-
Medical journals
- Career
A COMBINATION OF HERPES VIRUS INFECTION (HSV-1, HHV-6) AND MULTI-RESISTANT BACTERIAL INFECTION IN A SEVERELY BURNED PEDIATRIC PATIENT – A CASE REPORT
Authors: R. Zajíček 1; H. Hrbáčková 2; H. Šuca 1; I. Pafčuga 1; K. Labská 2; Mc Smula 3
Authors‘ workplace: Charles University, Third Faculty of Medicine, and Teaching Hospital Královské Vinohrady, Prague Burn Center, Prague, Czech Republic 1; National Institute of Health, Prague, Czech Republic 2; Teaching Hospital Královské Vinohrady, Institute of Laboratory Diagnostics, Prague, Czech Republic 3
Published in: ACTA CHIRURGIAE PLASTICAE, 60, 2-4, 2018, pp. 62-67
INTRODUCTION
Infections continue to be the main cause of mortality and morbidity in burn patients 1,2. Extensive burn trauma causes serious impairment of the immune system, primarily due to a loss of skin and serous barriers but secondarily in the context of systemic inflammatory response. Although the effect of bacterial and fungal pathogens on morbidity and mortality of burn patients is well established, the impact of viral infections is not yet well described. Herpes viruses constitute a significant group of DNA viruses with two important characteristics: an ability to adapt to the host after primary infection, and symptomatic reactivation after a period of latency. Despite the high seroprevalence of herpes viruses in the adult population (in Europe the seroprevalence of primary infections, particularly in childhood, follow a course that is either asymptomatic or resembles mild nonspecific symptoms similar to those of other viral infections), immunosuppression associated with serious burn injury can lead to reactivation of a latent herpes infection, or, in the setting of a primary infection, it can take a far more dramatic, even life-threatening course.
CASE REPORT
A 15-month-old boy sustained a scald injury with hot tea at his home, resulting in second-degree burn to his face, neck, chest, and back, encompassing 20% of total body surface area (Figure 1). According to his medical history, 11 months prior to the injury, he had experienced a primary HHV-6 infection (exanthema subitum). No other cases of serious disease were documented. The first point of care was at a regional hospital, where the patient was intubated as a result of burns to his neck and face. Ultimately, the patient was transferred to the Prague Burn Center. Upon arrival to the emergency room following an extended transport, hydrocolloid dressings were applied to the burn areas. Once admitted to the pediatric intensive care unit, comprehensive burn shock treatment was initiated. Fluid resuscitation consisted of administration of crystalloids and colloids. The patient was extubated after three hours due to his stable status. In the first three days after admission, the clinical picture was characterized by the emergence of an upper respiratory tract infection manifested by laryngeal stridor in combination with post-intubation laryngitis. Visible signs of peribronchitis were present on the chest X-ray and the patient was febrile. Treatment was symptomatic in the first 48 hours and included inhalation therapy with mucolytics and antipyretics. Due to elevation of inflammatory markers (rise in C-reactive protein from 8 mg/l up to 40 mg/l, procalcitonin from 0.8 ng/ml up to 1.2 ng/ml), empiric antibiotic therapy (sultamicillin [Unasyn®, Pfizer Inc., USA]) was started for three days. Bacterial swabs from the burn wound taken on admission were positive for oxacillin‑resistant Staphylococcus aureus (ORSA) and Acinetobacter species. Alarmingly, Enterococcus faecalis was identified in urine, most likely as a consequence of urinary catheterization at the regional hospital. Blood cultures were positive for coagulase‑negative S. aureus and Acinetobacter species. In view of these microbiological findings, the antibiotic strategy was modified to include amikacin. These changes led to an overall clinical improvement as confirmed by the regression of inflammatory parameters. Dressing changes were performed at the operating room, and the burn surfaces were reassessed as mixed second-degree burns. Silver sulfadiazine (Flammazine®, Smith & Nephew, UK) (Figure 2) was applied due to deepening of the wound and presence of infection. The patient’s febrile status persisted (39–40°C) in spite of a normalizing trend of inflammatory markers and absence of a significant bacterial burn wound infection. Consequently, further examinations were carried out as a part of differential diagnosis. These included: echocardiography, aspergillus and candida antigens, polymerase chain reaction (PCR) examination of influenza viruses, all with negative findings. On post-burn day 12, a macular–vesicular rash appeared on the edges of the burn wound (Figure 3). Treatment of burn surfaces with antibacterial cream continued. Swabs taken from burn surfaces on the chest and from pustules outside of the burn area were positive for HSV-1 DNA virus, confirming an active HSV-1 infection. HSV-1 DNA was also detectable in peripheral blood (106 copies/ml of whole blood). Moreover, PCR examination revealed active systemic HHV-6 infection in peripheral blood (2515 HHV-6 DNA copies/ml) and borderline positivity for HHV-6 DNA in swabs from pustules. These findings, along with information from the patient’s history, indicated high suspicion of clinical reactivation of HHV-6 after primary infection at the beginning of year 2014. HHV-6 type B was confirmed in all HHV-6 positive samples by a second test (end-point PCR with restriction fragment length polymorphism), and germline chromosomal integration of HHV-6 was excluded by PCR testing of hair follicles.
1. A 15-month-old boy admitted due to superficial seconddegree burns over 20% of total body surface area 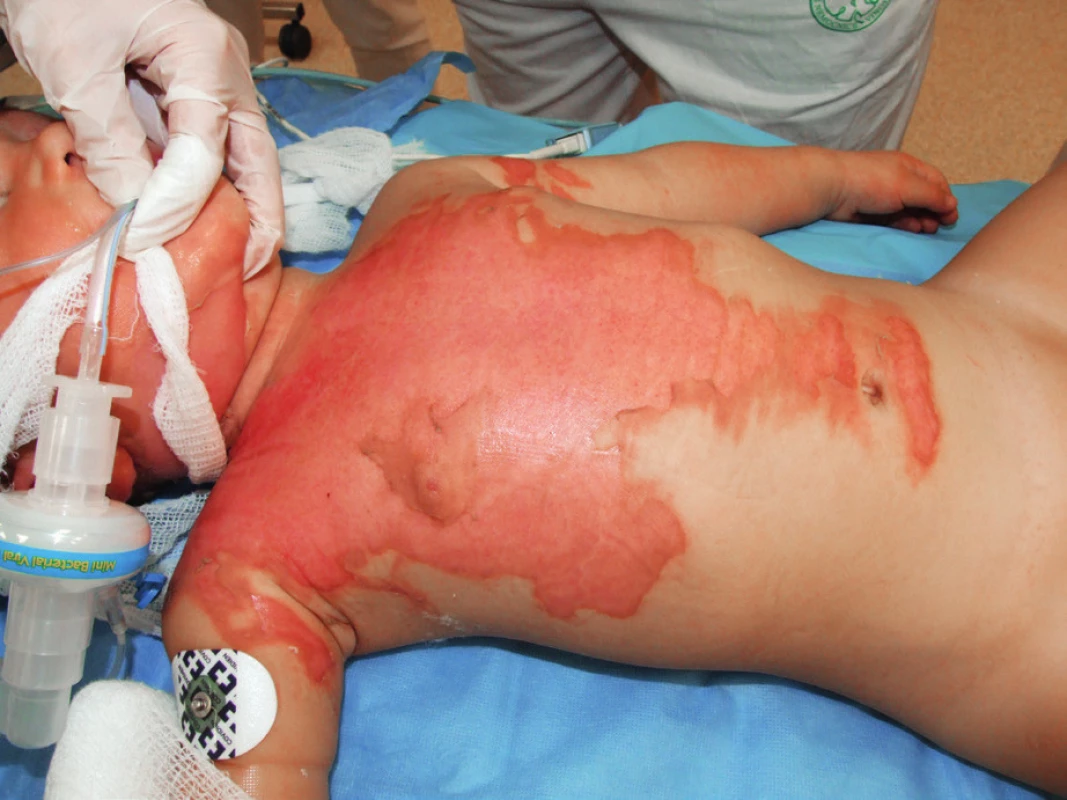
2. Post-burn day 3: Deepening of the wound to mixed second-degree burns 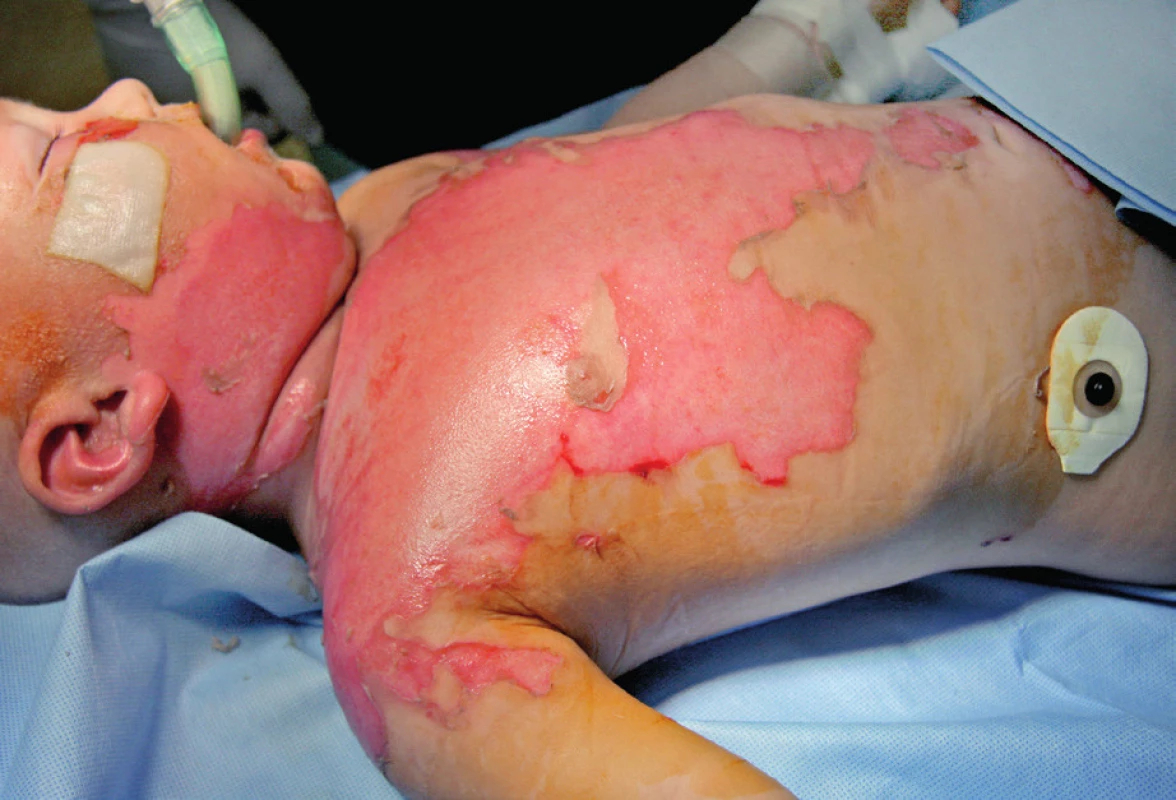
3. Post-burn day 12: Macular–vesicular herpetic rash appeared on the edges of the burn wound (arrow) 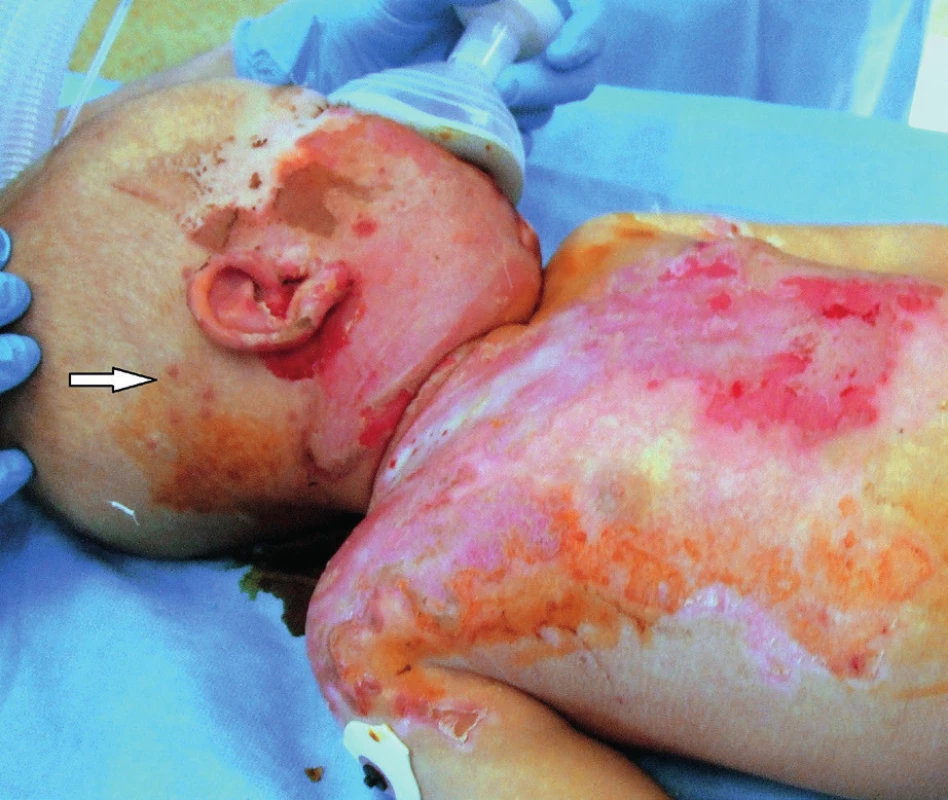
Due to the presence of both systemic and local HSV-1 infections, acyclovir at a dose of 5 mg/kg was administered for 10 days. Marked clinical improvement was obvious after three days, with subsiding fever and improvement of herpetic rash. The burn surfaces no longer bled on contact and no new vesicles were appearing. It was possible to observe healing of the vesicles in different stages from rupture, drying, scab formation to sloughing of the scabs. Spontaneous healing of the burn areas began, (Figure 4) and by post-admission day 23 complete healing and spontaneous epithelization of the injured areas was obvious. On post-admission day 26, the patient was discharged to home care (Figure 5).
4. Post-burn day 15: Wound healing after three days of acyclovir therapy 
5. Post-burn day 26: Full healing. Patient was discharged from hospital 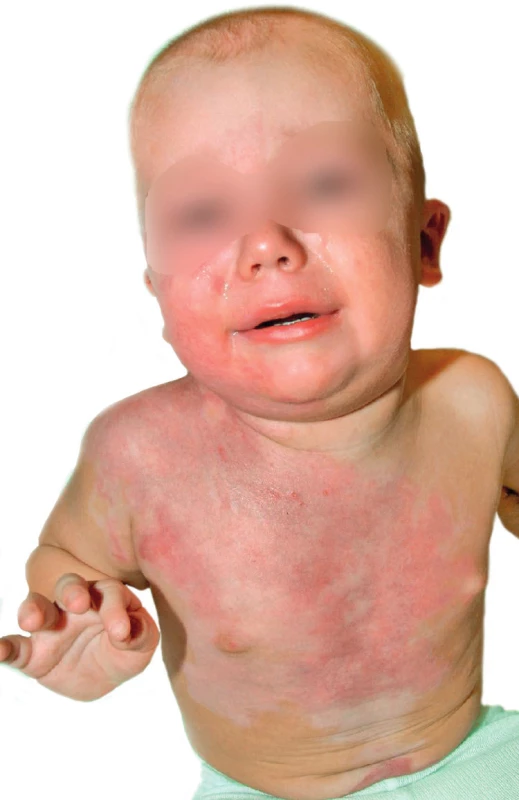
DISCUSSION
The immune system is significantly impaired in cases of severe burn trauma. Burn-induced immunosuppression is associated with negative consequences for the whole host. These include a myriad of immunological abnormalities not limited to antibodies but involving cellular immunity as well. Pathology of cellular immunity seems to be the most probable factor leading to the reactivation of latent herpes simplex 1 and 2 viral infections 4. Additionally, the literature highlights the important causal role of elevated levels of suppressor T lymphocytes in temporary suppression of immunity induced by thermal injury 5,6. In experimental animal models, burned mice had a 100 times higher probability of activation or primary infection with HSV viruses compared to controls 5. The incidence of active herpes simplex infections in a population of burn patients is surprisingly high. In their work, Linnemann and MacMillan report an incidence of 8–25% in pediatric burn patients 7. In a review article, Hayden reports that herpes virus infections of the burn surface occur in 60% of children younger than 10 years of age with extensive burns (mean body surface area 36%), typically localized to the neck and face 8.
It is not uncommon for burn patients to develop either a primary HSV infection upon exposure to the virus or, in the case of patients that are seropositive, reactivation of a latent HSV infection. The most frequent route of exposure is transfer of the infection from parents in the active phase of labial herpes, although infection without skin eruption is also possible due to viral shedding 9. The nosocomial spread of HSV has also been described 10. In the case presented here, the parents had a negative history of oral herpes. We speculate that the most probable source of infection were the primary caregivers in the first days after the burn injury. Systemic infection was indicated by the presence of the virus in peripheral blood. Primary HSV-1 infection was serologically confirmed by documenting the seroconversion of HSV-1 IgG from borderline to positive (21.7 and 96.4 arbitrary units/ml) – a four times increase during a period of two weeks form the 12th day post-injury. Anti-HSV2 IgG antibodies remained negative. In both examinations, anti-HSV IgM antibodies were present.
Kegan determined significant correlations of HSV-infected patients with burns in association with age (over 50 years), location (face), mechanism of injury (inhalation trauma), burn depth, and length of hospitalization (greater than three weeks) 4. In the case presented here, our patient had suffered burns to the face and had been treated at a regional hospital where he was intubated at the admission room. The prolonged transport of the patient from the regional hospital to Prague could have had an effect on the patient’s general state, as adverse weather conditions made air transportation of the patient impossible.
The clinical manifestations of viral infection are quite variable, ranging from mild symptoms to severe disseminated disease involving a systemic inflammatory reaction. In the case of HSV disseminated disease, the respiratory system and liver are the most frequently affected 11–13. A less frequently occurring but very serious complication is infection of the central nervous system 14. The typical clinical picture in burn patients includes a protracted febrile state of unknown etiology occurring before the appearance of the vesicles 15. In the presented case, the patient was continually febrile even when signs of inflammation were low. Only after pustules had appeared in the area of the burn, we started to consider herpes viruses as a diagnosis. Furthermore, the general state of the patient was modified by the presence of resistant bacterial strains (confirmed by swabs and urine cultivation upon admission to the Prague Burn Center). These bacterial findings most likely point to infectious contamination of the burn surfaces as a result of the initial treatment the patient had received at the regional hospital. A relationship between bacterial infection and the presence of HSV infection was reported by Sen et al. in 2012 16. They showed, among others, that HSV infection increased the risks of Acinetobacter Baumannii in the respiratory tract as well as of infection with oxacillin-resistant strains of S. aureus in wounds 16. Surprisingly, both bacteria were isolated in the presented patient, with A. Baumannii also being positively identified in blood cultures. According to the 2012 taxonomic classification of HHV-6, the virus is divided into two distinct variants: HHV-6A and HHV-6B 17. HHV-6A tends to be detected in the seriously ill, for example in cases of transplant patients with AIDS, neurological illnesses, and others 18. Primary infection with HHV-6B presents most commonly in older infants and toddlers as febrile condition, sometimes associated with febrile seizures. Exanthema occurs in only 20% of children, where it is described as exanthema subitum or 6th disease 19. After the initial primary infection, the virus typically remains latent in the host’s body for the remainder of his or her life. The virus can, however, reactivate and clinically reemerge as a serious disease in an immunocompromised host. In the general population, seroconversion is dependent on geographic location and occurs in 70% to 100% of adult individuals 20. There are many potential causes for reactivation, such as trauma, serious or multiple concurrent illnesses, immunosuppressive therapies, or any other therapy that may temporarily weaken the immune system. Although the mechanism for HHV-6 reactivation has not yet been precisely defined, it nevertheless has been suggested that inflammatory mediators and cytokines play an important role 21,22. Burn trauma is associated with the release of large quantities of cytokines and inflammatory mediators that may serve as the trigger reactivating the latent virus 23. Reactivation of HHV-6 infection has been observed in approximately 50% of organ transplant patients, and it is linked to a whole spectrum of serious illnesses like pneumonia, encephalitis, and giant cell hepatitis 24.
Returning to the case presented earlier, the toddler had been ill with 6th disease 11 months prior to the injury. The course of the primary infection was without any complications (febrile condition with exanthema). Twelve days after the injury, active HHV-6 infection was confirmed by positive but low findings in swabs from pustules and intermediate viral load in peripheral blood (2515 copies of HHV-6 DNA/ml). The presence of HHV-6 in swabs was likely due to blood contamination of the swabs, as subsequent swabs were negative and the wound was no longer bleeding. Conversely, the presence of HHV-6 DNA remained detectable in peripheral blood 26 days after the injury. The findings increased slightly in control samples (3880 DNA copies HHV-6/ml) but did not exceed the accepted reference range for significant increase in viral load.
Another well-known source of high HHV-6 DNA viral load in all types of biological samples is germline integration (also known as chromosomal integration), which has incidence of 0.8–2% in the general population 25,26. That was not our case, as indicated by HHV-6 DNA negativity of hair follicles.
To date, the presence of the HHV-6 DNA virus in the context of pediatric burn trauma has not been described in the available literature. In a study by Razonable et al., DNA viruses were found to be present in peripheral blood leukocytes in 65% of adult patients admitted to an intensive care unit for various medical reasons. Only adult patients were evaluated, and of those, just 7% were hospitalized for trauma 27.
Serological identification of HHV-6 antibodies was performed by indirect immunofluorescence test (both IgG and IgM). Assessing antibodies is especially useful regarding herpes viruses in the diagnosis of primary infection, whereas the value of serological testing in cases of viral reactivation, especially in chronically ill or immunodeficient patients, is unclear and ambiguous at the best. In such circumstances, direct identification of the viral agent is preferred.
In our patient, we demonstrated seroconversion to HSV-1. Because HHV-6 IgG antibodies were detectable in a titer of 1 : 40 on day 12 after injury (day 1 of vesicle eruption) and the patient has a history of exanthema subitum, we think this was not a primary infection. Activity of HHV-6 infection was confirmed by the presence of anti-HHV-6 IgM antibodies, whose positivity slightly increased in control samples (from 1 : 10 to 1 : 20 titer).
Whether viral activity and the slight increase in concentration of HHV-6 DNA can be linked to the administration of antibiotics even though major immunological and dermatological manifestations (e.g., Drug Reaction with Eosinophilia and Systemic Symptoms or Diffuse Idiopathic Skeletal Hyperostosis ) never occurred is open to debate 28,29. The combination of two viral infections on top of a mixed multi-resistant bacterial infection in the setting of the immature immune system of an 11-month-old toddler presents a unique starting point for discussion and future study to further explore the nature of infectious complications in severe burn injury.
The observed herpetic lesions consisting of fluid-filled vesicles appeared within two days in the areas where burn healing was slow: on the face, chest, and neck (see Figure 3). Over the next five days from their first appearance, the vesicular contents became clouded and ruptured spontaneously. Skin lesions changed into small, bleeding ulcerations and scabs with a potential for spontaneous healing (Figure 6). The location of skin lesions at the edges of the healing burn surfaces is interpreted as a feature of the rapidly replicating DNA in the regenerating epithelial cells 30. Local signs of infection in burn patients are less often observed in the areas distant from the thermal injury 31. According to the literature, it takes up to four days for complete resolution of skin lesions 32. In the case presented, it took 14 days from the first appearance of skin lesions to the complete healing and disappearance of scabs. This is indicative of the high variability in healing within the pediatric population and corresponds well with the treatment by acyclovir.
6. Post-burn day 18, after five days of acyclovir therapy: herpetic skin lesions healing spontaneously 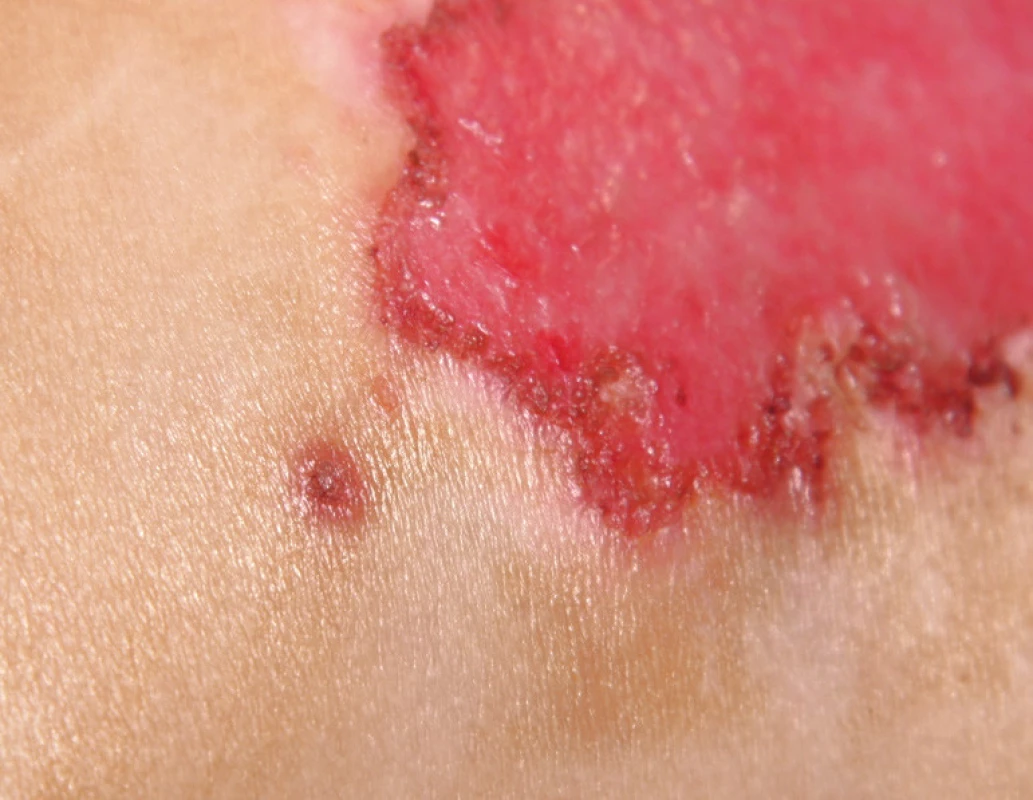
Regarding local therapy, topical application of antibacterial creams and silver sulfadiazine (Flammazine®, Smith & Nephew, UK) was chosen because a mixture of gram-positive and gram-negative resistant bacteria was present in the wounds. Although silver sulfadiazine is primarily indicated for bacterial and fungal infections, it has been demonstrated that inclusion of silver sulfadiazine into primary cultures of varicella zoster virus led to a significant decrease of viral pathogenicity in vitro 33. In the presence of herpetic infections, the superficial surface of second-degree burns can be converted into deep burns. Moreover, a loss of skin grafts due to the presence of herpetic infections on operated surfaces has been described 23. In our patient, the repeated application of silver sulfadiazine to burn surfaces facilitated healing in approximately 26 days from admission. Topical acyclovir ointment was applied to the burn edges where pustules were present. Acyclovir acts as an inhibitor of viral DNA synthase without affecting noninfected cells. Application of silver sulfadiazine occurred every 10 days and was coordinated with dressing changes once every two days. Administration of local therapy at more frequent intervals was not possible due to pain during manipulation with the patient. After complete healing, the child was referred to the outpatient clinic at Prague Burn Center, where follow-ups showed an absence of hypertrophic healing except at the edges of the burn surface in the vicinity of the navel and shoulder (Figure 7). It is noteworthy that, in their study of 11 adult burn patients with herpetic skin infection, Bourdarias and colleagues describe excellent healing without the use of any additional therapy 34. Their study was carried out on a small patient population without confirmation of viremia. In the present case, systemic therapy with acyclovir was fully warranted due to the concurrent primary HSV-1 infection and active HHV-6, because the risk of other organ system involvement was quite high. In their study, Byers et al. report a possible link between HSV immunopositivity and presence of adult respiratory distress syndrome 35. Immediately at the time of the diagnosis, we initiated systemic administration of acyclovir in 5 mg/kg dosages i.v. every eight hours over a period of 10 days. Renal toxicity was not documented, and after five days with clinical improvement was the treatment converted to oral administration. The question whether an acyclovir-based preventative strategy would be of benefit to burn patients with a history of oral herpes is unclear and necessitates further study 36.
7. One year after trauma at outpatient clinic 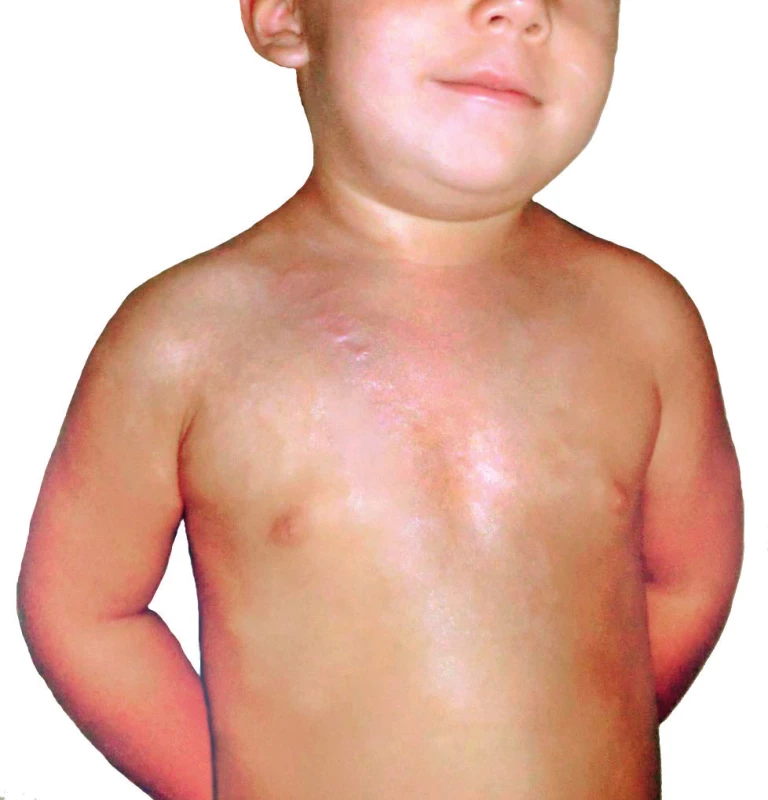
CONCLUSION
Primary infection as well as reactivation of herpes viruses can play a part in the infectious complications associated with extensive burn trauma. Clinical manifestation of infection ranges from an asymptomatic course to life-threatening disseminated systemic infection. The most common presentation is primary infection or the reactivation of a latent HSV-1 infection (HSV-2 infection is also possible). Not as common, but central to the case presented, is HHV-6 reactivation in the setting of extensive burn trauma. The typical clinical picture consists of vesicles in the area surrounding the burn surface with a predilection for the neck and face plus fever of unclear etiology. Therapy is based on local treatment and systemic administration of acyclovir in the setting of HSV-1,2.
Acknowledgments
The project was supported by grant from the Charles University in Prague, Progres – Q37.
Robert Zajíček, MD
Charles University, Third Faculty of Medicine, and Teaching Hospital Královské Vinohrady, Prague Burn Center,
Šrobárova 50
100 34 Prague 10
Czech Republic
E-mail: robert.zajicek@fnkv.cz
Sources
1. Appelgren P, Björnhagen V, Bragderyd K, Jonsson CE, Ransjö U. A prospective study of infections in burn patients. Burns. 2002;28(1):39–46.
2. Robson MC. Burn sepsis. Crit Care Clin. 1988;4(2):281–98.
3. Okuno T, Takahashi K, Balachandra K, Shiraki K, Yamanishi K, Takahashi M, et al. Seroepidemiology of human herpesvirus 6 infection in normal children and adults. J Clin Microbiol. 1989;27 : 651–3.
4. Kagan RJ, Naraqi S, Matsuda T, Jonasson OM. Herpes simplex virus and cytomegalovirus infections in burned patients. J Trauma. 1985;25(1):40–5.
5. Kobayashi M, Herndon DN, Pollard RB, Suzuki F. CD4+ contrasuppressor T cells improve the resistance of thermally injured mice infected with HSV. J Leukoc Biol. 1995;58(2):159–67.
6. Miller CL, Baker CC. Changes in lymphocyte activity after thermal injury. The role of suppressor cells. J Clin Invest. 1979;63(2):202–10.
7. Linnemann Jr CC, MacMillan BG. Viral infections in pediatric burn patients. Am J Dis Child. 1981;135(8):750–3.
8. Hayden FG, Himel HN, Heggers JP. Herpesvirus infections in burn patients. Chest. 1994;106(1 Suppl):15S–21S; discussion 34S–35S. PubMed PMID:8020328.
9. Mark KE, Wald A, Magaret AS, Selke S, Olin L, Huang M, et al. Rapidly cleared episodes of herpes simplex virus reactivation in immunocompetent adults. J Infect Dis. 2008;198(8):1141–9.
10. Boudarias B, Perro G, Cutillas M, Castede JC, Lafon ME, Sanchez R. Herpes simplex virus infection in burned patients: epidemiology of 11 cases. Burns. 1996;22 : 287–90.
11. Nash G, Foley FD. Herpetic infection of the middle and lower respiratory tract. Am J Clin Pathol. 1970;54 : 857–63.
12. Nash G. Necrotizing tracheobronchitis and bronchopneumonia consistent with herpetic infection. Human Pathol. 1972;3 : 283–91.
13. Foley FD, Greenwald KA, Nash G, Pruitt Jr BA. Herpesvirus infection in burned patients. N Engl J Med. 1970;282 : 652–6.
14. Gnann Jr JW, Whitley RJ. Herpes simplex encephalitis: an update. Curr Infect Dis Rep. 2017;19(3):13.
15. Edgar P, Kravitz M, Heggers J, Desai M, Herndon DN. Herpes simplex in pediatric burn patients [Abst.]. Proc Am Burn Assoc. 1990;22 : 56.
16. Sen S, Szoka N, Phan H, Palmieri T, Greenhalgh D. Herpes simplex activation prolongs recovery from severe burn injury and increases bacterial infection risk. J Burn Care Res. 2012;33 : 393–7.
17. Adams MJ, Lefkowitz EJ, King AM, Carstens EB. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2014). Arch Virol. 2014;159(10):2831–41. doi: 10.1007/s00705-014-2114-3.
18. Caserta MT, Mock DJ, Dewhurst S. Human herpesvirus 6. Clin Infect Dis. 2001;33(6):829–33.
19. Caserta MT. Roseola (Human Herpes Viruses 6 and 7). In: Kliegman RM, Stanton BF, Schor NF, St. Geme III JW, Berhman RE, editors. Nelson Textbook of Pediatrics (19th Edition), Philadelphia: Elsevier Saunders; 2011, p. 1117–20.
20. De Bolle L, Naesens L, De Clercq E. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin Microbiol Rev. 2005;18(1):217–45.
21. Prosch S, Wendt CE, Reinke P, Priemer C, Oppert M, Kruger DH, et al. A novel link between stress and human cytomegalovirus (HCMV) infection: sympathetic hyperactivity stimulates HCMV activation. Virology. 2000;272 : 357–65.
22. Ritter T, Brandt C, Prosch S, Vergopoulos A, Vogt K, Kolls J, et al. Stimulatory and inhibitory action of cytokines on the regulation of hCMV-IE promoter activity in human endothelial cells. Cytokine. 2000;12 : 1163–70.
23. Gallagher JJ, Branski LK, Williams-Bouyer N, Villarreal C, Herndon DN. Treatment of infection in burns. In: Herndon DN, editor. Total Burn Care (4th Edition), Philadelphia: Elsevier Saunders; 2016, p.143–5.
24. Pilmore H, Collins J, Dittmer I, Williams L, Carpenter L, Thomas S, et al. Fatal human herpesvirus-6 infection after renal transplantation. Transplantation. 2009;88 : 762–5.
25. Ward KN, Leong HN, Nacheva EP, Howard J, Atkinson CE, Davies NW, et al. Human herpesvirus 6 chromosomal integration in immunocompetent patients results in high levels of viral DNA in blood, sera, and hair follicles. J Clin Microbiol. 2006;44(4):1571–4.
26. Hubacek P, Muzikova K, Hrdlickova A, Cinek O, Hyncicova K, Hrstkova H, et al. Prevalence of HHV-6 integrated chromosomally among children treated for acute lymphoblastic or myeloid leukemia in the Czech Republic. J Med Virol. 2009;81(2):258–63.
27. Razonable RR, Fanning C, Brown RA, Espy MJ, Rivero A, Wilson J, Kremers W, Smith TF, Paya CV. Selective reactivation of human herpesvirus 6 variant A occurs in critically ill immunocompetent hosts. J Infect Dis. 2002;185(1):110–3.
28. Wolz MM, Sciallis GF, Pittelkow MR. Human herpesviruses 6, 7 and 8 from dermatologic perspective. Mayo Clin Proc. 2012;87(10):1004–14.
29. Mardivirin L, Valeyrie-Allanore L, Branlant-Redon E, Beneton N, Jidar K, Barbaud A, et al. Amoxicillin-induced flare in patients with DRESS (Drug Reaction with Eosinophilia and Systemic Symptoms): report of seven cases and demonstration of direct effect of amoxicillin on Human Herpesvirus 6 replication in vitro. Eur J Dermatol. 2010;20(1):68–73.
30. McGill SN, Cartotto RC. Herpes simplex virus infection in a paediatric burn patient: case report and review. Burns. 2000;26(2):194–9.
31. Brandt SJ, Tribble CG, Lakeman AD, Hayden FG. Herpes simplex burn wound infections: epidemiology of a case cluster and responses to acyclovir therapy. Surgery. 1985;98 : 338–43.
32. Hayden FG, Himel HN, Heggers JP. Herpesvirus infections in burn patients. Chest. 1994;106 : 15S–35S.
33. Montes LF, Muchinik G, Fox Jr CL. Response of varicella zoster virus and herpes zoster to silver sulfadiazine. Cutis. 1986;38(6):363–5.
34. Boudarias B, Perro G, Cutillas M, Castede JC, Lafon ME, Sanchez R. Herpes simplex virus infection in burned patients: epidemiology of 11 cases. Burns. 1996;22 : 287–90.
35. Byers RJ, Hasleton PS, Quigley A, Dennett C, Klapper PE, Cleator GM, et al. Pulmonary herpes simplex in burns patients. Eur Resp J. 1996;9 : 2313–7.
36. Haik J. Is prophylactic acyclovir treatment warranted for prevention of herpes simplex virus infections in facial burns? A review of the literature. J Burn Care Res. 2011;32(3):358–62.
Labels
Plastic surgery Orthopaedics Burns medicine Traumatology
Article was published inActa chirurgiae plasticae

2018 Issue 2-4-
All articles in this issue
- ČESKÉ SOUHRNY
- Editorial
- TRANSECTION OF INFERIOR ALVEOLAR NERVE IN THE RAT – NEUROANATOMICAL STUDY AND EXPERIMENTAL MODEL
- FAMILIAL HYPERCHOLESTEROLAEMIA – A DIAGNOSIS THAT EVERY PLASTIC SURGEON CAN EXPERIENCE
- EOSINOPHILIC ANGIOCENTRIC FIBROSIS AS A CAUSE OF NASAL OBSTRUCTION. A CASE REPORT
- A COMBINATION OF HERPES VIRUS INFECTION (HSV-1, HHV-6) AND MULTI-RESISTANT BACTERIAL INFECTION IN A SEVERELY BURNED PEDIATRIC PATIENT – A CASE REPORT
- Monika Adámková, MD, from the Ostrava Burn Center has been awarded Professor Radana Königová Prize
- In Memoriam of Associate Professor Jiří Kozák, MD, PhD
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- TRANSECTION OF INFERIOR ALVEOLAR NERVE IN THE RAT – NEUROANATOMICAL STUDY AND EXPERIMENTAL MODEL
- A COMBINATION OF HERPES VIRUS INFECTION (HSV-1, HHV-6) AND MULTI-RESISTANT BACTERIAL INFECTION IN A SEVERELY BURNED PEDIATRIC PATIENT – A CASE REPORT
- FAMILIAL HYPERCHOLESTEROLAEMIA – A DIAGNOSIS THAT EVERY PLASTIC SURGEON CAN EXPERIENCE
- EOSINOPHILIC ANGIOCENTRIC FIBROSIS AS A CAUSE OF NASAL OBSTRUCTION. A CASE REPORT
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

