87)90153-7/abstract.
44. Slipczuk L, Rafique AM, Davila CD, et al. The role of medical therapy in moderate to severe degenerative mitral regurgitation. Rev Cardiovasc Med 2016; 17 (1–2): 28–39.
45. Frenneaux M, Stewart RA, Newman CM, et al. Enalapril for severe heart failure in infancy. Arch Dis Child 1989; 64 (2): 219–223.
46. Lin M, Chiang HT, Lin SL, Chang MS, et al. Vasodilator therapy in chronic asymptomatic aortic regurgitation: enalapril versus hydralazine therapy. J Am Coll Cardiol 1994; 24 (4): 1046–1053.
47. Calabrò R, Pisacane C, Pacileo G, et al. Hemodynamic effects of a single oral dose of enalapril among children with asymptomatic chronic mitral regurgitation. Am Heart J 1999; 138 : 955–961.
48. Mori Y, Nakazawa M, Tomimatsu H, et al. Long-term effect of angiotensin-converting enzyme inhibitor in volume overloaded heart during growth: a controlled pilot study. J Am Coll Cardiol 2000; 36 (1): 270–275.
49. Evangelista A, Tornos P, Sambola A, et al. Long-term vasodilator therapy in patients with severe aortic regurgitation. N Engl J Med 2005; 353 (13): 1342–1349.
50. Gisler F, Knirsch W, Harpes P, et al. Effectiveness of angiotensin-converting enzyme inhibitors in pediatric patients with mid to severe aortic valve regurgitation. Pediatr Cardiol 2008; 29 (5): 906–909.
51. Knirsch W, Tlach L, Stambach D, et al. Angiotensin-converting enzyme inhibitors in pediatric patients with mitral valve regurgitation – case-control study and review of the literature. Congenit Heart Dis 2010; 5 (3): 278–284.
52. Hsu DT, Zak V, Mahony L, Sleeper LA, et al. Enalapril in infants with single ventricle: results of a multicenter randomized trial. Circulation 2010; 122 (4): 333–340.
53. Ohnemus D, Oster ME, Gatlin S, et al. The effect of angiotensin-converting enzyme inhibitors on the rate of ascending aorta dilation in patients with bicuspid aortic valve. Congenit Heart Dis 2015; 10 (1): E1–E5.
54. Hsu DT, Pearson GD. Heart failure in children. Part I: History, etiology, and pathophysiology. Circ Heart Fail 2009; 2 (1): 63–70.
55. Bregagnollo EA, Okoshi K, Bregagnollo IF, et al. Effects of the prolonged inhibition of the angiotensin-converting enzyme on the morphological and functional characteristics of left ventricular hypertrophy in rats with persistent pressure overload. Arq Bras Cardiol 2005; 84 (3): 225–232.
56. Colan SD. Hypertrophic cardiomyopathy in childhood. Heart Fail Clin 2010; 6 (4): 433–444.
57. Denfield SW, Webber SA. Restrictive cardiomyopathy in childhood. Heart Fail Clin 2010; 6 (4): 445–452.
58. Kantor PF, Lougheed J, Dancea A, et al. Presentation, diagnosis, and medical management of heart failure in children: Canadian Cardiovascular Society Guidelines. Can J Cardiol 2013; 29 (12): 1535–1552.
59. Eronen M, Pesonen E, Wallgren EI, et al. Enalapril in children with congestive heart failure. Acta Paediatr Scand 1991; 80 (5): 555–558.
60. Lewis AB, Chabot M. The effect of treatment with angiotensin-converting enzyme inhibitors on survival of pediatric patients with dilated cardiomyopathy. Pediatr Cardiol 1993; 14 (1): 9–12.
61. Kantor PF, Abraham JR, Dipchand AI, et al. The impact of changing medical therapy on transplantation-free survival in pediatric dilated cardiomyopathy. J Am Coll Cardiol 2010; 55 (13): 1377–1384.
62. Silber JH, Cnaan A, Clark BJ, et al. Enalapril to prevent cardiac function decline in long-term survivors of pediatric cancer exposed to anthracyclines. J Clin Oncol Off J Am Soc Clin Oncol 2004; 22 (5): 820–828.
63. Janbabai G, Nabati M, Faghihinia M, et al. Effect of enalapril on preventing anthracycline-induced cardiomyopathy. Cardiovasc Toxicol 2017; 17 (2): 130–139.
64. Gupta V, Kumar Singh S, Agrawal V, et al. Role of ACE inhibitors in anthracycline-induced cardiotoxicity: A randomized, double-blind, placebo-controlled trial. Pediatr Blood Cancer 2018; 65 (11): e27308.
65. Harrington JK, Richmond ME, Fein AW, et al. Two-dimensional speckle tracking echocardiography – derived strain measurements in survivors of childhood cancer on angiotensin converting enzyme inhibition or receptor blockade. Pediatr Cardiol 2018; 39 (7): 1404–1412.
66. Ohuchi H, Hasegawa S, Yasuda K, et al. Severely impaired cardiac autonomic nervous activity after the Fontan operation. Circulation 2001; 104 (13): 1513–1518.
67. Yim DLS, Jones BO, Alexander PMA, et al. Effect of anti-heart failure therapy on diastolic function in children with single-ventricle circulations. Cardiol Young 2015; 25 (7): 1293–1299.
68. Yimgang DP, Sorkin JD, Evans CF, et al. Angiotensin converting enzyme inhibitors and interstage failure in infants with hypoplastic left heart syndrome. Congenit Heart Dis 2018; 13 (4): 533–540.
69. Jefferies JL, Hoffman TM, Nelson DP. Heart failure treatment in the intensive care unit in children. Heart Fail Clin 2010; 6 (4): 531–558.
70. Tume SC, Schwartz SM, Bronicki RA. Pediatric Cardiac Intensive Care Society 2014 consensus statement: Pharmacotherapies in cardiac critical care – treatment of acute heart failure. Pediatr Crit Care Med 2016; 17 (3 Suppl 1): S16–19.
71. White CM. Pharmacologic, pharmacokinetic, and therapeutic differences among ACE inhibitors. Pharmacotherapy 1998; 18 (3): 588–599.
72. Lloyd TR, Mahoney LT, Knoedel D, et al. Orally administered enalapril for infants with congestive heart failure: a dose-finding study. J Pediatr 1989; 114 (4): 650–654.
73. Nakamura H, Ishii M, Sugimura T, et al. The kinetic profiles of enalapril and enalaprilat and their possible developmental changes in pediatric patients with congestive heart failure. Clin Pharmacol Ther 1994; 56 (2): 160–168.
74. Momma K. ACE inhibitors in pediatric patients with heart failure. Pediatr Drugs 2006; 8 (1): 55–69.
75. Ku E, McCulloch CE, Vittinghoff E, et al. Use of antihypertensive agents and association with risk of adverse outcomes in chronic kidney disease: Focus on angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. J Am Heart Assoc 2018; 7 (19): e009992.
76. Gantenbein MH, Bauersfeld U, Baenziger O, et al. Side effects of angiotensin converting enzyme inhibitor (captopril) in newborns and young infants. J Perinat Med 2008; 36 (5): 448–452.
77. Orchard EA, Apps A, Wilson N. Use of captopril in paediatric congestive cardiac failure: early effects on blood pressure and renal function. Arch Dis Child 2010; 95 (7): 566–567.
78. Lindle KA, Dinh K, Moffett BS, et al. Angiotensin-converting enzyme inhibitor nephrotoxicity in neonates with cardiac disease. Pediatr Cardiol 2014; 35 (3): 499–506.
79. Incidence of and risk factors for severe acute kidney injury in children with heart failure treated with renin–angiotensin system inhibitors. Springer Link 2020. http://link-springer-com-443.webvpn.fjmu.edu.cn/article/10.1007%2Fs00431-015-2680-8.
80. Roche SL, Timberlake K, Manlhiot C, et al. Angiotensin-converting enzyme inhibitor initiation and dose uptitration in children with cardiovascular disease: A retrospective review of standard clinical practice and a prospective randomized clinical trial. J Am Heart Assoc Cardiovasc Cerebrovasc Dis 2016. https://www.ncbi.nlm.nih.gov/pmc//articles/PMC4889193/
81. Moffett BS, Mattamal R, Ocampo EC, et al. Impact of pharmacotherapy on interstage outcomes in single ventricle infants: medication influence on interstage weight gain. Congenit Heart Dis 2011; 6 (4): 286–293.
82. Meulen M van der, Läer S, Castro C, et al. Safety of ACE inhibitors in children with heart failure. Arch Dis Child 2016; 101 (1): e1.
Labels
Neonatology Paediatrics General practitioner for children and adolescents
Article was published inCzech-Slovak Pediatrics

2020 Issue 5-
All articles in this issue
- COVID-19 pandemy from the perspective of regional children´s clinic
- Abdominal pain caused by retrocaval ureter
- Anemia in children with inflammatory bowel diseases
- Management of children with infantile hemangioma treated by systemic propranolol
- Recommendations for diagnosis and treatment of JIA associated uveitis: Czech-Slovak adaptation of European recommendation SHARE
- Prof. MUDr. Jiří ZEMAN, DrSc. – jubileum
- Zprávy
- Lucia Časnocha Lúčanová: Infekcie novorodencov
- Angiotensin converting enzyme inhibitors in paediatric cardiac patients – current experience and practice
- Czech-Slovak Pediatrics
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Prof. MUDr. Jiří ZEMAN, DrSc. – jubileum
- COVID-19 pandemy from the perspective of regional children´s clinic
- Anemia in children with inflammatory bowel diseases
- Abdominal pain caused by retrocaval ureter
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

