-
Medical journals
- Career
Incidence of uterine sarcomas in West Bohemia, at the Department of Gynaecology and Obstetrics, University Hospital in Pilsen, Czech Republic
Authors: J. Kosťun 1; J. Bouda 1; P. Vlasák 1; A. Bartáková 1; D. Berezovskiy 1; Vít Weinberger 2; Jiří Presl 1
Authors‘ workplace: Gynekologicko-porodnická klinika LF UK a FN, Plzeň, přednosta doc. MUDr. Z. Novotný, CSc. 1; Gynekologicko-porodnická klinika LF MU a FN, Brno, přednosta prof. MUDr. P. Ventruba, DrSc., MBA 2
Published in: Ceska Gynekol 2017; 82(6): 436-442
Overview
Objective and setting:
A retrospective review of women of the West Bohemian population was performed at the Department of Gynaecology and Obstetrics, University Hospital in Pilsen, Czech Republic from 1/2005 – 1/2017.Methods:
The following data was analysed: patients age, histological type, tumour size, FIGO stage, body mass index, previous irradiation, Tamoxifen usage, and other possible risk factors. A total number of 20 uterine sarcoma patients were detected in the period from 1/2005 to 1/2015, and these cases were followed until 1/2017.Results:
The histological types identified were: leiomyosarcoma in 12 cases, endometrial stromal sarcoma in 7 cases and one case of high-grade undifferentiated uterine sarcoma. This only patient diagnosed with high-grade undifferentiated uterine sarcoma showed distant metastases 12 months after the surgical treatment and died one month later. The whole group of uterine sarcoma patients regardless histological subtype reached two years in 50% of cases, with the median disease-free interval 18 months and the median follow up of 38 months.
The diagnosis of sarcoma was already known in 25% of cases from dilatation and curettage histology. There were suspicious sonographic findings suggestive of sarcoma in 10% of cases. Multiparity was associated with 48.2% of endometrial stromal sarcoma cases. The leading clinical symptom was postmenopausal bleeding in 55% of patients.Conclusion:
We confirmed uterine sarcomas to be rare malignancies, even in the Czech population with high uterine malignancy incidence. Uterine cold knife morcellation during the vaginal part of laparoscopically assisted vaginal hysterectomy didn’t worsen the prognosis of our patients.Keywords:
uterine sarcoma, incidence, risk factors, cold knife morcellation, Czech RepublicINTRODUCTION
The Czech Republic ranks among the countries with the highest incidence of uterine malignancies in the world [13]. According to the Czech National Oncological Database, the female population of West Bohemia shows the highest nationwide incidence of uterine malignancies with an incidence of 34/100 000 and a mortality of 13.1/100 000. This database retrospectively covers the years from 1977–2013 [33].
Primary uterine malignancies are most commonly represented by epithelial tumours and only rarely by mesenchymal or mixed histological manifestations. Malignant mesenchymal tumours originating from uterine connective tissue are a histologically diverse group associated with a very poor prognosis [2]. According to literature, uterine sarcomas (US) account for approximately 3% to 7% of all uterine malignancies [32].
According to the WHO classification published in 2014 the uterine sarcomas are divided in two main groups: the mesenchymal and the mixed epithelial and mesenchymal tumours. The former group contains leiomyosarcoma, endometrial stromal sarcoma, undifferentiated endometrial sarcoma and smooth muscle cells of uncertain malignant potential. The latter group is including carcinosarcoma and adenosarcoma [27].
Approximately 40% of uterine sarcomas are represented by leiomyosarcomas. These tumours have typically pink or grey surface, necrotic and haemorrhagic areas and their margins are either infiltrative or well defined. The basic histological features defining the grade of the tumour are cellular atypia, mitotic activity and the presence of tumour cell necrosis. According to morphology we can distinguish following main categories: spindeled, epithelioid and myxoid [6].
The second most frequent uterine sarcoma is represented by endometrial stromal sarcoma. It forms soft, yellow nodules infiltrating the endometrium and myometrium. The tumour necrosis and areas of haemorrhage may be observed. The microscopic characteristic of this lesion resembles the proliferative-phase endometrium. Very uncommon tumour is the undifferentiated endometrial stromal sarcoma. It is a high-grade sarcoma without any specific histological features. Thus, the histological diagnosis is made after excluding all other possibilities [36].
In 2010 the International Federation of Gynaecology and Obstetrics (FIGO) published a new staging system for US including the size of the tumour as an important prognostic marker for disease limited to the uterus [16]. Detailed knowledge of risk factors is lacking due to the low incidence of US. The only risk factor present in 10–25% of cases is previous pelvic radiation therapy. In addition, the incidence of US was proven to be associated with the use of Tamoxifen [7, 8, 35] .
The aim of this report is to retrospectively collect data from our patients diagnosed with any type of uterine malignancy, and then in detail to characterize those with malignant mesenchymal tumours to find any specific clinical features or possible risk factors. In doing so this analysis could improve the identification of patients with a higher risk of US, and lower the number of unexpected sarcomas with inadequate primary treatment or inappropriate usage of power morcellation.
Uterine carcinosarcoma (malignant Mullerian mixed tumour), is no longer considered a purely mesenchymal tumour but a mixed epithelial and mesenchymal tumour and so is not included in this analysis of sarcomas [28].
PATIENTS AND METHODS
A search of the medical database of the Department of Obstetrics and Gynaecology at the University Hospital in Pilsen, Charles University in Prague between January 2005 and January 2015 was performed to collect data of all the patients with uterine malignancies and identify those with diagnosed US for a more detailed analysis. Their medical records, including follow up data until January 2017, were systematically reviewed for the following variables; date of diagnosis, histological subtype, age, parity, tumour size, association with Tamoxifen use or previous pelvic radiation therapy, FIGO staging, clinical manifestation, disease free interval and overall survival. The staging was performed retrospectively according to current FIGO revision from 2009. The following US subtypes were distinguished by histology: leiomyosarcoma (LMS), endometrial stromal sarcoma (ESS) and high-grade undifferentiated US. Furthermore, the medical records were searched for any types of uterine morcellation technique used and any suspicion of malignancy prior to surgical treatment (i.e. sonographic findings, histology etc.). Given the character of the study, a patient informed consent was not considered necessary.
RESULTS
Between January 2005 and January 2015, 1954 new cases of malignant gynaecologic diseases were diagnosed. The total count of uterine malignancies in the above mentioned period was 668 cases (34.2%); among them were 20 US (1% of all malignancies, 3% of uterine malignancies). For US, hysterectomy with bilateral salpingo-oophorectomy (BSO) was the primary treatment in all cases, except in two LMS cases where BSO was not performed due to the low age of the patient and because malignancy was not suspected. In one case of ESS it was necessary to add further debulking with intestinal resection, and in one case of LMS omentectomy was performed due to tumoral infiltration. There were no cases of primary lymph-node dissection in this US study group.
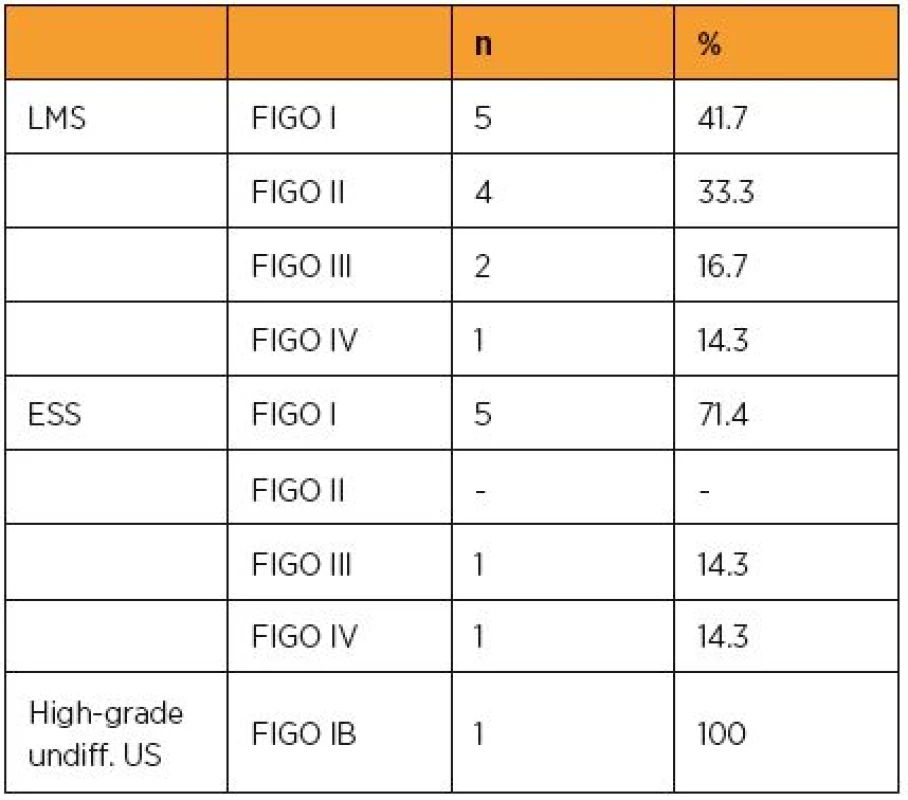
The vast majority of US were represented by LMS in 12 cases (60%), ESS in 7 cases (35%) and high-grade undifferentiated US in one case (5%). Most of the women were postmenopausal (65%, n=13). The median age of the patient at the time of diagnosis was 55 irrespective of histological subtype (see Table 1a, 1b and 1c for more details).
Table 1a. Patient characteristics, general 
Table 1b. Patient characteristics irrespectively to histological subtype 
Table 1c. Patient characteristics, sites of metastasis 
The diagnosis of US was already known in five cases (25%) due to histology from dilation and curettage (D&C). In one case the histological examination from D&C showed a poorly differentiated endometrioid adenocarcinoma; nevertheless the final histology from a uterine specimen showed the result of endometrial stromal sarcoma with infiltrative growth to other pelvic structures. It was a 75-year-old patient, obese (BMI 37), presented with postmenopausal bleeding. The sonographic examination showed a suspicious tumorous mass greater than 10 cm in diameter with hyperechogen unhomogeneous features.
In two cases (10%) there was a suspicion of US according to expert sonographic examination (oval inhomogeneous solid structure of uterine mass with atypical vessels and necrosis - identified in both cases). In 13 cases the diagnoses of US was not expected prior to surgery (65%). All patients had one of the following clinical symptoms: pre or postmenopausal vaginal bleeding was present in 11 (55%) of the cases, abdominal discomfort in 8 (40%) cases and two patients suffered from fatigue (10%).
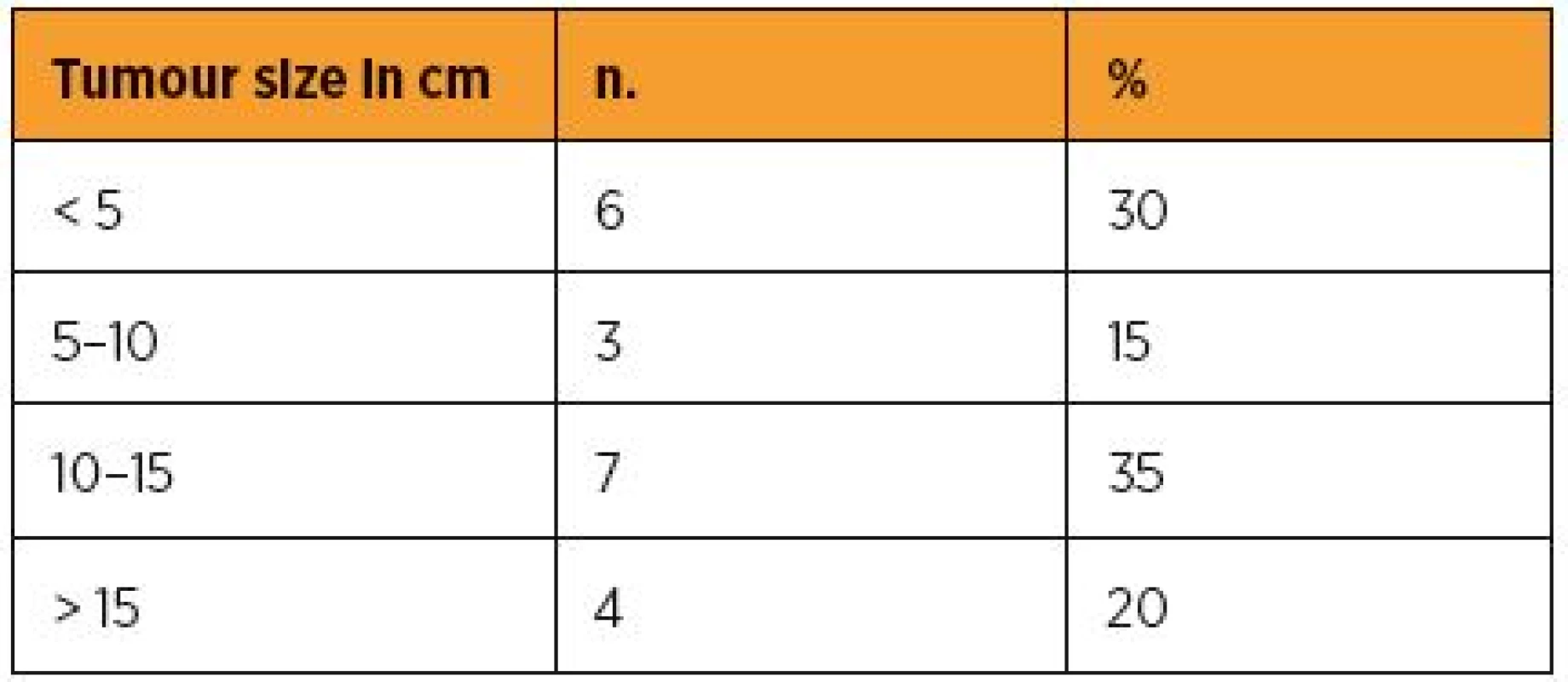
As reflected in the current FIGO classification of US, the size of the tumour does matter. Table 2 summarizes the size of the diagnosed malignancy irrespective of its histological type and Table 3 the staging according to the latest FIGO revision (2009) in our patients.
In patients with LMS, 16 months was the median disease free interval, the overall survival longer two years was 58%, the median follow up was 63 months. Two ESS patients died during the observed period. The first one however, not because of ESS, but due to polymorbidity and the consequences of extreme obesity, the second cause of death was the generalisation of the malignant process. Thus, the ESS patients showed better outcome comparing to LMS group with reaching 2 years in 5 of 7 cases (71,4%), the median follow up was 35,5 months, the median disease free interval was 35,5 moths as well. The only patient diagnosed with high-grade undifferentiated US showed distant metastases 12 months after the surgical treatment and died one month later. The whole group of 20 patients regardless histological subtype of US showed overall survival of longer than 2 years in 50% of cases, the median disease free interval of 18 months, and the median follow up of 38 months.
There were no cases of power morcellation in our group of patients. However, there were two cases of morcellation (10%) of the specimen by cold knife during the vaginal part of laparoscopically assisted vaginal hysterectomy (LAVH). In both cases the diagnosis of US was made after the hysterectomy. The first of them was a patient diagnosed with LMS in 2010 at the age of 43, where the LAVH was indicated because of uterine fibroids. The patient showed no signs of recurrence in January 2017. The second case was a patient diagnosed with ESS in 2013 at the age of 61 and was also operated on due to uterine fibroids. This patient has shown no signs of recurrence either.
DISCUSSION
Our analysis of US cases retrospectively diagnosed at our centre reflects the incidence of US in our region over the period of ten years. The total number of 20 cases confirms the rarity of this type of malignancy and is in accordance with the data so far reported. Although the total amount of uterine neoplasia in western Bohemia is well above average, the percentage of US is not higher and corresponds to global data with 3% of all uterine malignancies and 1% of all gynaecological malignancies. Corresponding numbers are presented also in the analysis of German authors, where our western neighbour Bavaria is showing incidence for uterine sarcoma of 1.30 for every 100 000 women averaged for years 2002–2011 [4].
LMS was acknowledged as the most frequent histological subtype with most cases diagnosed at FIGO stage I [1, 26]. Vaginal bleeding was the leading clinical symptom in agreement with other studies [17, 19, 21]. Patients diagnosed with ESS showed significantly better prognoses due to the low-grade behaviour of this malignancy [18, 31]. In the majority of cases the diagnosis of US was made following surgery for a benign condition such as uterine fibroids or vaginal bleeding [9, 29]. On the other hand, we couldn´t confirm Tamoxifen usage as a risk factor although it has been debated and publications made on this subject [22]. Neither were there any cases associated with previous pelvic radiation. In one patient, there was a histological examination made from D&C pointing towards endometrioid adenocarcinoma. Nevertheless, the following examination of the specimen from the hysterectomy showed ESS. Given the suspicious indices (age, sonographic findings, obesity, Tamoxifen, pelvic radiation, …) it seems we shouldn´t absolutely exclude the possibility of the patient having had uterine sarcoma before the final histological result, even though the D&C histology directs us towards another type of malignancy or even a benign result. In this study the histological diagnoses from D&C and the histology from the subsequent surgery had a consensus in five of six cases (83.3%).
In cases of ESS, 48.2% (3) of patients were quartiparas. Despite the small number of patients in the study group this could point to an association between the multiparity and the increased risk of ESS. The median of 5 for parity in ESS patients is described in an article from Behtash et al [5]. Yet this high number might be associated with the cultural background of the study population. Therefore, more research and data collection in this field needs to be carried out. The treatment in all cases was primarily surgical, with emphasis on zero residual disease in order to achieve the maximum therapeutic efficacy [24]. Lymph node dissection was not routinely performed as previously recommended [10, 14, 25, 30]. Amant et al published a paper in 2007 commenting on the importance of systematic lymphadenectomy in patients with ESS. In a multicentre study, 31 cases were reviewed retrospectively. No improved outcomes were found for patients with systematic lymphadenectomy or surgical castration [3]. Low incidence of nodal metastasis in soft tissue sarcomas was already postulated by Weingrad et al in 1978 in a retrospective study of 374 patients [34]. Bilateral salpingo-oophorectomy was performed in the majority of patients, although there is no clear scientific evidence regarding the benefit of BSO in the treatment of LMS and/or ESS [3, 12, 19, 20, 23, 30].
According to published data and based on FDA recommendations from 2014, uterine morcellation should not be performed during surgery for benign gynaecological conditions, as it may result in intraabdominal dissemination of a potential malignant disease [11]. In two cases, cold knife morcellation was performed during the vaginal part of the LAVH, as a benign condition was expected. No negative prognostic effect was found in these patients because the risk of intraabdominal dissemination during this type of specimen morcellation in the vagina is extremely low. A study by Zhang et al recently reported no additional increase in risk of sarcoma dissemination in the abdominal cavity in patients diagnosed postoperatively with uterine sarcoma where the transvaginal scalpel morcellation was used. Nevertheless, in accordance with most studies concerning uterine sarcomas, this study documents just a small cohort of patients undergoing this type of procedure, and in addition there was only a short-term follow up [37]. In 2017 ESGO published a statement on fibroid and uterine morcellation concluding morcellation techniques should keep their place in gynaecologic surgery. Usage of endobag and proper preoperative workup should help to decrease the risk of tumour cells spillage [15].
In conclusion, we confirmed that uterine sarcomas are rare malignancies, even in a population with one of the highest risks of uterine neoplasia in the world. The aggressive behavioural pattern is expressed most by LMS and high-grade undifferentiated US (even when detected in FIGO stage I). A BMI of greater than 27 seems to be associated with an increased risk of US, and there might be a connection between multiparity and ESS. However, this study retrospectively evaluated just a small number of patients. As our analysis has shown, we shouldn´t absolutely exclude the possibility of US in patients presenting risk factors for it, even if the uterus curettage histology offers other results.
MUDr. Jan Kosťun
Gynekologicko-porodnická klinika
FN a LFP UK
Alej Svobody 80
304 60 Plzeň
e-mail: jan.kostun@gmail.com
Sources
1. Abeler, VM., Royne, O., Thoresen, S., et al. Uterine sarcomas in Norway. A histopathological and prognostic survey of a total population from 1970 to 2000 including 419 patients. Histopathology, 2009, 54(3), p. 355–364.
2. Amant, F., Coosemans, A., Debiec-Rychter, M., et al. Clinical management of uterine sarcomas. Lancet Oncol, 2009, 10(12), p. 1188–1198.
3. Amant, F., De Knijf, A., Van Calster, B., et al. Clinical study investigating the role of lymphadenectomy, surgical castration and adjuvant hormonal treatment in endometrial stromal sarcoma. Brit J Cancer, 2007, 97(9), p. 1194–1199.
4. Beckmann, MW., Juhasz-Boss, I., Denschlag, D., et al. Surgical methods for the treatment of uterine fibroids – risk of uterine sarcoma and problems of morcellation: position paper of the DGGG. Geburtshilfe und Frauenheilkunde, 2015, 75(2), p. 148–164.
5. Behtash, N., Akhavan, S., Gilani, MM., et al. Low grade endometrial stromal sarcoma of uterine: review of 17 cases. Acta Med Iranica, 2011, 49(9), p. 619–624.
6. Bell, SW., Kempson, RL., Hendrickson, MR. Problematic uterine smooth muscle neoplasms. A clinicopathologic study of 213 cases. Amer J surg Pathol, 1994, 18(6), p. 535–558.
7. Bergman, L., Beelen, ML., Gallee, MP., et al. Risk and prognosis of endometrial cancer after tamoxifen for breast cancer. Comprehensive Cancer Centres‘ ALERT Group. Assessment of liver and endometrial cancer risk following tamoxifen. Lancet, 2000, 356(9233), p. 881–887.
8. Cohen, I. Endometrial pathologies associated with postmenopausal tamoxifen treatment. Gynecol Oncol, 2004, 94(2), p. 256–266.
9. D‘Angelo, E., Prat, J. Uterine sarcomas: a review. Gynecol Oncol, 2010, 116(1), p. 131–139.
10. Dos Santos, LA., Garg, K., Diaz, JP., et al. Incidence of lymph node and adnexal metastasis in endometrial stromal sarcoma. Gynecol Oncol, 2011, 121(2), p. 319–322.
11. FDA, C.f.D.a.R.H. Safety Communications – Laparoscopic Uterine Power Morcellation in Hysterectomy and Myomectomy: FDA Safety Communication. 2014.
12. Giuntoli, RL. 2nd, Metzinger, DS., DiMarco, CS., et al. Retrospective review of 208 patients with leiomyosarcoma of the uterus: prognostic indicators, surgical management, and adjuvant therapy. Gynecol Oncol, 2003, 89(3), p. 460–469.
13. GLOBOCAN. Uterine cancer, incidence and mortality. 2012; http://globocan.iarc.fr.
14. Goff, BA., Rice, LW., Fleischhacker, D., et al. Uterine leiomyosarcoma and endometrial stromal sarcoma: lymph node metastases and sites of recurrence. Gynecol Oncol, 1993, 50(1), p. 105–109.
15. Halaska, MJ., Haidopoulos, D., Guyon, F., et al. European Society of Gynecological Oncology Statement on Fibroid and Uterine Morcellation. Intern J Gynecol Cancer, 2017, 27(1), p. 189–192.
16. Horn, LC., Schmidt, D., Fathke, C., et al. [New FIGO staging for uterine sarcomas]. Pathology, 2009, 30(4), p. 302–303.
17. Chan, JK., Kawar, NM., Shin, JY., et al. Endometrial stromal sarcoma: a population-based analysis. Brit J Cancer, 2008, 99(8), p. 1210–1215.
18. Chang, KL., Crabtree, GS., Lim-Tan, SK., et al. Primary uterine endometrial stromal neoplasms. A clinicopathologic study of 117 cases. Amer J surg Pathol, 1990, 14(5), p. 415–438.
19. Kapp, DS., Shin, JY., Chan, JK. Prognostic factors and survival in 1396 patients with uterine leiomyosarcomas: emphasis on impact of lymphadenectomy and oophorectomy. Cancer, 2008, 112(4), p. 820–830.
20. Kim, WY., Lee, JW., Choi, CH., et al. Low-grade endometrial stromal sarcoma: a single center‘s experience with 22 cases. Inter J Gynecol Cancer, 2008, 18(5), p. 1084–1089.
21. Koivisto-Korander, R., Martinsen, JI., Weiderpass, E., et al. Incidence of uterine leiomyosarcoma and endometrial stromal sarcoma in Nordic countries: results from NORDCAN and NOCCA databases. Maturitas, 2012, 72(1), p. 56–60.
22. Lavie, O., Barnett-Griness, O., Narod, SA., et al. The risk of developing uterine sarcoma after tamoxifen use. Inter J Gynecol Cancer, 2008, 18(2), p. 352–356.
23. Leitao, MM., Jr., Hensley, ML., Barakat, RR., et al. Immunohistochemical expression of estrogen and progesterone receptors and outcomes in patients with newly diagnosed uterine leiomyosarcoma. Gynecol Oncol, 2012, 124(3), p. 558–562.
24. Leitao, MM., Jr., Zivanovic, O., Chi, DS., et al. Surgical cytoreduction in patients with metastatic uterine leiomyosarcoma at the time of initial diagnosis. Gynecol Oncol, 2012, 125(2), p. 409–413.
25. Leitao, MM., Sonoda, Y., Brennan, MF., et al. Incidence of lymph node and ovarian metastases in leiomyosarcoma of the uterus. Gynecol Oncol, 2003, 91(1), p. 209–212.
26. Nordal, RR., Thoresen, SO. Uterine sarcomas in Norway 1956–1992: incidence, survival and mortality. Eur J Cancer, 1997, 33(6), p. 907–911.
27. Oliva, E., Carinelli, SG., Ip, P., et al. WHO classification of tumours of female reproductive organs. Kurman, RJ., Carcangiu, ML., Herrington, CS., Young, RH., ed. Lyon: IARC Press 2014.
28. Prat, J., Mbatani, S. Uterine sarcomas. Inter J Gynaecol Obstet, 2015, 131, Suppl. 2S105–110.
29. Sagae, S., Yamashita, K., Ishioka, S., et al. Preoperative diagnosis and treatment results in 106 patients with uterine sarcoma in Hokkaido, Japan. Oncology, 2004, 67(1), p. 33–39.
30. Shah, JP., Bryant, CS., Kumar, S., et al. Lymphadenectomy and ovarian preservation in low-grade endometrial stromal sarcoma. Obstet Gynecol, 2008, 112(5), p. 1102–1108.
31. Schick, U., Bolukbasi, Y., Thariat, J., et al. Outcome and prognostic factors in endometrial stromal tumors: a Rare Cancer Network study. Inter J Radiation Oncol Biol Physics, 2012, 82(5), p. e757–763.
32. Toro, JR., Travis, LB., Wu, HJ., et al. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978–2001: An analysis of 26,758 cases. J Inter Cancer, 2006, 119(12), p. 2922–2930.
33. ÚZIS. Epidemiologie zhoubných nádorů v České republice. 2013; www.svod.cz.
34. Weingrad, DN., Rosenberg, SA. Early lymphatic spread of osteogenic and soft-tissue sarcomas. Surgery, 1978, 84(2), p. 231–240.
35. Wickerham, DL., Fisher, B., Wolmark, N., et al. Association of tamoxifen and uterine sarcoma. J Clin Oncol, 2002, 20(11), p. 2758–2760.
36. Xue, WC., Cheung, AN. Endometrial stromal sarcoma of uterus. Best Practice: Clin Obstet Gynaecol, 2011, 25(6), p. 719–732.
37. Zhang, J., Li, T., Zhu, L., et al. Clinical characteristics and prognosis of unexpected uterine sarcoma after hysterectomy for presumed myoma with and without transvaginal scalpel morcellation. Inter J Gynecol Cancer, 2016, 26(3), p. 456–463.
Labels
Paediatric gynaecology Gynaecology and obstetrics Reproduction medicine
Article was published inCzech Gynaecology

2017 Issue 6-
All articles in this issue
- Cure effect and persistence of treatment with Mirabegron in patients with symptoms of overactive bladder: a multicentre clinical study
-
Vaginal reconstruction for the remedy of pelvic organ prolapse: the effect, influence on urinary and sexual function and quality of life in two-years follow-up
Part I. Vaginal status and complications - External cephalic version of breech fetus after 36 weeks of gestation – evaluation of efectiveness and complications
- Aspiration of functional ovarian cyst
- The hypercholesterolemias in pregnancy: their etiology and diagnostic significance considerations
- Are women‘s attitudes towards pregnancy, childbirth and motherhood associated with length of labour?
- Cystic adenomatoid malformation of fetus
- Placenta percreta as a cause of massive intraabdominal bleeding
- Current limits of cervical cancer prevention in the Czech republic
- The role of T-regulatory lymphocytes in pathogenesis of preterm delivery
- DNA quality of spermatozoa is negatively affected by male age and represents a risk factor for conception
- Incidence of uterine sarcomas in West Bohemia, at the Department of Gynaecology and Obstetrics, University Hospital in Pilsen, Czech Republic
- Czech Gynaecology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- External cephalic version of breech fetus after 36 weeks of gestation – evaluation of efectiveness and complications
- Aspiration of functional ovarian cyst
- DNA quality of spermatozoa is negatively affected by male age and represents a risk factor for conception
- Placenta percreta as a cause of massive intraabdominal bleeding
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career