-
Medical journals
- Career
EOSINOPHILIC ANGIOCENTRIC FIBROSIS AS A CAUSE OF NASAL OBSTRUCTION. A CASE REPORT
Authors: I. Němec 1; M. Mikolaj 1; P. Hrabal 2
Authors‘ workplace: Charles University, Third Faculty of Medicine, and the Military University Hospital Prague, Department of Otorhinolaryngology and Maxillofacial Surgery, Prague, Czech Republic 1; Military University Hospital Prague, Department of Pathology, Prague, Czech Republic 2
Published in: ACTA CHIRURGIAE PLASTICAE, 60, 2-4, 2018, pp. 59-61
INTRODUCTION
Eosinophilic angiocentric fibrosis (EAF) is a rare benign fibrotic lesion of unknown aetiology, found predominantly in the upper respiratory tract where it causes obstruction1. The first case of this lesion was described by Holmes and Panje in 1983 who named it intranasal granuloma faciale2. In 1985, Roberts and McCann described three cases of lesions in the upper respiratory tract as mucous variants of granuloma faciale. Based on detailed histology, the authors recommended the name eosinophilic angiocentric fibrosis1. Additional cases of this disease have been published3–20. We present a case of a patient with this diagnosis.
CASE REPORT
A 49-year-old male patient observed deformation of his nose gradually worsening for 20 years. In the last five years the patient observed a gradually increasing nasal obstruction, more apparent on the left. The patient suffered an unspecified nose injury in his childhood and had a history of allergies to dust and pollen. He had a surgery of the nasal septum five years ago. The patient had no other history of problems. Examination showed septal perforation and septal thickening in its frontal part. Spherical, elastic resistance was felt in this area of the septum and nasal pathways, which deformed the external nose, particularly on the left. There were no cutaneous lesions. Computed tomography (CT) showed a soft-tissue, expansive lesion in the frontal part of the nasal cavity, sized 41 x 31 mm. Partial destruction of nasal bones was apparent, more distinct on the left (Figure 1).
1. Computed tomographic axial scan showing a softtissue, expansive lesion in the frontal part of the nasal cavity (arrow) 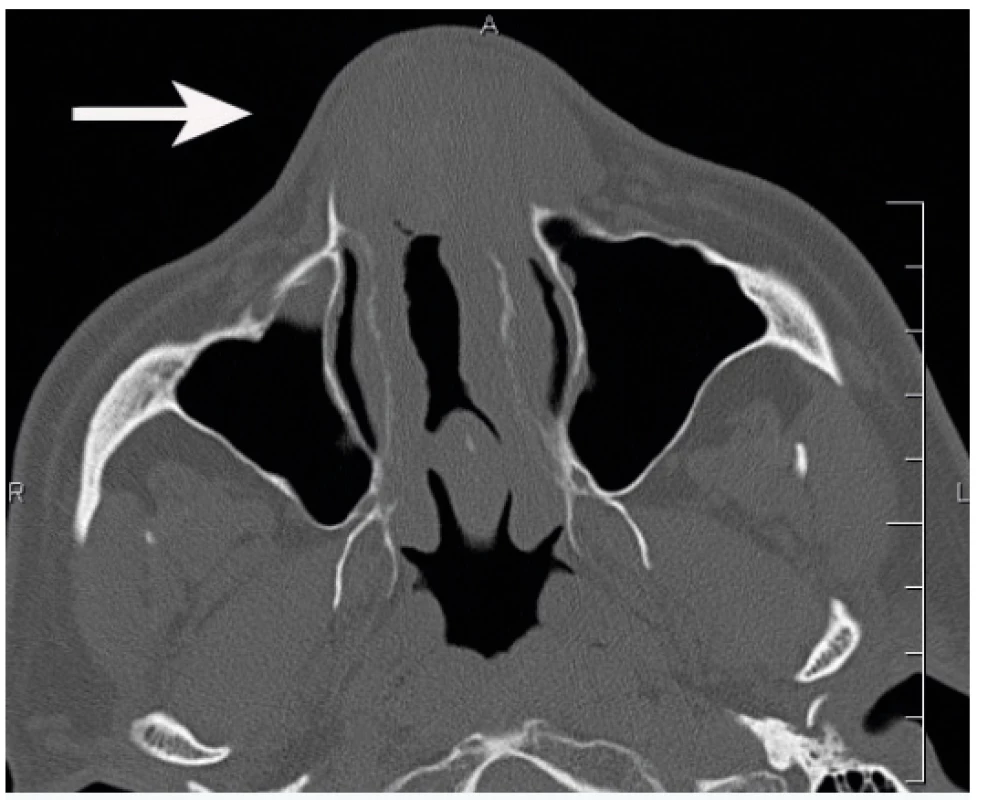
Biopsy of the lesion showed evidence of chronic inflammatory changes. We performed removal of the tumour from lateral rhinotomy including resection of parts of the nasal bones and lateral nasal cartilages on both sides. Furthermore, we performed partial resection of the frontal process of the maxilla on the left. The lesion caused partial destruction of lateral nasal cartilages on both sides of the nose. At the same time, we performed partial resection of the cartilaginous and bony part of the septum. Subsequently, corticosteroids were administered locally for three months.
Histology examination revealed dense collagenous sclerosing lesion with obliterative and concentric perivascular fibrosis and inflammatory infiltrate with significant numbers of eosinophils. Histologically the lesion can be separated into early and late phases: The early phase consists of eosinophilic vasculitis without fibrinoid necrosis, occurring in a patchy fashion and involving groups of capillaries and/or venules. Eosinophils create a cluster and migrate through the vessel wall and are accompanied by a mixture of plasma cells, lymphocytes, and fibroblasts (Figure 2a). The late lesion is characterized by a dense fibrous thickening of stroma and a characteristic obliterative perivascular onion skin whorling of collagen fibres and reticulin. The inflammatory infiltrate becomes progressively hypocellular with increasing fibrosis (Figure 2b). The typical histological findings with both early and late phases of the disease, which could both be seen in a single biopsy, were noted in our patient.
2. Histological findings.
a) Dense fibrous connective tissue with chronic inflammatory cells and eosinophils surrounding small blood vessels (haematoxylin and eosin, 200 x)
b) Dense fibrotic stroma with concentric onion-skin perivascular fibrosis (haematoxylin and eosin, 100 x)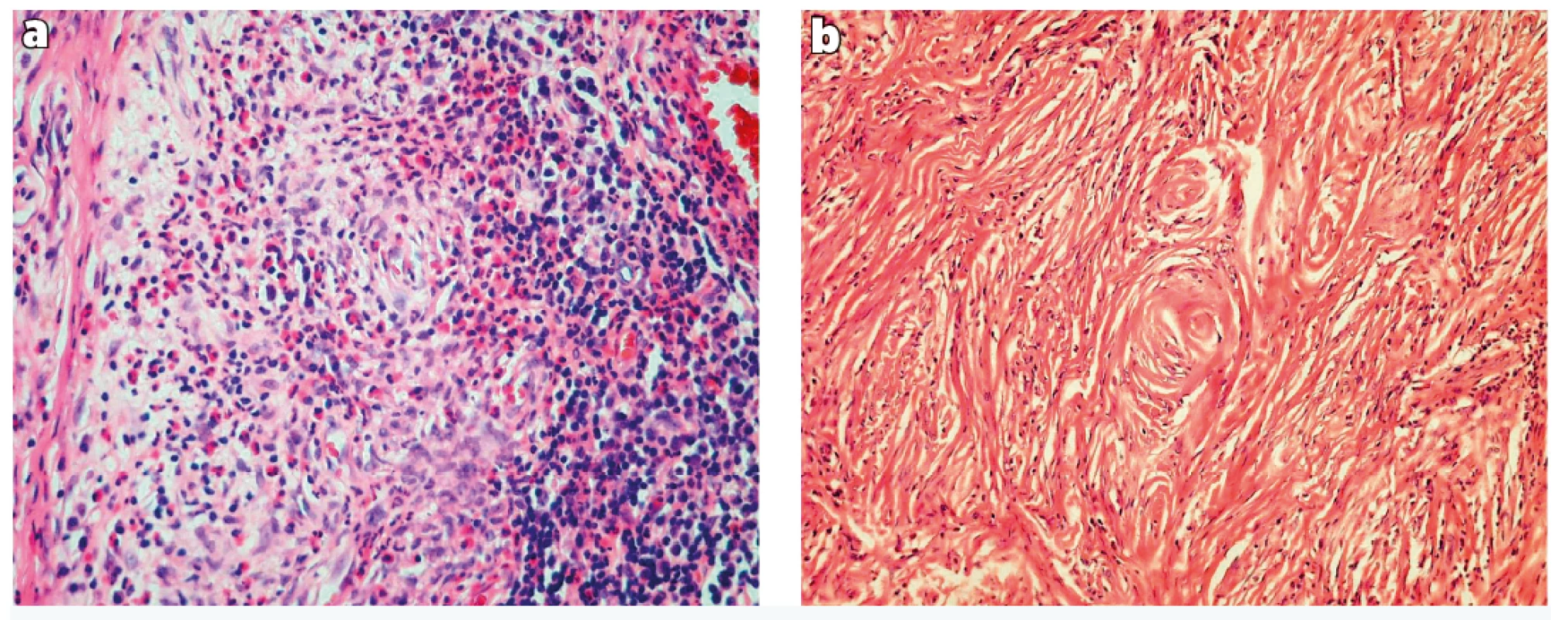
Two years later, a lesion recurrence was observed in the left lower edge of the orbit, in its medial part. Extirpation of the lesion was performed. We also reconstructed the saddle-like deformation of the nose after the previous surgery by inserting an alloplastic implant (Medpor). We used this implant for augmentation of the nasal dorsum and as a tip support. The application of the Medpor implant for facial reconstruction was described for example by Niechajev21. The patient was free of any problems thereafter. Five years later, a lesion recurrence was observed in the lateral part of the nose on the right and in the frontal part of the septum. The recurrence was not in contact with the implant. The lesion was gradually removed from lateral rhinotomy on the right, including expanded resection of a part of the nasal bone on the right, partial resection of the frontal process of the maxilla and of the affected part of the septum. No recurrence of the lesion was observed at regular follow-up visits in the course of subsequent two years. Reconstruction of the nose by Medpor implant was successful in restoring aesthetics and function.
DISCUSSION
The PubMed database was searched using the following keywords: eosinophilic angioncetric fibrosis, nasal cavity, nasal obstruction. Articles published in English from 1983 to 2015. We have selected only a few main articles for this publication.
EAF is a slowly progressing fibrotic lesion3–7. The aetiology is unknown.
Associations with trauma, allergy and nasal surgery are reported in the literature7,8. Fang et al. (2014) published review of 52 cases with sinonasal EAF8.
Typical symptoms of the disease include nasal obstruction, epistaxis, pain and swelling6,8. As shown by research studies, EAF is a part of the spectrum of IgG4-related systemic diseases9.
Non-enhanced CT revealed the lesion to be of homogenous isodensity compared with grey matter and rarely has calcifications. As the lesion progresses, it may cause adjacent bone remodelling, thinning, slight absorption or even sclerosis. EAF is generally isointense relative to grey matter on Tl-weighted images, with moderate inhomogeneous enhancement after contrast administration. Most lesions exhibit hypointense signal intensity on T2-weighted images in comparison to fibrosis seen in the late stage lesions10. In our case, CT showed clear destruction of nasal bones particularly on the left including partial destruction of the frontal process of the maxilla on the left.
The histological features were described by Roberts and McCann in 19851. The histology shows gradual progression of the lesion from an early inflammatory lesion to a fibrotic lesion. Both features may be found in a single biopsy. The early lesion is characterized by the presence of eosinophilic vasculitis without fibrinoid necrosis, occurring in a patchy fashion involving groups of capillaries and/or venules in the submucosa. The eosinophils cluster around and migrate through the vessel wall, show degranulation and are accompanied by a variable number of plasma cells and lymphocytes in the perivascular space. There is no true granulomatous reaction. The late lesion is characterised by obliterative perivascular onionskin whorling of collagen fibres and reticulin. The inflammatory infiltrate becomes progressively scanty as fibrosis increases1. The typical histological findings with both early and late phases of the disease, which can both be seen in a single biopsy, were noted in our patient.
Granuloma faciale, Wegener‘s granulomatosis, Churg-Strauss syndrome, Kimura‘s disease3,5,9,11–13, angiolymphoid hyperplasia with eosinophilia3,5, and nasal NK/T-cell lymphoma10,14 should be considered in the differential diagnosis.
The treatment of EAF is predominantly surgical; corticosteroids provide no improvement3–5,10–13,15,16, while the same applies to antibiotics3,11,12,16 and immunosuppressants5. Recurrences of the disease are often observed3,10,13. Our patient had repeated resections of the lesion for its recurrences. As shown in literature, radical resection is crucial for the treatment of EAF. This procedure may be difficult at times given the extent and localization of the disease7.
CONCLUSION
EAF is a rare benign lesion of unknown aetiology, found predominantly in the upper respiratory tract. Fibrosis must be confirmed by considering other lesions in the differential diagnosis. The typical histological finding provides the basis for determining the correct diagnosis. CT and magnetic resonance imaging combined with history may contribute to making the right diagnosis and also provide closer specification of the extent of the lesion. The most common treatment method is surgical resection. The published case is unique in our country.
Conflict of interest: The authors state that there are no conflicts of interest regarding the publication of this article.
Funding: The authors declare that this study has received no financial support.
Ethical requirements: All procedures performed in this study involving human participants were in accordance with ethical standards of the institutional and/or national research committee and with the Helsinki declaration and its later amendments or comparable ethical standards.
Ivo Němec, MD
Department of Otorhinolaryngology and Maxillofacial Surgery, Third Faculty of Medicine of Charles University Prague and the Military University Hospital Prague
U Vojenské nemocnice 1200
169 02 Prague 6
Czech Republic
E-mail: Ivo.Nemec@uvn.cz
Sources
1. Roberts PF, McCann BG. Eosinophilic angiocentric fibrosis of the upper respiratory tract: a mucosal variant of granuloma faciale? A report of three cases. Histopathology. 1985, 9 : 1217–25.
2. Holmes DK, Panje WR. Intranasal granuloma faciale. Am J Otolaryngol. 1983, 4 : 184–6.
3. Thompson LD, Heffner DK. Sinonasal tract eosinophilic angiocentric fibrosis. A report of three cases. Am J Clin Pathol. 2001, 115 : 243–8.
4. Paun S, Lund VJ, Gallimore A. Nasal fibrosis: long-term follow up of four cases of eosinophilic angiocentric fibrosis. J Laryngol Otol. 2005, 119 : 119–24.
5. Tabaee A, Zadeh MH, Proytcheva M, LaBruna A. Eosinophilic angiocentric fibrosis. J Laryngol Otol. 2003, 117 : 410–13.
6. Sunde J, Alexander KA, Reddy VV, Woodworth BA. Intranasal eosinophilic angiocentric fibrosis: a case report and review. Head Neck Pathol. 2010, 4 : 246–8.
7. Rimmer J, Andrews P, Lund VJ. Eosinophilic angiocentric fibrosis of the nose and sinuses. J Laryngol Otol. 2014, 128 : 1071–7.
8. Fang CH, Mady LJ, Mirani NM, Baredes S, Eloy JA. Sinonasal eosinophilic angiocentric fibrosis: a systematic review. Int Forum Allergy Rhinol. 2014, 4 : 745–52.
9. Deshpande V, Khosroshahi A, Nielsen GP, Hamilos DL, Stone JH. Eosinophilic angiocentric fibrosis is a form of IgG4-related systemic disease. Am J Surg Pathol. 2011, 35 : 701–6.
10. Yang BT, Wang YZ, Wang XY, Wang ZC. Nasal cavity eosinophilic angiocentric fibrosis: CT and MR imaging findings. AJNR Am J Neuroradiol. 2011, 32 : 2149–53.
11. Clauser L, Mandrioli S, Polito J, Marchetti E. Eosinophilic angiocentric fibrosis. J Craniofac Surg. 2006, 17 : 812–4.
12. Nguyen DB, Alex JC, Calhoun B. Eosinophilic angiocentric fibrosis in a patient with nasal obstruction. Ear Nose Throat J. 2004, 83 : 183–6.
13. Jain R, Robblee JV, O’Sullivan-Mejia E, Lea J, Heller A, Faquin WC, Powers CN. Sinonasal eosinophilic angiocentric fibrosis: a report of four cases and review of literature. Head Neck Pathol. 2008, 2 : 309–15.
14. Li Y, Liu H, Han D, Zang H, Wang T, Hu B. Eosinophilic angiocentric fibrosis of the nasal septum. Case Rep Otolaryngol. 2013; 2013 : 267285.
15. Narayan J, Douglas-Jones AG. Eosinophilic angiocentric fibrosis and granuloma faciale: analysis of cellular infiltrate and review of literature. Ann Otol Rhinol Laryngol. 2005, 114 : 35–42.
16. Faramarzi M, Dadgarnia MH, Moghimi M, Sharouny H, Behniafard N. Nasal Eosinophilic Angiocentric Fibrosis with Orbital Extension. Head Neck Pathol. 2015, 9 : 426–9.
17. Burns BV, Roberts PF, De Carpentier J, Zarod AP. Eosinophilic angiocentric fibrosis affecting the nasal cavity. A mucosal variant of the skin lesion granuloma faciale. J Laryngol Otol. 2001, 115 : 223–6.
18. Loane J, Jaramillo M, Young HA, Kerr KM. Eosinophilic angiocentric fibrosis and Wegener´s granulomatosis: a case report and literature review. J Clin Pathol. 2001, 54 : 640–1.
19. Matai V, Baer S, Barnes S, Boxer M. Eosinophilic angiocentric fibrosis. J Laryngol Otol. 2000, 114 : 563–4.
20. Owa AO, Boyle S, Gallimore AP. Eosinophilic angiocentric fibrosis as a cause of nasal obstruction. Rhinology. 2002, 40 : 41–3.
21. Niechajev I. Facial reconstruction using porous high-density polyethylene (medpor): long-term results. Aesthetic Plast Surg. 2012, 36 : 917–27.
Labels
Plastic surgery Orthopaedics Burns medicine Traumatology
Article was published inActa chirurgiae plasticae

2018 Issue 2-4-
All articles in this issue
- ČESKÉ SOUHRNY
- Editorial
- TRANSECTION OF INFERIOR ALVEOLAR NERVE IN THE RAT – NEUROANATOMICAL STUDY AND EXPERIMENTAL MODEL
- FAMILIAL HYPERCHOLESTEROLAEMIA – A DIAGNOSIS THAT EVERY PLASTIC SURGEON CAN EXPERIENCE
- EOSINOPHILIC ANGIOCENTRIC FIBROSIS AS A CAUSE OF NASAL OBSTRUCTION. A CASE REPORT
- A COMBINATION OF HERPES VIRUS INFECTION (HSV-1, HHV-6) AND MULTI-RESISTANT BACTERIAL INFECTION IN A SEVERELY BURNED PEDIATRIC PATIENT – A CASE REPORT
- Monika Adámková, MD, from the Ostrava Burn Center has been awarded Professor Radana Königová Prize
- In Memoriam of Associate Professor Jiří Kozák, MD, PhD
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- TRANSECTION OF INFERIOR ALVEOLAR NERVE IN THE RAT – NEUROANATOMICAL STUDY AND EXPERIMENTAL MODEL
- A COMBINATION OF HERPES VIRUS INFECTION (HSV-1, HHV-6) AND MULTI-RESISTANT BACTERIAL INFECTION IN A SEVERELY BURNED PEDIATRIC PATIENT – A CASE REPORT
- FAMILIAL HYPERCHOLESTEROLAEMIA – A DIAGNOSIS THAT EVERY PLASTIC SURGEON CAN EXPERIENCE
- EOSINOPHILIC ANGIOCENTRIC FIBROSIS AS A CAUSE OF NASAL OBSTRUCTION. A CASE REPORT
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

