-
Medical journals
- Career
TRANSECTION OF INFERIOR ALVEOLAR NERVE IN THE RAT – NEUROANATOMICAL STUDY AND EXPERIMENTAL MODEL
Authors: I. Němec 1; V. Smrčka 2,3; J. Mazánek 4; J. Pokorný 5
Authors‘ workplace: Charles University, Third Faculty of Medicine, and the Military University Hospital Prague, Department of, Otorhinolaryngology and Maxillofacial Surgery, Prague, Czech Republic 1; Charles University, First Faculty of Medicine, Institute for History of Medicine and Foreign Languages, Prague, Czech Republic 2; Charles University, First Faculty of Medicine, and Bulovka Hospital, Department of Plastic Surgery, Prague, Czech Republic 3; Charles University, First Faculty of Medicine, and General University Hospital in Prague, Department of Stomatology, Prague, Czech Republic 4; Charles University, First Faculty of Medicine, Institute of Physiology, Prague, Czech Republic 5
Published in: ACTA CHIRURGIAE PLASTICAE, 60, 2-4, 2018, pp. 48-53
INTRODUCTION
The inferior alveolar nerve (IAN) is a branch of the mandibular nerve, which is itself the third branch of the trigeminal nerve. It enters the mandible via the mandibular foramen and continues in the mandibular canal. The nerve anatomy in rat was described in the study of Naftel et al.1. IAN enters the mandibular canal as a single large trunk. The nerve is accompanied by smaller nerves that emerged from the IAN at or near the mandibular foramen. These branches innervate the first and partially the second molar; third molars and the distal part of the second molar are innervated by a branch of the lingual nerve. After entering the mandibular foramen (approximately 2 mm anterior to the mandibular foramen), the main trunk of IAN divides into two branches: the larger dorsal branch, the mental nerve, to exit via the mental foramen anterior to the first molar, and the ventral branch designated here as the incisor nerve1.
Experimental IAN lesions are used to study the peripheral and central alterations caused by its transection, by an excision of a part of the nerve, by contusion or ligation. Harada et al. studied regeneration of periodontal Ruffini endings following transection of the IAN2. Atsumi et al. studied nerve regeneration in connection with various types of injuries3. Yamashiro et al. described the effect of transection of the nerve on bone remodelling during an experimental movement of teeth4. Iwata et al. determined alterations in medullary dorsal horn neuronal activity after IAN transection5. Saito et al. studied the modulation of trigeminal spinal subnucleus caudalis neuronal activity in connection with regeneration of the transected IAN6. Lv et al. studied the effect of transectioning IAN on the healing of a periodontal defect7. Yu et al. also studied the effect of a lesion caused to the nerve on periodontal tissue regeneration8.
The purpose of our study was to contribute to closer specification of approaching the IAN and creating a lesion in the nerve. In the observation period, persistent denervation was verified by surgical revision and by histological and immunohistochemical analysis of a tissue sample taken from the lesion in the nerve. Subsequently, the method will be used to determine the effect of transectioning the sensory nerve on the inorganic component of the bone and teeth in the mandible.
MATERIAL AND METHODS
Experimental animals
Twenty-six males of Wistar laboratory rats having the weight of 320–405 g were used for the study. The animals were obtained from the breed of the Institute of Physiology of the First Faculty of Medicine, Charles University, Prague. The experiment was approved by the Ethical Committee of the First Faculty of Medicine (Charles University) and was compliant with the Guidelines of the Animal Protection Law of the Czech Republic and Guidelines for the treatment of laboratory animals (EU Guidelines 86/609/EEC). In each case, the surgical procedure was performed under general anaesthesia with intraperitoneal application of thiopental 4 mg/100 g of rat weight. The animals were kept in boxes at 20–23 °C with the application of the standard 12h-light/12h-dark cycle. The animals had normal food and water available ad libitum. The rats were divided into three groups. The control (intact) group included 6 rats. The experimental group included 12 rats (group with the nerve transection on the left side). The sham group included 8 rats (the nerve was only dissected without transectioning it).
Transection of the inferior alveolar nerve
The anatomical dissection provides a view of the lateral side of the left mandible including the IAN (Figure 1a,b). Our description of the lateral area of the mandible is based on the data of Kassab et al.9, Retief and Dreyer10. The microsurgical technique was used to approach the nerve and to perform its excision (we used the microscope: Carl Zeiss OPTON S4, Germany). This modification of the method has not been published yet.
a,b. Anatomical dissection of the course of the inferior alveolar nerve (IAN) in the mandibular canal (view of the lateral surface of the mandible – left).<br> a) 1 – prominence above the apex of the incisor root; 2 – bone ridge running from the prominence to the condylar process; 3 – condylar process<br> b) Partially removed cortical bone above the nerve between the mandibular foramen and the third molar. The arrow indicates the IAN branched out to the mental nerve (1) and the incisor nerve (2).<br> (Author’s archive) 
prolekare.web.text.media.image_type_figure.en The bone dissection site was identified according to the position of the bony prominence found at the site of the incisor root apex. Line 1 runs from the corner of the mouth to the lower edge of the external acoustic meatus, line 2 runs from the external corner of the eye to line 1 and is perpendicular to line 1. The point where the lines intersect (marked with a cross) identifies the site of muscle dissection and an approach to the bony prominence (Figure 2). To establish the approach, an arc-shaped 12 mm long incision was performed in the left face, in the direction from the corner of the mouth to the lower edge of the external acoustic meatus. The centre of the incision was found in the middle of the distance between the corner of the mouth and the lower edge of the external acoustic meatus. We exposed the masseter muscle fascia and cut the fascia along the direction of the muscle fibres between the dorsally oriented facial nerve and the ventrally located parotid duct (Figure 3a)11, 12. We performed blunt dissection of the muscles and reached the lateral part of the mandible, at the site of the prominence (Figure 3b). The bone ridge was identified in the area from the prominence to the condylar process. Caudally from the prominence, a round dental bur, size 1.2 mm, was used to remove a part of the bone, exposing the nerve in a length of 2–3 mm (Figure 3c). The scheme provides a better specification of the site of bone removal and nerve dissection (Figure 3d). The nerve was partially pulled out from the mandibular canal. The nerve can be moved especially in its proximal part found at the site of the mandibular foramen. The nerve was excised along the length of 3 mm (Figure 3e). After its excision, the proximal part slightly sank into the soft tissues adjacent to the mandibular foramen. In the process of dissecting the nerve, an effort was made to minimise any injuries to the vasculature. The wound was rinsed with 1 ml of saline. Edges of the muscle were adapted using one non-absorbable suture (Prolene 4/0). The same non-absorbable material was used to close the skin.
1. Bone prominence identification scheme. 1 – line from the corner of the mouth to the lower edge of the external acoustic meatus; 2 – perpendicular line from the external corner of the eye to line 1. The cross indicates the point of muscle dissection and approach to the bone prominence. The course of the incision is indicated using the dashed line.
(Author’s archive)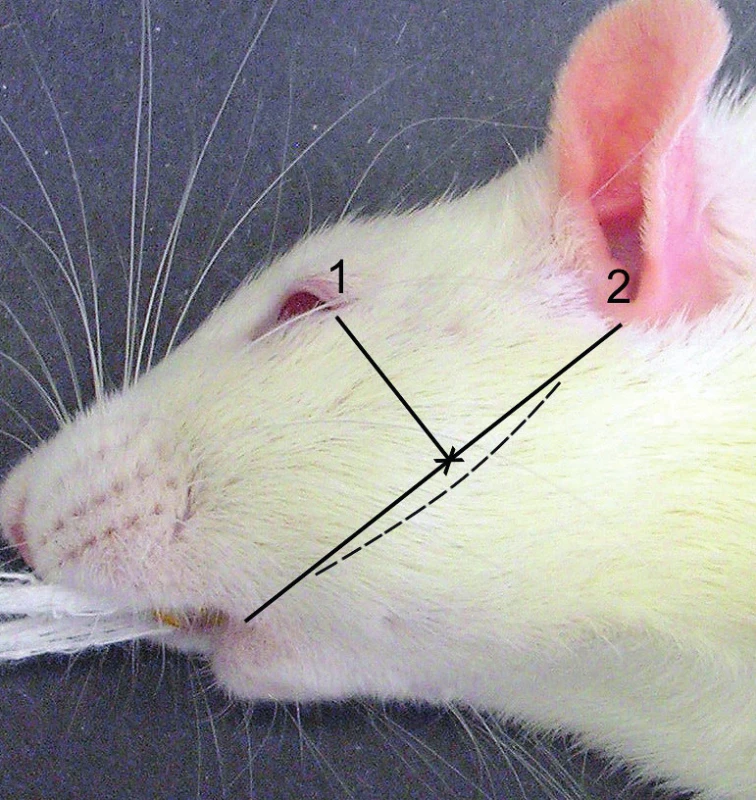
2. Figure 3a,b,c,d,e. Surgical approach and resection of a part of the nerve.
a) 1 – Exposed fascia of the masseter muscle. 1 – course of the facial nerve. 2 – course of the parotid duct
b) Dissected masseter muscle, exposed bone prominence (1) and part of the bone ridge (2) of the left mandible
c) The arrow indicates the partially exposed and slightly pulled out IAN on the left
d) Scheme of the mandible – the black area located caudally from the prominence (indicated with the arrow) shows the site of the nerve dissection
e) Resected 3 mm of the nerve
(Author’s archive)
Tissue dissection
The animals were weighed four weeks later and they were killed by overdose of intraperitoneal thiopental. The surgical microscope (Carl Zeiss OPTON S4, Germany) was used to revise the nerve excision site, while at the same time taking tissue samples for histological and immunohistochemical analysis. Material was fixed in 10% formaldehyde solution. Then it was drained with ascending set of alcohols and acetone, brightened with the xylene and immersed in paraffin wax. Then several tissue sections were cut to 3µm thickness, which were stretched on microscope slides.
Haematoxylin and eosin staining
Tissue sections were deparaffinised with xylene and alcohol, stained with Mayer´s haematoxylin stain and washed in tap water. Next step was staining with eosin, washing in tap water, draining with alcohol and acetone and in the finish brightening with xylene. Tissue sections were fixed with Pertex medium (Histolab Products AB, Sweden). Then the sections were examined and photographed with an optical microscope (Olympus BX 53, Japan).
Immunohistochemical staining (anti S100 protein)
Tissue sections were deparaffinised with xylene and alcohol. Then we put tissue sections for 35 minutes in to the water bath of Tris – EDTA buffer with pH 9 tris(hydroxymethyl)aminomethane and ethylenediaminetetraacetic acid, disodium salt, dihydrate; Made by Department of Clinical Pharmacology, Military university hospital, Prague) heated up to 98 degrees for 35 minutes. Then we washed cut sections with distilled water. Next step was blocking of endogen peroxidase with 3% solution of the H2O2 for 10 minutes. Then we washed cut sections with PBS – phosphate-buffered saline, pH 7.2 (made by Department of Clinical Pharmacology, Military university hospital, Prague). We applied primary antibody – Polyclonal Rabbit Anti – S100 (Dako, USA) on the sections, which reacted in room temperature for 60 minutes. Then we washed cut sections with the PBS substance and applied detection system – EnVision+System Labelled polymer rabbit (Dako, USA), which reacted for 30 minutes at room temperature. Then we washed sections with PBS substance again. Reaction between antigen and antibody was shown through DAB chromogen (3,3´-diaminobenzidine; Dako, USA). The sections were washed in distilled water and cell nuclei were stained with Mayer´s haematoxylin. Redundant stain was reduced; alcohol and acetone were used for draining and xylene for brightening of the sections. Tissue sections were fixed with Pertex medium (Histolab Products AB, Sweden). Then the sections were examined and photographed with an optical microscope (Olympus BX 53, Japan).
Statistical analysis
The comparison of mean weight increase of the animals after 4 weeks among the groups was done using the t-test. GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA, USA) was used for the statistical analysis. The statistical significance level of 5% was used.
RESULTS
The surgical wound healed in one week in all animals. At the time of the nerve excision site revision (after 4 weeks), persistent transection of IAN was clearly visible. The proximal stump of the nerve was sunk in the soft tissues, medially from the mandibular foramen. The distal stump of the nerve was not found.
We found no statistically significant differences in mean weight gains between individual groups during the observation period (Figure 4a,b,c).
3. a,b,c. Mean weight gains of the animals in 4 weeks.
a) Axis x: C = control group (C=127.2 g ± SEM 5.7); E = experimental group (E=122.0 g ± SEM 7.064). b) Axis x: C = control group; S = sham group (S=123.3 g ± SEM 8.958). c) Axis x: E = experimental group; S = sham group. Axis y: Mean weight gains of the animals in grams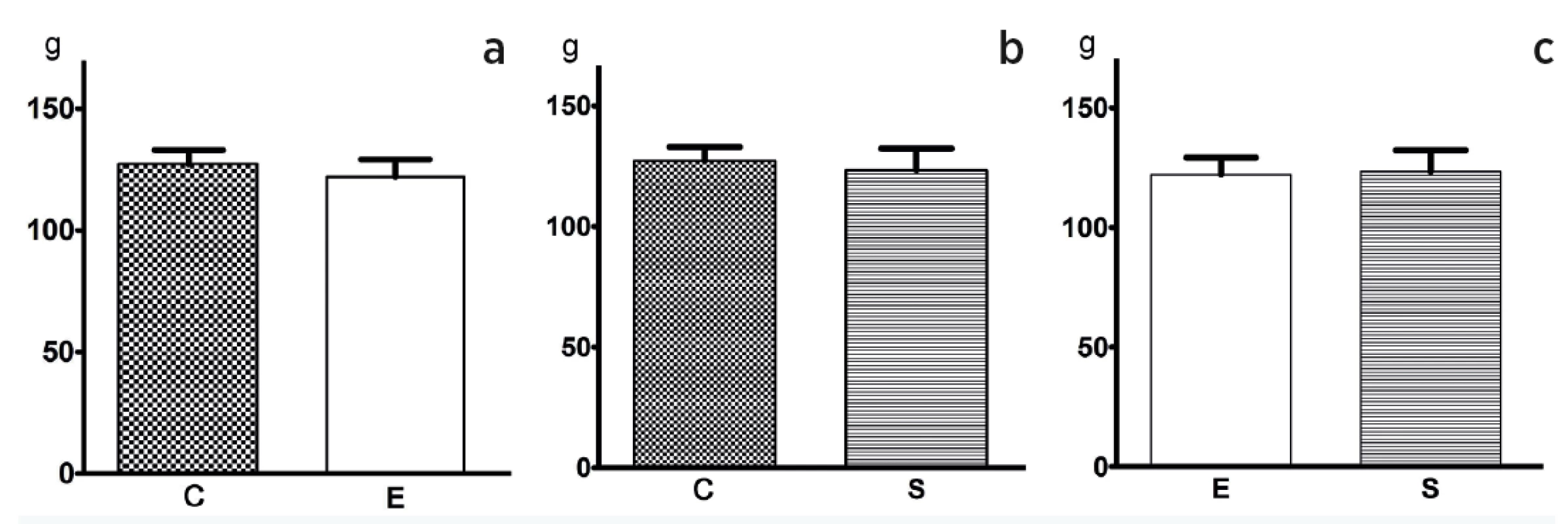
Histological and immunohistochemical analysis – microscopic finding was analogical in all of the samples. There were small portions of the muscle tissue with adjacent connective tissue, which grew between muscle fibres with a character of reactive proliferation of connective tissue. Samples contain a few very small fragments of bone tissue. Any structures of the peripheral nerve were not shown even with the immunohistochemical staining (S-100 protein) (Figure 5a,b).
4. a,b. Histological and immunohistochemical findings.
a) Haematoxylin and eosin staining (magnification 400 x). Muscle fibres and reactive proliferation of the connective tissue are present
b) Immunohistochemical staining: S-100 protein negative (magnification 400 x)
(With permission of Department of Pathology of the Military University Hospital Prague, Czech Republic)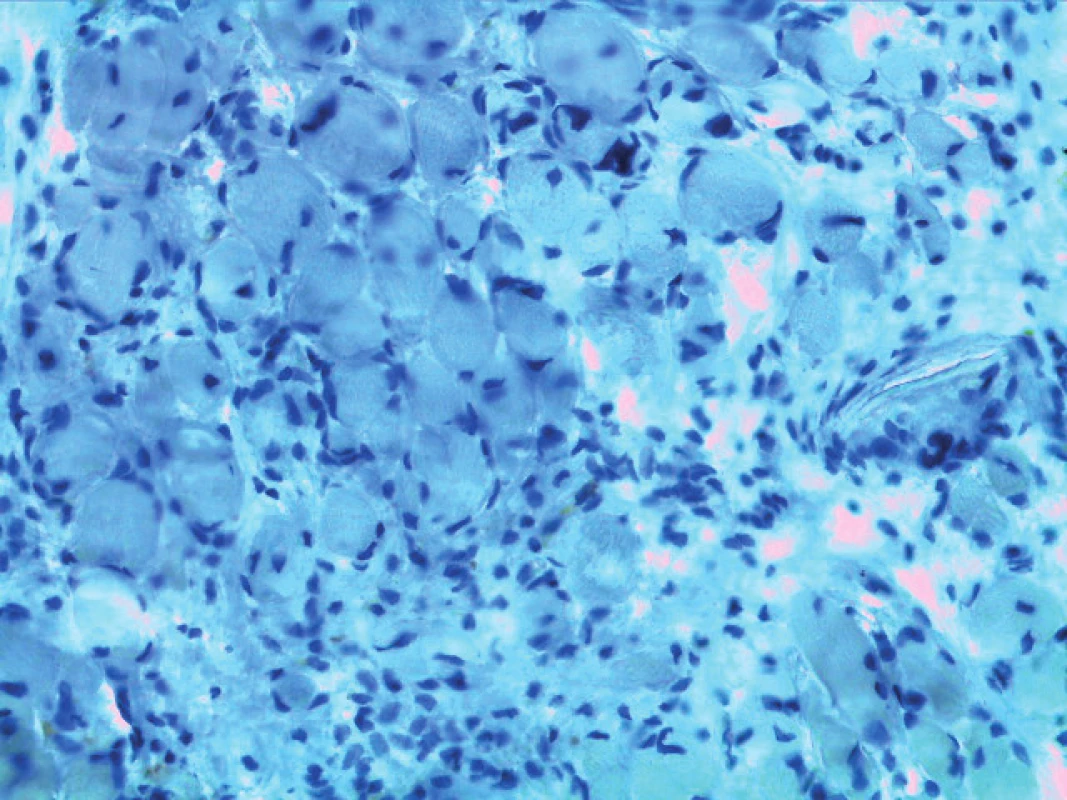
DISCUSSION
Experimental procedures aimed to create a nerve lesion have been associated with various complications13,14. For example, as reported by Stewart, the approach and transectioning of the nerve between the oval foramen and the mandibular foramen is associated with bleeding. The author himself described the process of dissecting and transectioning the nerve along its course in the mandibular canal without causing any injury to the vasculature13.
Retief and Dreyer described another, a relatively simple technique to approach the inferior alveolar nerve while causing a minimal trauma and injury to the blood vessels. The authors used four lines to determine the approach point to the lateral area of the mandible. The authors identified the prominence and bone ridge in the direction to the condyle. The nerve was exposed caudally from the prominence. As reported by the authors, no animals died during the surgery compared to other methods. The authors did not observe any complications after the surgery, either10.
Furthermore, a surgical method of transectioning the inferior alveolar nerve is described by Torneck and Harnett who incised the muscles between the facial nerve and the parotid duct. The incision line was parallel to the muscle fibres14.
Hoffmann et al. described an approach and transectioning of the nerve from the medial side. As reported by the authors, the transosseal approach poses a risk of causing a vascular injury, a risk of easier regeneration of the nerve given that the proximal stump remains in the mandibular canal, and moreover, the process of obtaining approach to the nerve is time-consuming. All these complications can be avoided when extraosseal approach is used15.
Iwata et al.5 and Saito et al.6 described dissection of the alveolar bone and removal of a part of the cortical bone. They reached the nerve above the angle of the mandible. Iwata et al. ligated the nerve at two points5. Saito et al. used the approach to transection the nerve6.
Kassab et al. described another approach to the nerve, anterior to the prominence. These authors see an advantage in preventing any perforation of the medial cortical bone. They dissected the muscle without sectioning its fibres, thereby providing better conditions for healing. The authors applied capsaicin to the exposed nerve9.
Lv et al. described an approach to the nerve from the medial side. At the site of the mandibular foramen, the authors dissected the nerve and excised the nerve in the length of 5 mm7. The same procedure was used by Yu et al.8.
In our set, we decided to approach the nerve through the lateral area of the mandible. The surgery was performed using the microsurgical technique. A modified procedure was used to identify the prominence as described by Retief and Dreyer10 and Kassab et al.9. The position of the bony prominence was determined at the intersection point of the two lines. Blunt dissection of the muscles was performed. The bone was prepared caudally from the prominence (see Figure 2). No perforation of the medial cortical bone was observed. In our opinion, this approach provides the advantage of enabling the surgeon to pull out the nerve from the mandibular canal of the mandibular foramen and to perform an easy excision of the nerve. The lesion itself was created directly at the site of the main trunk of the nerve and at the point where it splits to the mental nerve and the incisive nerve. In our set, we excised 3 mm of the nerve. After its excision, the proximal stump of the nerve partially sank into the soft tissues medially from the mandibular foramen. This fact prevents subsequent reinnervation. As shown by the results in our set, no reinnervation occurred after 4 weeks. We showed no statistically significant difference in the mean weight increase among individual groups during the observation period of 4 weeks.
CONCLUSION
The presented study provides closer specification of the anatomical situation and approach to IAN in the mandibular canal. At the same time, the described method provides one of the possible ways to create a lesion in the nerve at the site of its main trunk. Persistent transectioning of the nerve was verified by surgical revision and by histological and immunohistochemical analysis of a tissue sample taken from the lesion of the nerve. The procedure of transectioning the nerve had no effect on weight increase of the animals during the observation period.
Acknowledgement
The authors thank I. Tučková, MD (Department of Pathology of the Military University Hospital Prague, Czech Republic) for histological and immunohistochemical examination of the samples.
Conflict of interest: The authors have no conflicts of interest to disclose.
Funding: The authors declare that this study has received no financial support.
Ethical requirements: All procedures performed in this study were in accordance with ethical standards of the institutional and/or national research committee and with the Helsinki declaration and its later amendments or comparable ethical standards.
Ivo Němec, MD
Department of Otorhinolaryngology and Maxillofacial Surgery, Third Faculty of Medicine of Charles University and the Military University Hospital Prague
U Vojenské nemocnice 1200
169 02 Prague 6
Czech Republic
E-mail: Ivo.Nemec@uvn.cz
Sources
1. Naftel JP, Richards LP, Pan M, Bernanke JM. Course and composition of the nerves that supply the mandibular teeth of the rat. Anat Rec. 1999, 256 : 433–47.
2. Harada F, Hoshino N, Hanada K, Kawano Y, Atsumi Y, Wakisaka S, Maeda T. The involvement of brain-derived neurotrophic factor (BDNF) in the regeneration of periodontal Ruffini endings following transection of the inferior alveolar nerve. Arch Histol Cytol. 2003, 66 : 183–94.
3. Atsumi Y, Imai T, Matsumoto K, Sakuda M, Maeda T, Kurisu K, Wakisaka S. Effects of different types of injury to the inferior alveolar nerve on the behavior of Schwann cells during the regeneration of periodontal nerve fibers of rat incisor. Arch. Histol. Cytol. 2000, 63 : 43–54.
4. Yamashiro T, Fujiyama K, Fujiyoshi Y, Inaguma N, Takano-Yamamoto T. Inferior alveolar nerve transection inhibits increase in osteoclast appearance during experimental tooth movement. Bone. 2000, 26 : 663–9.
5. Iwata K, Imai T, Tsuboi Y, Tashiro A, Ogawa A, Morimoto T, Masuda Y, Tachibana Y. Hu J. Alteration of medullary dorsal horn neuronal activity following inferior alveolar nerve transection in rats. J Neurophysiol. 2001, 86 : 2868–77.
6. Saito K, Hitomi S, Suzuki I, Masuda Y, Kitagawa J, Tsuboi Y, Kondo M, Sessle BJ, Iwata K. Modulation of trigeminal spinal subnucleus caudalis neuronal activity following regeneration of transected inferior alveolar nerve in rats. J Neurophysiol. 2008, 99 : 2251–63.
7. Lv L, Wang Y, Zhang J, Zhang T, Li S. Healing of periodontal defects and calcitonin gene related peptide expression following inferior alveolar nerve transection in rats. J Mol Histol. 2014, 45 : 311–20.
8. Yu X, Lv L, Zhang J, Zhang T, Xiao C, Li S. Expression of neuropeptides and bone remodeling-related factors during periodontal tissue regeneration in denervated rats. J Mol Histol. 2015, 46 : 195–203.
9. Kassab A, Hage M, Jabbur SJ, Chidiac JJ. Modified technique for the exposure of the inferior alveolar nerve in rats. J Pharmacol Toxicol Methods. 2013, 67 : 182–6.
10. Retief DH, Dreyer CJ. Sectioning of the inferior dental nerve in rats. J Dent Res. 1969, 48 : 969.
11. Popesko P, Rajtová V, Horák J. Atlas anatómie malých laboratórnych zvierat. 2. Potkan, Myš, Chrček zlatý. Bratislava: Príroda; 1990.
12. Olds RJ, Olds JR. Farbatlas der Anatomie der Ratte – Sektionsanleitung. Hengersberg: Schober Verlags-GmbH; 1984.
13. Stewart JM. Sectioning the inferior alveolar nerve in rats. J. Dent. Res. 1965, 44 : 830.
14. Torneck CD, Harnett B. Surgical method for unilateral removal of the inferior dental nerve in the rat. J Dent Res. 1971, 50 : 167.
15. Hoffman DR, Tade WH. Improved technique for sectioning the inferior alveolar nerve in rats. J Dent Res. 1972, 51 : 668.
Labels
Plastic surgery Orthopaedics Burns medicine Traumatology
Article was published inActa chirurgiae plasticae

2018 Issue 2-4-
All articles in this issue
- ČESKÉ SOUHRNY
- Editorial
- TRANSECTION OF INFERIOR ALVEOLAR NERVE IN THE RAT – NEUROANATOMICAL STUDY AND EXPERIMENTAL MODEL
- FAMILIAL HYPERCHOLESTEROLAEMIA – A DIAGNOSIS THAT EVERY PLASTIC SURGEON CAN EXPERIENCE
- EOSINOPHILIC ANGIOCENTRIC FIBROSIS AS A CAUSE OF NASAL OBSTRUCTION. A CASE REPORT
- A COMBINATION OF HERPES VIRUS INFECTION (HSV-1, HHV-6) AND MULTI-RESISTANT BACTERIAL INFECTION IN A SEVERELY BURNED PEDIATRIC PATIENT – A CASE REPORT
- Monika Adámková, MD, from the Ostrava Burn Center has been awarded Professor Radana Königová Prize
- In Memoriam of Associate Professor Jiří Kozák, MD, PhD
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- TRANSECTION OF INFERIOR ALVEOLAR NERVE IN THE RAT – NEUROANATOMICAL STUDY AND EXPERIMENTAL MODEL
- A COMBINATION OF HERPES VIRUS INFECTION (HSV-1, HHV-6) AND MULTI-RESISTANT BACTERIAL INFECTION IN A SEVERELY BURNED PEDIATRIC PATIENT – A CASE REPORT
- FAMILIAL HYPERCHOLESTEROLAEMIA – A DIAGNOSIS THAT EVERY PLASTIC SURGEON CAN EXPERIENCE
- EOSINOPHILIC ANGIOCENTRIC FIBROSIS AS A CAUSE OF NASAL OBSTRUCTION. A CASE REPORT
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career






