-
Medical journals
- Career
Uterine tumors resembling ovarian sex cord tumors (UTROSCT). Report of a case with lymph node metastasis
Authors: Jirka Mačák 1; Pavel Dundr 2; Jana Dvořáčková 1,3; Jaroslav Klát 4
Authors‘ workplace: Department of Pathology, University Hospital and Faculty of Medicine, University of Ostrava, Czech Republic 1; Department of Pathology, First Faculty of Medicine and General University Hospital, Charles University in Prague, Czech Republic 2; CGB laboratory Inc. Ostrava, Czech Republic 3; Department of Gynecology and Obstetrics, University Hospital Ostrava, Czech Republic 4
Published in: Čes.-slov. Patol., 50, 2014, No. 1, p. 46-49
Category: Original Article
Overview
Uterine tumors resembling ovarian sex cord tumors (UTROSCT) have an uncertain histogenesis. Although generally considered to be benign, they metastasize in some cases. We report the case of a 53-year-old woman who presented with vaginal bleeding. Clinical examination revealed a tumor sized 1.5 cm in diameter localized in the subendometrial region of the uterine wall. Histologically, the tumor consisted of epithelioid oval cells arranged in solid nests, trabeculae and ribbons. Immunohistochemically, approximately 1% of tumor cells expressed strong desmin positivity, calponin in 10% of cells, WT1 in 80% cells, and Ki-67 was positive in about 5 % of tumor cells. All the other immunohistochemical reactions applied including anti-cytokeratin antibodies were negative. The RT-PCR method for identification of the JAZF1-JJAZ1 fusion transcript was negative. In one lymph node in the right iliac artery region, a metastasis of UTROSCT was found. This finding adds to the previously reported UTROSCT cases with metastatic spread.
Keywords:
uterine tumors resembling ovarian sex cord tumors – UTROSCT – metastasis – lymph nodeUterine tumors resembling ovarian sex cord tumors (UTROSCT) were first reported by Morehead and Bowman in 1945 (1). Later, Clement and Scully (2) classified the tumors into two subgroups. The first was comprised of tumors similar to endometrial stromal tumors with focal epithelioid formations resembling sex cord-like elements of an ovarian tumor making up 10 – 40 % of the overall tumor mass. These group I tumors, referred to as endometrial stromal tumors with sex cord-like elements (ESTSCLE), are considered to be endometrial stromal tumors. They are associated with an increased risk of recurrence and metastases. The other group contains more than 50 % of sex cord-like cells (3). Unlike group I, these tumors are clearly separated from the surrounding tissues, have distinct clinicopathologic features and are benign. Yet, cases were published with tumor recurrence or metastases (4-7). In this report, we present another case of UTROSCT with a metastasis in the pelvic lymph node.
MATERIAL AND METHODS
In the last year, one case of UTROSCT was noted at the Department of Pathology, University Hospital in Ostrava. The tissues were fixed in 10% buffered formalin and processed using the paraffin technique. The sections were stained by the standard hematoxylin-eosin technique.
Immunohistochemistry
Immunohistological examination was carried out using the avidin-biotin complex (ABC) method with antibodies against estrogen receptors (ER; NOVOCASTRA, dilution 1 : 50, clone GF11), progesterone receptors (PR; NOVOCASTRA, dilution 1 : 200, clone 16), CD10 (DB BIOTECH, dilution 1 : 100), desmin (BIOGENEX, prediluted, clone 33); antibodies produced by DIAGNOSTIC BIOSYSTEMS: HMB45 (dilution 1 : 50, clone HMB45), pancytokeratin AE1-AE3 (dilution 1 : 50, clone AE1+AE3), CK20 (prediluted, clone KS20.8); and antibodies produced by DAKO, Glostrup, Denmark: CD99 (prediluted, clone 12E7), calponin (dilution 1 : 50, clone CALP), WT1 (prediluted, clone 6F-H2), S-100 protein (dilution 1 : 600, polyclonal), CD117 (1 : 400, polyclonal), CK7 (dilution 1 : 100, clone OV-TL 12/30), calretinin (dilution 1 : 50, clone DAK-Calret1), Ki-67 (dilution 1 : 50, clone MIB-1), inhibin (dilution 1 : 10, clone R1), and smooth muscle actin (dilution 1 : 100, clone 1A4).
RT-PCR method for detecting the JAZF1-JJAZ1 fusion transcript
The presence of the JAZF1-JJAZ1 fusion transcript was analyzed using the RT-PCR method. RNA was extracted from paraffin-embedded tissue using the RecoverAll Total Nucleic Acid Isolation Kit (Ambion, Austin, USA). An amplification of a 247-bp product of the PGK gene and of 133-bp and 105-bp products of the beta2-microglobulin gene was used to test the quality of the extracted RNA as previously described (8-10). A 93-bp part of the JAZF1-JJAZ1 fusion transcript was amplified with primers JAZF1-369-FW with sequence 5´CCACCCATCACCCCCTCCT´3 (complementary to JAZF1) and JJAZ1-400-RV with sequence 5´TGCTATGAGATTCCGAGTTC´3 (complementary to JJAZ1) (11). The sample was run in duplicate. No fusion transcript was detected using the RT-PCR method with fusion-specific primers in the analyzed sample.
CASE REPORT
A 53-year-old woman presented at the hospital with metrorrhagia. The patient had regular gynecological check-ups, always with negative results. During her previous visit, a hyperechoic structure sized approximately 29 mm had been found in the uterine wall. The right ovary was small and the left ovary could not be visualized. Hysteroscopy revealed a polypoid mass with an uneven surface on the endometrium. UTROSCT was histologically diagnosed. Given the findings and persistent metrorrhagia, the patient gave consent for a hysterectomy.
Macroscopically, the right uterine horn was found to contain a spherical tumor, 1.5 cm in diameter, surrounded by uterine muscles.
A biopsy examination of the polypoid structure (sized 1.0 cm x 1.5 cm x 1.0 cm) removed during hysteroscopy was performed. The histological examination showed epithelioid structures forming trabeculae, ribbons or solid nests. Epithelioid cells predominated, making up more than 50 % of the tumor. The tumor cells contained light cytoplasm. The tumor met the criteria for ovarian sex cord tumors. A similar histological appearance was observed in the tumor detected by hysterectomy in the uterine wall. The tumor cells were surrounded by spindle cells with positivity to desmin and smooth muscle actin. Also approximately 1% of epithelioid sex cord-like cells expressed strong cytoplasmic positivity to desmin, calponin was positive in 10 % and WT1 in 80 % of cells (Fig. 1-3).
Fig. 1. Primary tumor in the uterus. Foci of tumor cells surrounded by fine septa. Hematoxylin - eosin stain. Bar scale = 200 μm. 
Fig. 2. Some cells of UTROSCT expressing desmin. Immunohistochemistry. Bar scale = 50 μm. 
Fig. 3. Tumor cells expressing WT1. Immunohistochemistry. Bar scale = 50 μm. 
There were no atypical cells, tumor invasion into the blood or lymph capillaries or necroses. The mitotic activity was very low (<1 mitosis/10 HPF).
Ki-67 was positive in about 5 % of tumor cells. The other antibodies did not react with the tumor (Tab. 1). Hysterectomy and a bilateral adnexectomy were performed. During the procedure, pelvic and paraaortic lymph nodes were also removed. One lymph node in the region around the right internal iliac artery was found to contain a metastasis of the same appearance as the tumor in the uterine wall (Fig. 4,5). Immunohistological examination showed the same positivity as the primary tumor in the uterus - i.e. the primary tumor lesion in the uterus and the lymph node metastasis had an identical phenotype.
1. Antibodies used and immunohistological results 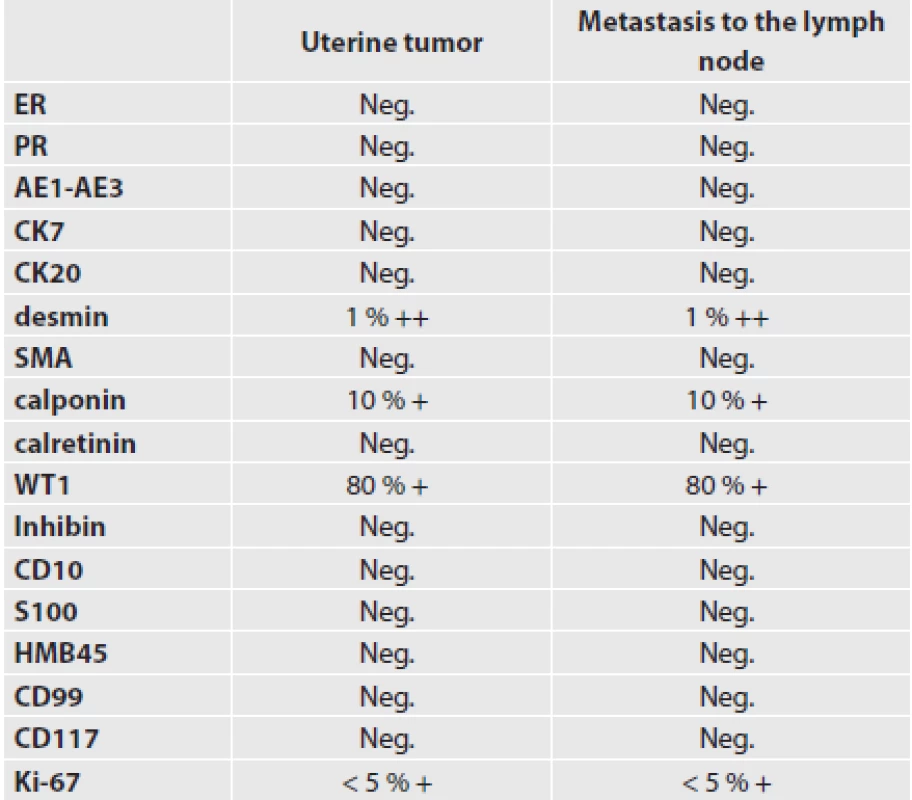
Fig. 4. Metastasis of UTROSCT to the lymph node. Hematoxylin - eosin stain. Bar scale = 1 mm. 
Fig. 5. Higher magnification of the lymph node metastasis. Hematoxylin - eosin stain. Bar scale = 20 μm. 
In the paraffin block, the JAZF1-JJAZ1 chimeric transcript was examined. The sample was run in duplicate. No fusion transcript was detected using the RT-PCR method with fusion-specific primers in the analyzed sample.
Since the hysterectomy procedure, the patient has been without signs of tumor progression for 10 months of follow-up.
DISCUSSION
UTROSCT is a rare tumor with an uncertain histogenetic origin. Whereas some authors suggest that these tumors belong to ESTSCLE, just with a higher presence of sex cord-like elements, others (3,5) consider them an individual category of tumors. According to the 2003 World Health Organization classification, these tumors belong to a group of miscellaneous neoplasms and the category of sex cord-like tumors, with a predominant pattern of sex cord-like elements (12). The tumor is made up of epithelioid cells with the appearance of ovarian sex cord-like elements. In the histological pattern, there are ribbons, trabeculae, small nests or tubules resembling granulosa or Sertoli cell tumors of the ovary. UTROSCT with retiform sex cord-like differentiation was also reported (13). Rarely, Call-Exner bodies may be present (14). This component is considered to be tumorous.
Histogenetically, the tumors are likely to arise from multipotent mesenchymal cells of the uterus. These cells may differentiate in various directions. This corresponds with expression of individual markers as seen in these tumors (14). Hillard et al. (15) suggests that uterine tissue at the endomyometrial junction exhibits a pluripotent capacity to differentiate into a range of cells composing epithelial, stromal, or myometrial tissue.
UTROSCT are made up of sex cord-like trabeculae and tubules as well as elongated cells. The relationship of these elongated cells to the tumor itself has not been clearly elucidated. Apart from the opinion that these are myoid cells are randomly incorporated into the tumor, it is hypothesized that these may be tumor cells (16-18). Zamecnik et al. (19,20) detected immunohistologically myoid differentiation not only in elongated cells but also in epithelioid sex cord-like elements. On the other hand, the sex cord-like marker calretinin was expressed by not only epithelioid sex cord-like elements but also by the myoid elongated cells.
Some authors (8,17,21) assume that UTROSCT belong among the ESTSCLE tumors, with only an enhanced presence of sex cord-like elements. Others (22,16) admit that it is a special tumor category. Staats et al. (23) found that endometrial stromal tumors including ESTSCLE contain the JAZF1-JJAZ1 chimeric gene that has not been detected in UTROSCT. The authors cannot fully rule out that UTROSCT is an ESTSCLE variant lacking that translocation. On the other hand, this is suggestive of a different genetic mechanism of UTROSCT development. This justifies the classification of these tumors as a special category unrelated to ESTSCLE. In this case, the JAZF1-JJAZ1 chimeric gene was not identified by the RT-PCR method.
Distinguishing UTROSCT from other tumors may be difficult, especially in a small biopsy specimen. The differential diagnosis of UTROSCT includes tumors showing only focal sex cord-like features and tumors which can show overlapping morphological features throughout the whole lesion. Tumors with only focal sex cord-like areas include endometrial stromal tumor with sex cord-like differentiation, endometroid carcinoma, leiomyoma, and malignant mixed müllerian tumor (29,30). However, these tumors show other typical areas different from UTROSCT and, if apparent, recognition of these areas together with immunohistochemical analysis allow us to reach a correct diagnosis. The other tumors entering the differential diagnosis are those showing diffuse features resembling UTROSCT. These tumors include uterine Sertoli-Leydig cell tumor, epithelioid leiomyoma and PEComa (31,32). The correct diagnosis of these lesions can be based on morphological features and immunohistochemistry as well.
UTROSCT are polyphenotypic and polyimmunophenotypic tumors (3,24,25). The immunophenotypic heterogeneity has led to various opinions on its histogenesis. Some derived the tumor from endometrial stroma (25,26), others from epithelium (27) or smooth muscles (28). The immunohistological profile of UTROSCT is comprised of the following four groups of markers: smooth muscle markers, epithelial markers, sex cord markers and miscellaneous markers. According to Pusiol et al. (29), at least two immunohistological markers should be present. Our case showed positive findings with antibodies against desmin in approximately 1 %, against calponin in 10 % and against WT1 in approximately 80 % of tumor cells. The Ki-67 proliferation marker was positive in less than 5 % of cells. Although the immunophenotype was rather poor, it met the criterion of two positive markers as stated by Pusiol et al. (29). Similarly, some other reported cases showed low antigen expression. In a group of 13 cases, Gupta et al. (25) found one tumor that was positive with antibodies against cytokeratins KL1, calretinin and S100. Retrospectively, Abdullazade et al. (3) reported other authors’ cases with very limited immunophenotypes.
In most cases, UTROSCT do not relapse or spread to adjacent tissues and organs. In the past, however, several cases with metastatic spread were noted (4-7). O’Meara (7) reported multiple metastatic lesions in the peritoneal omentum, subcutaneous tissue and lymph nodes in a patient three years after hysterectomy.
Given the fact that UTROSCT are rare tumors, there are no mandatory guidelines as to how radical surgical therapy should be. Generally, a hysterectomy is performed in these cases. Uterus-sparing surgical procedures are not the method of choice. They only preserve the fertility of women who are strongly motivated and with to become pregnant in the future. These patients should be informed that the condition may recur (33). Lymphadenectomy remains a discussed issue. It must be borne in mind that the biological behavior of UTROSCT is uncertain and metastases cannot be ruled out. In the presented case, the patient was indicated for a hysterectomy with bilateral adnexectomy and pelvic and paraaortic lymphadenectomy.
In conclusion, we report another case of metastasizing UTROSCT. The possibility of aggressive behavior of this tumor should be borne in mind when considering the therapeutic approach including the extent of surgery.
ACKNOWLEDGEMENTS
The work was supported by the grant NT/13519-4/2012 from the Internal Grant Agency of the Ministry of Health of the Czech Republic.
The authors are grateful to RNDr. T. Vaněček, Ph.D. from Bioptická laboratoř, s.r.o. and Šikl’s Institute of Pathology in Pilsen for the RT-PCR analysis of the JAZF1-JJAZ1 chimeric gene.
Correspondence address:
Prof. MUDr. Jirka Mačák, CSc.
Department of Pathology, University Hospital Ostrava
17. listopadu 1790, 708 52 Ostrava, Czech Republic
e-mail: macak.jirka@seznam.cz
Sources
1. Morehead RP, Bowman MC. Heterologous mesodermal tumors of the uterus: report of a neoplasm resembling a granulosa cell tumor. Am J Pathol 1945; 21 : 53-61.
2. Clement PB, Scully RE. Uterine tumors resembling ovarian sex-cord tumors. A clinicopathologic analysis of fourteen cases. Am J Clin Pathol 1976; 66 : 512-525.
3. Abdullazade S, Kosemehmetoglu K, Adanir I, Kutluay L, Usubtun A. Uterine tumors resembling ovarian sex cord-stromal tumors: synchronous uterine tumors resembling ovarian sex cord-stromal tumors and ovarian sex cord tumor. Ann Diagn Pathol 2010; 14 : 432-437.
4. Kantelip B, Cloup N, Dechelotte P. Uterine tumor resembling ovarian sex cord tumors: report of a case with ultrastructural study. Hum Pathol 1986; 17 : 91-94.
5. Biermann K, Heukamp LC, Büttner R, Zhuo H. Uterine tumor resembling an ovarian sex cord tumor associated with metastasis. Int J Gynecol Pathol 2008; 27 : 58-60.
6. Leiser AL, Hamid AM, Blanchard R. Recurrence of prolactin-producing endometrial stromal sarcoma with sex-cord stromal component treated with progestin and aromatase inhibitor. Gynecol Oncol 2004; 94 : 567-571.
7. O’Meara AC, Giger OT, Kurrer M, Schaer G. Case report: Recurrence of a uterine tumor resembling ovarian sex-cord tumor. Gynecol Oncol 2009; 114 : 140-142.
8. Viswanatha DS, Foucar K, Berry BR. Blastic mantle cell leukemia: an unusual presentation of blastic mantle cell lymphoma. Mod Pathol 2000;13 : 825-833.
9. Gaffney R, Chakerian A, O’Connell JX. Novel fluorescent ligase detection reaction and flow cytometric analysis of SYT-SSX fusion in synovial sarcoma. J Mol Diagn 2003; 5 : 127-135.
10. Antonescu CR, Kawai A, Leung DH. Strong association of SYT-SSX fusion type and morphologic epithelial differentiation in synovial sarcomas. Diagn Mol Pathol 2000; 9 : 1-8.
11. Hrzenjak A, Moinfar F, Tavassoli FA. JAZF1/JJAZ1 gene fusion in endometrial stromal sarcomas: molecular analysis by reverse transcriptase-polymerase chain reaction optimized for paraffin-embedded tissue. J Mol Diagn 2005; 7 : 388-395.
12. Czernobilsky B. Uterine tumors resembling ovarian sex cord tumors: an update. Int J Gynecol Pathol 2008; 27 : 229-235.
13. Zamecnik M, Michal M. Endometrial stromal nodule with retiform sex-cord-like differentiation. Pathol Res Pract 1998; 194 : 449-453.
14. Giordano G, Lombardi M, Brigati F, Mancini C, Silini EM. Clinicopathologic features of 2 new cases of uterine tumors resembling ovarian sex cord tumors. Int J Gynecol Pathol 2010; 29 : 459-467.
15. Hillard JB, Malpica A, Ramirez PT. Conservative management of a uterine tumor resembling an ovarian sex cord-stromal tumor. Gynecol Oncol 2004; 92 : 347-352.
16. Irving JA, Carinelli S, Prat J. Uterine tumors resembling ovarian sex cord tumors are polyphenotypic neoplasms with true sex cord differentiation. Modern Pathol 2006; 19 : 17-24.
17. Oliva E, Clement PB, Young RH. Endometrial stromal tumors: an update on a group of tumors with a protean phenotype. Adv Anat Pathol 2000; 7 : 257-281.
18. Oliva E, Young RH, Amin MB, Clement PB. An immunohistochemical analysis of endometrial stromal and smooth muscle tumors of the uterus: a study of 54 cases emphasizing the importance of using a panel because of overlap in immunoreactivity for individual antibodies. Am J Surg Pathol 2002; 26 : 403-412.
19. Zamecnik M, Staník M. Uterine tumor resembling ovarian sex cord tumor (UTROSCT). Report of case suggesting neoplastic origin of intratumoral myoid cells. Cesk Patol 2006; 42 : 145-149.
20. Zamecnik M, Stanik M, Michal M. Smooth muscle/myoid differentiation in uterine tumour resembling ovarian sex-cord tumour (UTROSCT). Histopathology 2009; 55 : 619-628.
21. Sutak J, Lazic D, Cullimore JE. Uterine tumour resembling an ovarian sex cord tumour. J Clin Pathol 2005; 58 : 888-890.
22. Hurrell DP, McCluggage WG. Uterine tumour resembling ovarian sex cord tumour is an immunohistochemically polyphenotypic neoplasm which exhibits coexpression of epithelial, myoid and sex cord markers. J Clin Pathol 2007; 60 : 1148-1154.
23. Staats PN, Garcia JJ, Dias-Santagata DC, et al. Uterine tumors resembling ovarian sex cord tumors (UTROSCT) lack the JAZF1-JJAZ1 translocation frequently seen in endometrial stromal tumors. Am J Surg Pathol 2009; 33 : 1206-1212.
24. de Leval L, Lim GS, Waltregny D, Oliva E. Diverse phenotypic profile of uterine tumors resembling ovarian sex cord tumors: an immunohistochemical study of 12 cases. Am J Surg Pathol 2010; 34 : 1749-1761.
25. Gupta M, de Leval L, Selig M, Oliva E, Nielsen GP. Uterine tumors resembling ovarian sex cord tumors: an ultrastructural analysis of 13 cases. Ultrastruct Pathol 2010; 34 : 16-24.
26. Clement PB, Scully RE. Uterine tumors resembling ovarian sex-cord tumors. A clinicopathologic analysis of fourteen cases. Am J Clin Pathol 1976; 66 : 512-525.
27. Fekete PS, Vllios F, Patterson BD. Uterine tumor resembling an ovarian sex-cord tumor: report of a case of an endometrial stromal tumor with foam cells and ultrastructural evidence of epithelial differentiation. Int J Gynecol Pathol 1985; 4 : 378-387.
28. McCluggage WG, Burton J, Maxwell P, Sloan JM. Immunohistochemical staining of normal, hyperplastic, and neoplastic adrenal cortex with a monoclonal antibody against alpha inhibin. J Clin Pathol 1998; 51 : 114-116.
29. Pusiol T, Parolari AM, Pisciolo F. Uterine leiomyoma with tubules. Int Semin Surg Oncol 2008; 5 : 15. doi: 10.1186/1477-7800-5-15.
30. Murray SK, Clement PB, Young RH. Endometrioid carcinomas of the uterine corpus with sex cord-like formations, hyalinization, and other unusual morphologic features: a report of 31 cases of a neoplasm that may be confused with carcinosarcoma and other uterine neoplasms. Am J Surg Pathol 2005; 29 : 157-166.
31. Simon RA, Sung CJ, Lawrence WD, Quddus MR. Vascular plexiform leiomyoma mimicking uterine tumor resembling ovarian sex cord tumor. Ann Diagn Pathol 2010; 14 : 355-357.
32. Czernobilsky B, Mamet Y, David MB, Atlas I, Gitstein G, Lifschitz-Mercer B. Uterine retiform sertoli-leydig cell tumor: report of a case providing additional evidence that uterine tumors resembling ovarian sex cord tumors have a histologic and immunohistochemical phenotype of genuine sex cord tumors. Int J Gynecol Pathol 2005; 24 : 335-340.
33. Garuti G, Gonfiantini C, Mirra M, Galli C, Luerti M. Uterine tumor resembling ovarian sex cord tumors treated by resectoscopic surgery. J Minim Invasive Gynecol 2009; 16 : 236-240.
Labels
Anatomical pathology Forensic medical examiner Toxicology
Article was published inCzecho-Slovak Pathology

2014 Issue 1-
All articles in this issue
- Lynch syndrome in the hands of pathologists
- Detection of chromosome changes by CGH, array-CGH and SNP array techniques in tumours
- Cell cultures
- Granular cell variant of atypical fibroxanthoma. A case report
- Expression of the active caspase-3 in children and adolescents with classical Hodgkin lymphoma
- Uterine tumors resembling ovarian sex cord tumors (UTROSCT). Report of a case with lymph node metastasis
- Gynecomastia with pseudoangiomatous hyperplasia and multinucleated giant cells in a patient without neurofibromatosis
- Czecho-Slovak Pathology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Lynch syndrome in the hands of pathologists
- Cell cultures
- Detection of chromosome changes by CGH, array-CGH and SNP array techniques in tumours
- Uterine tumors resembling ovarian sex cord tumors (UTROSCT). Report of a case with lymph node metastasis
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

