-
Medical journals
- Career
Response by an automated inspired oxygen control system to hypoxemic episodes: assessment of damping
Authors: Thomas E. Bachman; Jakub Ráfl
Authors‘ workplace: Department of Biomedical Technology, Faculty of Biomedical Engineering, Czech Technical University in Prague, Kladno, Czech Republic
Published in: Lékař a technika - Clinician and Technology No. 2, 2018, 48, 41-45
Category:
Overview
Manual titration of inspired oxygen necessary to adequately respond to respiratory fluctuations of the neonate is a challenging task. Furthermore, exposure to high and low levels of oxygen saturations are associated with significant morbidity and mortality. Ventilators that automatically control inspired oxygen based on pulse oximeter signals are becoming available and seem to be safe and effective when compared to manual control. However, the potential to overshoot in response to a hypoxemic episode, thus causing excess hyperoxemia, has not been carefully studied. We evaluated the response of one automated FiO2-SpO2 control system to 7,667 desaturations in 21 infants over 113 days. We found that the sustained response to desaturations resulted primarily in achievement of normoxemia with balance between high and low saturations. We concluded that this closed loop control system was adequately damped. We suggest that this kind of analysis might be helpful is refining control algorithms.
Keywords:
oxygen control, physiological closed loop controller, neonatal
Background
In the neonatal ICU oxygenation is continuously monitored noninvasively with the pulse oximeter (SpO2). Titration of inspired oxygen (FiO2) in the neonatal ICU is a challenging task as sick neonates have unstable respiratory systems. Apnea and disordered breathing result in frequent episodes of hypoxemia. It is generally accepted that these infants spend only half the time in the desired SpO2 target range [1]. Clinicians respond to prolonged desaturations with a temporary increase in the inspired FiO2. They often do not respond quickly enough not only to the need for an increase, but also with a return to baseline FiO2 after recovery. The latter increases the exposure to hyperoxemia. Importantly exposure to SpO2 extremes, high and low, is associated with significant morbidity and mortality [2].
Systems that automatically control the FiO2, based on the SpO2 level (Auto-FiO2) are now available as an integral part of some mechanical ventilators [3]. These systems are continually vigilant and make adjustments as often as every second. In contrast, during manual control nurses average about 2–5 adjustments per hour. Controlled trials have shown that Auto-FiO2 increases the time in the desired SpO2 target range and reduces time at SpO2 extremes [4–7]. Examples of the relative improvements in oxygenation for the system used in this study are shown in Table 1.
1. Relative Safety and Effectiveness of the AVEACLiO2 according to published studies. 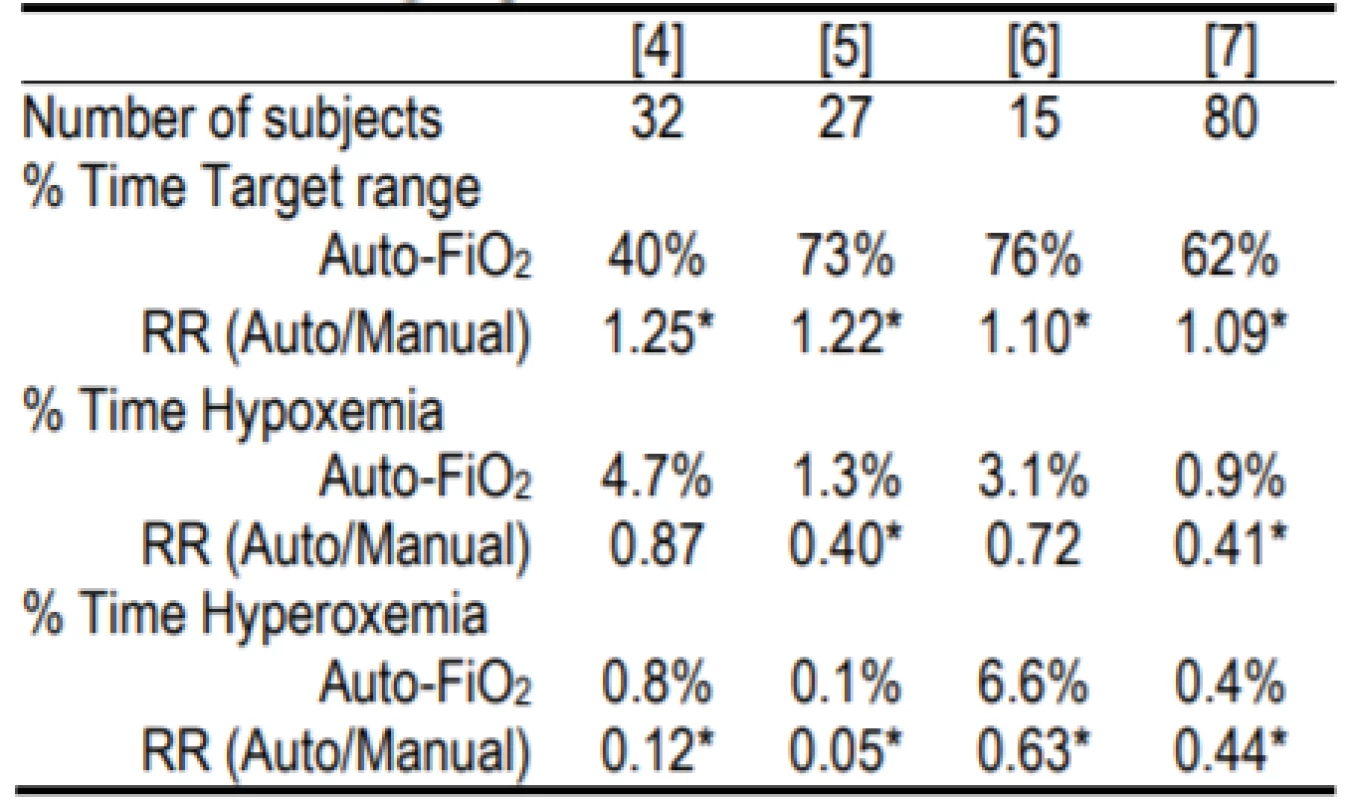
RR is relative risk. Hypoxemia and hyperoxemia were as defined in the study. The former was either <80% or <75% [4] SpO2, and the latter either >98% or >97% [5] or >96% [6]. Two of the studies [4, 7] were multicenter.
* indicates statistical significance within the studyThese Auto-FiO2 control systems are classified as Physiological Closed Loop Controllers (PCLC) [8]. This classification is defined by the use of one measured physiological parameter (SpO2) to determine the system response/change to the treatment (FiO2). This is in contrast to control systems that measure and modify the same parameter; for instance ventilator airway pressure or volume. It is difficult to simulate the complex physiological domain in which PCLC operate. In our case no validated model is available so bench tests are of limited value and extensive clinical testing is needed to verify safety and effectiveness.
One important element of the implementation of PCLC systems must be consideration of appropriate damping of the response to a physiological change. In the case of Auto-FiO2 control for neonates, while perhaps shortening the degree of hypoxemia, an underdamped response to hypoxemia would lead to increased hyperoxemia. Both hyperoxemia and hypoxemia increase outcomes risk. Further swinging from the latter to the former exacerbate the risk by causing additional reperfusion injury to the previous hypoxic tissues [9]. While studies suggest these Auto-FiO2 systems are safe and effective [4–7], nothing has been published characterizing their specific overshoot to hypoxemic episodes. Using data from a recently completed clinical study our goal was to document the response of one commonly used Auto-FiO2 system to hypoxemic episodes.
Methods
The response of Auto-FiO2 to hypoxemic episodes was extracted from data gathered in a study evaluating the frequency of alarms during use of Auto-FiO2 [10]. The institution’s Bioethics Committee approved this study. The Auto-FiO2 system (AVEA-CLiO2, Vyaire Medical, USA) has been in routine clinical use since 2012 at the study center, the Independent Public Hospital of Prof W. Orlowski, in Warsaw Poland.
The AVEA is a widely used full function mechanical ventilator. A summary of the clinical validations of the CLiO2 Auto-FiO2 option is provided in Table 1. The system is a rules based, multi-parameter, variable gain control system. It continuously monitors the SpO2 and makes a decision every second whether an adjustment in FiO2 should be made. The system’s response characteristics are different depending on whether the SpO2 is above, below or within the selected control range. For SpO2 excursions below the set SpO2 control range, which is the focus of this analysis, the magnitude of the adjustment is based on 5 metrics. The first 4 are the size of the drop below the control range, the duration of the drop, the rate of change of SpO2 and whether the SpO2 is increasing or decreasing. In addition when the SpO2 is recovering (increasing) it targets a system derived baseline FiO2 level. The later is intended to reduce the probability of overshoot above the desired target range into hyperoxemia. Figure 1 provides examples of the response to desaturations in one subject over an 11-minute period. A rapid marked increase in FiO2 is evident with significant decreases in SpO2. Likewise the decrease in FiO2 towards the baseline FiO2 in response to increasing SpO2 is also apparent.
1. Auto-FiO2 Response to changes in SpO2. The markers are 5-second data points, the line implies the interim values (1-second adjustments). The shaded area is the set SpO2 target range. This epoch includes one episode of SpO2 <86% and one <80%. It also includes the less pronounced adjustments in FiO2 associated with small changes in SpO2. 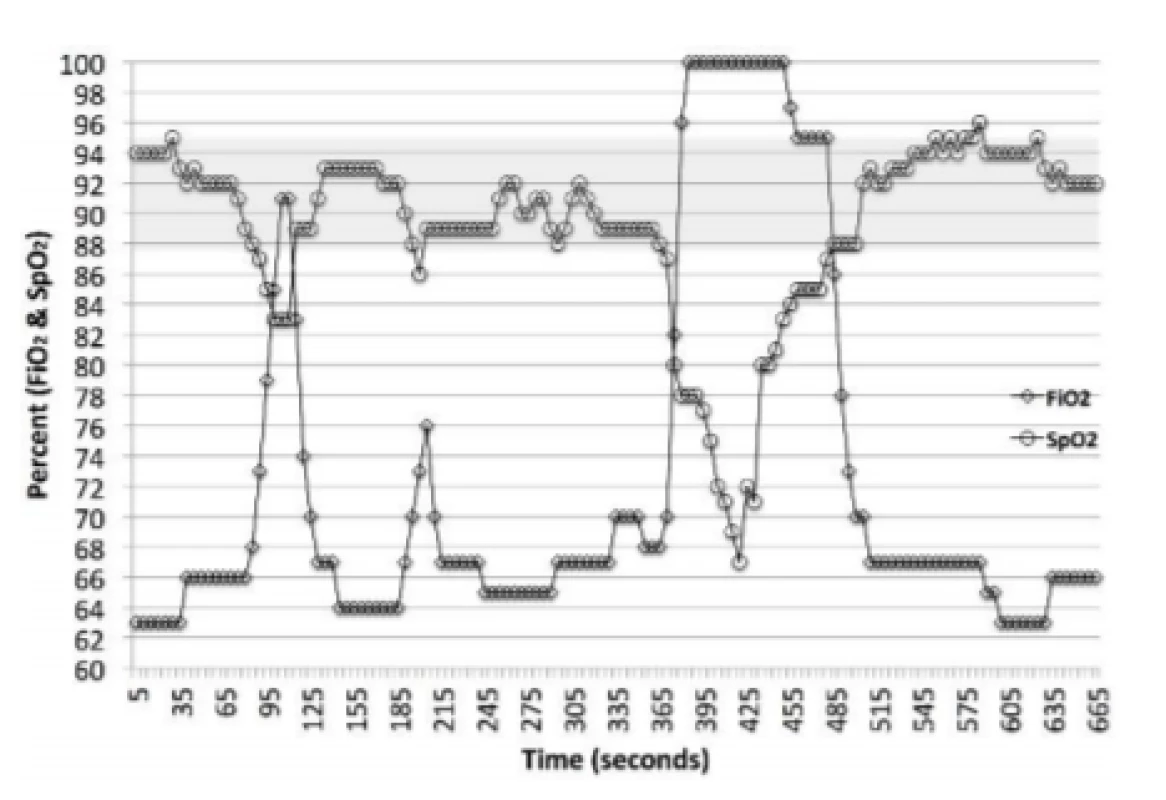
During the study, data was transmitted from the ventilator every 5 seconds to an electronic data-logger. Endpoints were extracted by purpose-built software. Both have been used in other clinical studies. The alarm study from which the response data was extracted included 21 neonates, evaluated for a total of 113 days.
Two levels of potential hypoxemic episodes (HE) were prospectively defined. These were: HE<80% (hypoxemia, SpO2<80%) and HE<86% (low-oxemia, SpO2<86%). Four response categories of oxemia status were also prospectively defined: Hypoxemia (SpO2<80%) Low-oxemia (SpO2 80–85%), Normoxemia (86–96% SpO2) and Hyperoxemia (>96% SpO2).
Two metrics of response were also prospectively defined. These were Initial and Sustained. Initial response was the first reading after the end of an episode and reflects the FiO2 changes made during the HE. The Sustained response evaluates the 2 minutes after the HE. Sustained identifies the category of oxemia associated with greater than 50% of the 2 minutes following the end of the episode. The latter being our primary measure of damping. An episode was defined as at least two 5-second data points, that is 6–9 seconds. Figure 2 illustrated examples of 3 of the 4 categories of response.
2. Prototypical Examples of Sustained Response. The curve with the circles is a Sustained response of Hyperoxemia, with an Initial response of Normoxemia. The curve with the diamonds is a Sustained response of Normoxemia, with an Initial response of Normoxemia. The curve with the squares is a Sustained response of Low-oxemia, with an Initial response of Low-oxemia. The first and third both spend part of the time, but less than half, in Normoxemia. 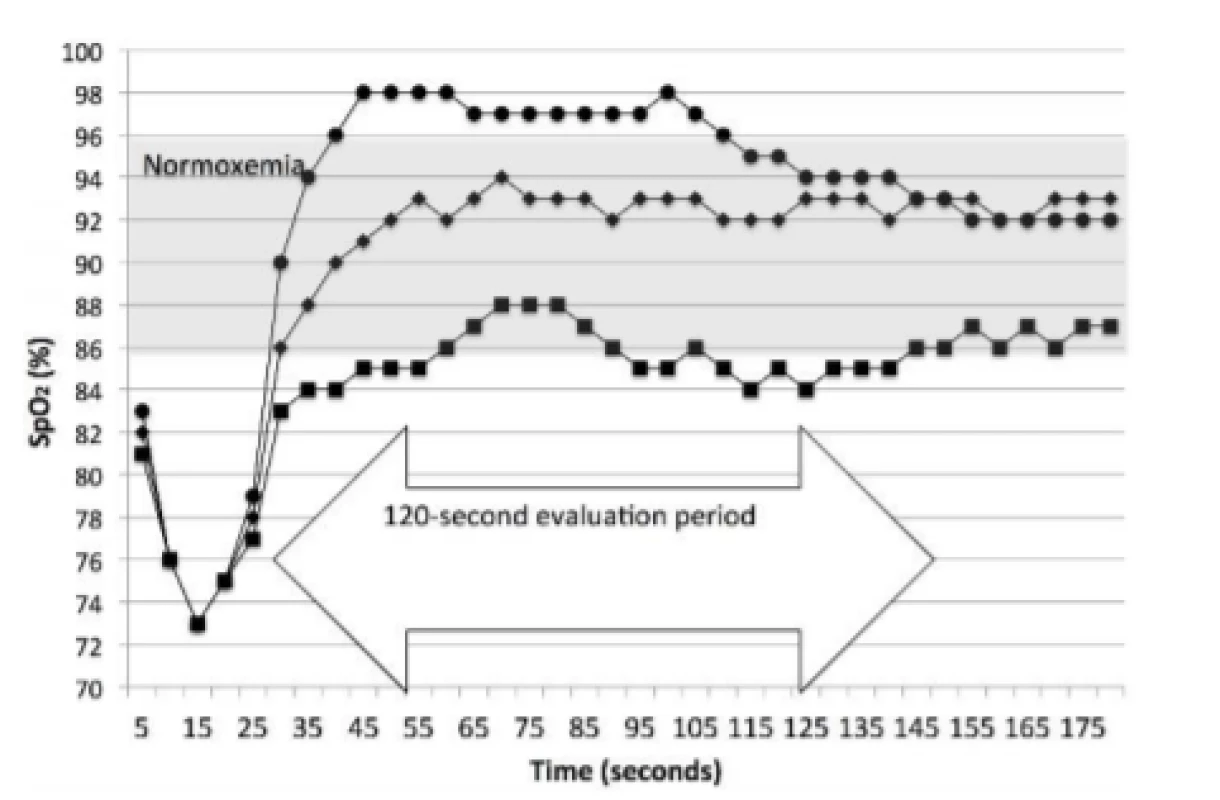
It should also be noted that more than one episode with SpO2<80% could occur in one prolonged episode with SpO2 <86%, and that some of the hypoxemic responses might not fit into the Sustained definition, in that they did not spend at least 60 seconds in any one of the 4 oxemia ranges.
Data were analyzed using XLSTAT v19.02 (Addinsoft, Paris, France). Chi2 was used to evaluate the differences in the overall response to episodes of potential hypoxemia, and z-test to evaluate differences in the specific categories of oxemia. A p<0.05 was considered to be statistically significant.
Results
We identified nearly 10,000 potential hypoxemic episodes during the 113 days of the study. Of these we were able to categorize the response in nearly all (>95%). Thus we evaluated the response to 7,667 low-oxemic episodes (HE<86%) and 1,819 hypoxemic episodes (HE<80%). The median (IQR) of the rate of HE<86% was 1.82/hour (1.13–2.76) and for HE<80%, 0.29/hour (0.16–0.45). The median inspired oxygen of the infants varied among the days (range FiO2: 22.4–53.2%). The SpO2 was well maintained (daily median, range 90.4–95.5% SpO2), with 95% of the time spent in normoxemia.
The Initial and Sustained response to HE<86% and HE<80% are shown in Tables 2 and 3 respectively. In both cases the Initial response is clearly different than the Sustained response (p<0.001). As seen in Table 2 the initial response to episodes of HE<86% is almost entirely Normoxemia (86–96% SpO2). In the two minutes of continued response most (86% of episodes) remain normoxemic with some moving higher and some lower. As seen in Table 3 the response to HE<80% is similar. That is, the Initial response to episodes is primarily (84% of episodes) 80–85% SpO2, with some (15%) also initially in normoxemia. In the two minutes of continued response to HE<80% most (58% of episodes) became normoxemic with some moving to higher and some to lower levels of SpO2.
2. Categorization of Response to 7,667 episodes of SpO2 <86%. 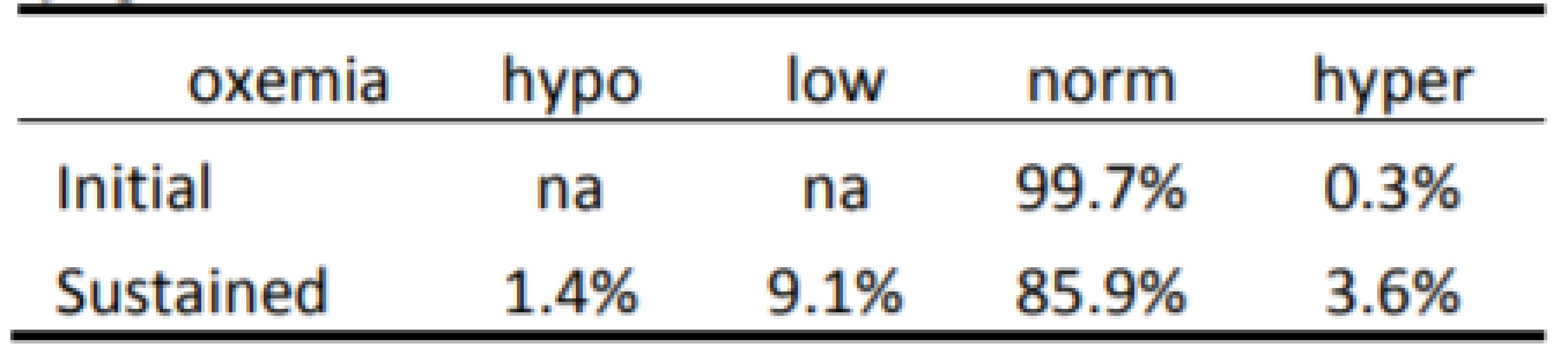
3. Categorization of Response to 1,819 episodes of SpO2 <80%. 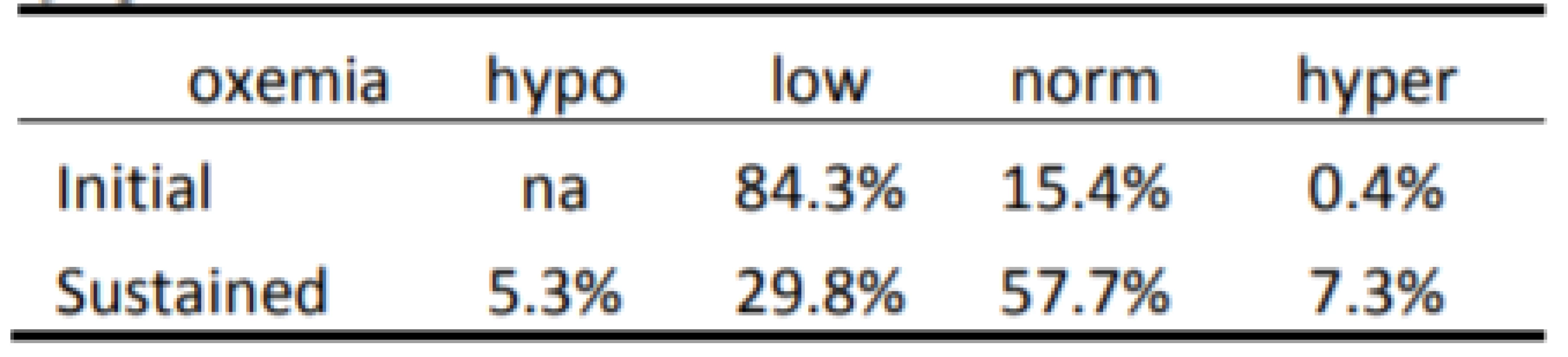
For purposes of comparison between HE<86% and HE<80% the same data is presented graphically, in Fig. 3 for the Sustained response. The two minutes of continued Sustained control reflect a similar balanced distribution, although the difference is statistically significant, p<0.001. Further the responses to hypoxemia result in a higher incidence of hyperoxemia and hypoxemia for HE<80% (7.25% and 5.25% respectively) than for HE<86% (3.63% and 1.37% respectively), p<0.001.
3. Sustained Response Status after Episodes <80% and <86% SpO2. 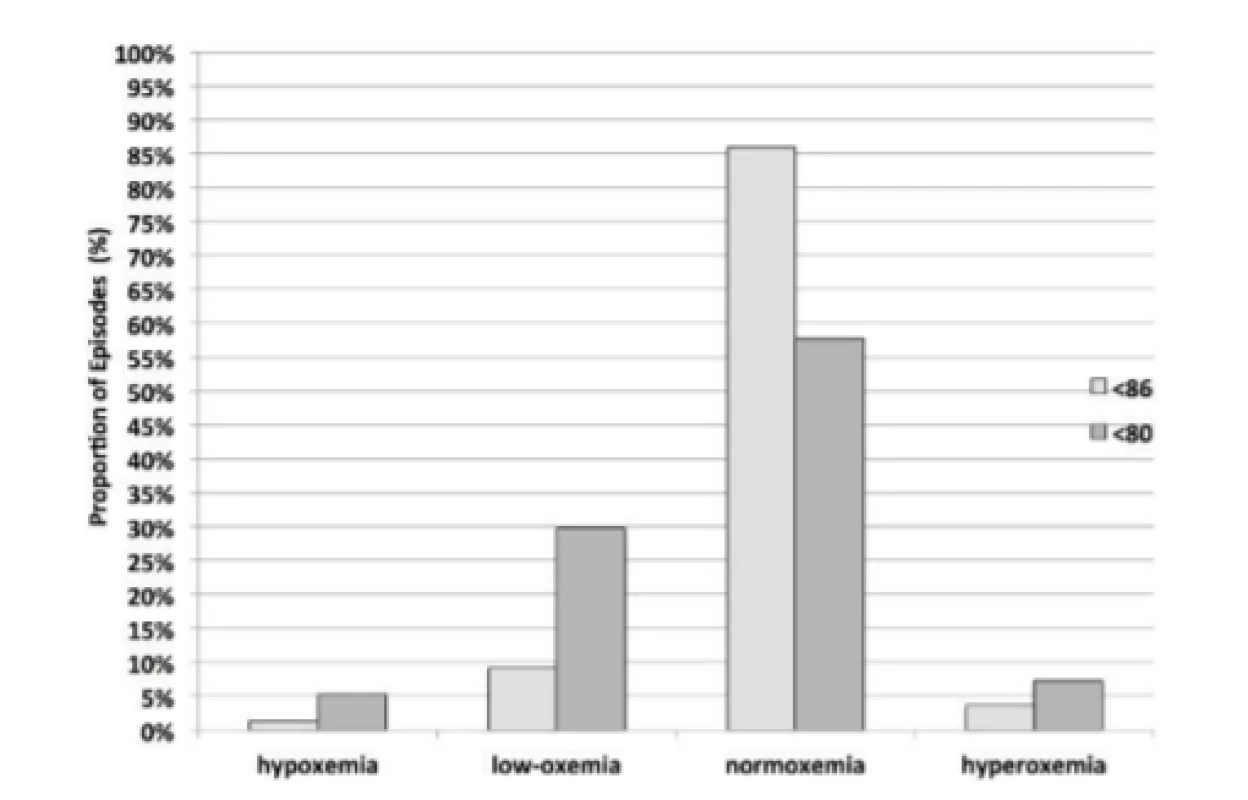
Discussion
We evaluated nearly 10,000 responses of an Auto-FiO2 system to two levels of SpO2 desaturations (HE<86% and HE<80%) that occurred in 21 neonates over 113 days. We found that in the two-minute period following the desaturation the SpO2 level predominately reflected normoxemia and that the presence of hypoxemia or hyperoxemia was infrequent and balanced. However, the incidence of hyperoxema was markedly higher when recovering from HE<80%, suggesting a more aggressive response of the algorithm to more significant desaturations. This effect was also apparent in the Initial response. We are unaware of any similar data from testing of this or any other Auto-FiO2 system.
One study did characterize the response of SpO2 to single manual step increases and decreases in FiO2 [11]. They found a typical 36–40 second 95%-response to a change. However, the response varied significantly among episodes, but most would be consistent with our 2-minute evaluation window. A review of Fig. 1 and 2 suggests that the two-minute window for Sustained response is adequate to capture an over or undershoot in response to a desaturation. During manual titration of FiO2 clinicians are encouraged to wait to make an adjustment to insure the desaturation is persistent and then after an adjustment, to focus on the response before making another adjustment. This full step response noted above is much longer than the 1-second adjustments of the Auto-FiO2 system and even the 5‑second data epochs we evaluated. This Auto-FiO2 approach is in obvious contrast to the standard practice for manual adjustments. One should expect that this could lead to less stable SpO2 if the controller was underdamped. In one early study where SpO2 stability was evaluated, the coefficient of variation of SpO2 was reduced during Auto-FiO2 and the duration of episodes of significant hypoxemic episodes and hyperoxemic episodes were shorter, suggesting the potential for overshoot is appropriately addressed [4].
During manual control of FiO2 most episodes of hypoxemia are short and resolve without intervention. However, when episodes are prolonged, the increase in FiO2 is generally appropriate, but there is often a failure to promptly wean the FiO2 back to a baseline level. In a study of manual FiO2 control, Van Zanten et al showed hyperoxemia occurred in 79% of the responses to marked hypoxemia [12]. These resulting episodes of hyperoxemia were very prolonged with a median duration of 13 minutes. While an Auto-FiO2 system does not forget to wean, they should be designed to wean slowly to avoid a swing back to hypoxemia. Van Zanten also reported, that during manual control, when the FiO2 was weaned back to baseline within 2–3 minutes hyperoxemia was rare. Studies of this Auto-FiO2 system have shown that prolonged episodes of hyperoxemia are infrequent [4–6]. It is unclear how much of the hyperoxemia seen during the use of this system is due to a short term transient overshoot in the first 2 minutes or weaning from hyperoxemia once established. Our analysis was not designed to evaluate response to hyperoxemic episodes. However, we speculate that while both are relevant factors, longer term weaning and not overshoot is the main contributor. This could be addressed in future analyses.
Studies of this Auto-FiO2 system have consistently shown a marked reduction in hyperoxemia when compared to routine manual adjustment of FiO2 (Table 1). While this supports the effectiveness of Auto-FiO2 at reducing hyperoxemia, it speaks more to the inadequacy of manual control. However, three multicenter studies have also suggested that the set control range might also impact the relative risk of hyperoxemia [7, 13, 14]. The report of van Kaam et al showed an increase in hyperoxemia associated with a higher target range (91–95%) as compared to a lower (89–93%). This effect was present in both Manual and Automated control. In the van den Heuvel study of three different automatically controlled target ranges with the same midpoint, there was a trend toward increased hyperoxemic episodes with a narrower target range. This suggested the possibility of increased overshoot with a narrower control range. The report of Wilinska et al is consistent with the findings of both. Therefore, it is not settled as to what degree that increased hyperoxemia, when using this Auto-FiO2, is associated with a narrowing or a shift of target range. We speculate that the frequency of hyperoxemic episodes associated with both the stability of the infant and the selected target range, as well as the propensity of increased overshoot with narrower target ranges are both factors. In this analysis the target range was fairly wide (88–95%). We suggest that additional evaluation of these factors is warranted.
Our analysis has some limitations. Different definitions of the ranges of oxemia would lead to different results. However, we believe the trend and conclusions would be consistent. Further the categories of oxemia we selected are consistent with PaO2 levels [15]. Finally, it is possible that less stable subjects might result in exacerbated responses. These were particularly stable patients, with only 3–4 episodes per hour. In a clinical study of subjects selected because they were unstable, the incidence was 5 times higher [4]. In such cases it is possible that the Sustained evaluation period would be confounded with subsequent hypoxemic episodes. Even with these more stable subjects the Sustained time period of 2 minutes is probably confounded to some degree by ongoing physiological variation.
Conclusion
We found no evidence of significant overshoot from hypoxemia to hyperoxemia in this group of subjects with this Auto-FiO2 system. In the two minutes following recovery, the risk of hypoxemia and hyperoxemia were low and balanced. This suggests that the control system is appropriately damped to address physiological variability. We suggest that further evaluation of specific response characteristics might be helpful in testing and refining Auto-FiO2 control algorithms.
Acknowledgement
We thank the Center for Medical Postgraduate Education in Warsaw for supporting this clinical research study and Drs. Warakomsa and Wilinska for sharing the data that made this analysis possible. The work was supported by grant SGS17/203/OHK4/3T/17 of the Czech Technical University in Prague.
Thomas E. Bachman MSc.
Department of Biomedical Technology
Faculty of Biomedical Engineering
Czech Technical University in Prague
nám. Sítná 3105, CZ-272 01 Kladno
&
Economedtrx
Lake Arrowhead, CA USA 92352-1269
E-mail: tbachman@me.com
Phone: +1 909 337-0828
Sources
- Hagadorn, J. I., Sink, D. W., Buus-Frank, M. E., et al.: Alarm safety and oxygen saturation targets in the Vermont Oxford Network iNICQ 2015 Collaborative. J Perinatol, 2017; vol. 37, no. 3, pp. 270–276.
- Cummings, J. J., Polin, R. A., Committee on Fetus and Newborn: Oxygen targeting in extremely low birth weight infants. Pediatrics, 2016, vol. 138, no. 2, e20161576.
- Claure, N., Bancalari, E.: Closed–loop control of inspired oxygen in preterm infants. Sem Fetal & Neonatal Med, 2015, vol. 20, no. 3, 198–204.
- Claure, N., Bancalari, E., D’Gard, C., et al.: Multicenter crossover study of automated control of inspired oxygen in ventilated preterm infants. Pediatrics, 2011, vol. 127, no. 1, pp. e76–83.
- Lal, M., Tin, W., Sinha, S.: Automated control of inspired oxygen in ventilated preterm infants: crossover physiological study. Acta Paediatr, 2015, vol. 104, no. 11, pp. 1084–1089.
- Waitz, M., Schmid, M., Fuchs, H., et al.: Effects of automated adjustment of the inspired oxygen on fluctuations of arterial and regional cerebral tissue oxygenation in preterm infants with frequent desaturations. J Pediatr, 2015, vol. 166, no. 2, pp. 240–244.
- Van Kaam, A. H., Hummler, H., Wilinska, M., et al.: Automated versus manual oxygen control with different saturation targets and modes of respiratory support in preterm infants. J Pediatr, 2015, vol. 167, no. 3, pp. 545–550.
- IEC 60601-1-10: Medical electrical equipment – Part 1-10: General requirements for basic safety and essential performance – Collateral standard: Requirements for the development of physiologic closed-loop controllers. The International Electrotechnical Commission, 2007.
- Sola, A.: Oxygen saturation in the newborn and the importance of avoiding hyperoxia-induced damage. NeoReviews, 2015, vol. 16, no. 7, e395.
- Warakomska, M., Bachman, T. E., Wilinska, M.: Randomized evaluation of two SpO2 alarm strategies during automated FiO2 control in the NICU. PAS 2018.
- Fathabadi, O. S., Gale, T. J., Lim, K., et al.: Characterisation of the oxygenation response to inspired oxygen adjustments in preterm infants. Neonatology, 2016, vol. 109, pp. 37–43.
- Van Zanten, H. A., Tan, R. N., Thio, M., et al.: The risk for hyperoxaemia after apnoea, bradycardia and hypoxaemia in preterm infants. Arch Dis Child Neonatal Ed, 2014, vol. 99, no. 4, pp. F269–273.
- Van den Heuvel, M. E., van Zanten, H. A., Bachman T. E., et al.: Optimal target range of closed-loop inspired oxygen support in preterm infants: a randomized controlled study. J Pediatr, 2018, vol. 197, pp. 36–41.
- Wilinska, M., Bachman, T. E., Swietlinski, J., et al.: Automated FiO2-SpO2 control system in neonates requiring respiratory support: a comparison of a standard to a narrow SpO2 control range. BMC Pediatr, 2014, vol. 14, 130.
- Bachman, T. E., Newth, C. J. L., Iyer, N. P., et al.: Hypoxemic and hyperoxemic likelihood in pulse oximetry ranges: NICU observational study. Arch Dis Child Neonatal Ed, 2018, Jun 20. pii: fetalneonatal-2017-314448. doi: 10.1136/archdischild-2017-314448. [Epub ahead of print].
Labels
Biomedicine
Article was published inThe Clinician and Technology Journal

2018 Issue 2-
All articles in this issue
- Response by an automated inspired oxygen control system to hypoxemic episodes: assessment of damping
- Workflow for bioprinting of cell-ladem bioink
- Application of sibgle wireless holter to simultaneous EMG, MMG and eim measurement of human muscles activity
- Biocompatibility of the human mesenchymal stem cells with bovine bone tissue at the cellular level in vitro
- Machine learning using speech utterances for parkinson disease detection
- The Clinician and Technology Journal
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Application of sibgle wireless holter to simultaneous EMG, MMG and eim measurement of human muscles activity
- Response by an automated inspired oxygen control system to hypoxemic episodes: assessment of damping
- Workflow for bioprinting of cell-ladem bioink
- Machine learning using speech utterances for parkinson disease detection
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

