-
Medical journals
- Career
Perineural cell differentiation in ganglioneuromas. Report of 8 cases with immunohistochemical expression of perineural cell markers
Authors: M. Zámečník 1; M. Staník 2; A. Chlumská 3,4; P. Mukenšnabl 4; F. Ondriaš 1
Authors‘ workplace: Alpha Medical Pathology, s. r. o., Bratislava, Slovak Republic 1; Department of Pathology, Suedharz Hospital, Nordhausen, Germany 2; Šikl’s Department of Pathology, Faculty Hospital, Charles University, Pilsen, Czech Republic 3; Laboratory of Surgical Pathology, Pilsen, Czech Republic 4
Published in: Čes.-slov. Patol., 48, 2012, No. 4, p. 215-217
Category: Original Articles
Overview
Eight cases of ganglioneuroma were examined for a presence of perineural cell differentiation, using the immunohistochemical markers epithelial membrane antigen (EMA), claudin-1 and GLUT-1. The mean age of the patients was 42.3 years (range 26–68 years), six patients were females and two were males. Five tumors were located in the adrenal gland and 3 tumors in the retroperitoneum. Morphology of the tumors was typical, i.e., they were composed of neuroid spindle cell population and scattered mature appearing ganglion cells. Spindle cells positive for perineural cell markers claudin-1 and GLUT-1 were found in all lesions, at least focally. EMA+ cells were seen in 2 of 8 tumors. These perineural-type cells were often arranged in organoid fashion around the schwannoid bundles or around the vessels. Our findings indicate that perineural cell differentiation is commonly present in ganglioneuromas.
Keywords:
ganglioneuroma – perineurioma – EMA – claudin-1 – GLUT-1Ganglioneuroma is a benign tumor that occurs usually in adults, and it is most often located in the posterior mediastinum and retroperitoneum (1). It is composed of ganglion cells and neuroid spindle cells. Ultrastructurally, the spindle cell component contains mostly Schwann cells (1). Rare ultrastructural studies found, in addition to Schwann cell population, some cells with perineural-type features (2,3). Recently, we reported a case of ganglioneuroma with perineural cell differentiation proven with the immunohistochemical markers epithelial membrane antigen (EMA), claudin-1 and GLUT-1 (4). After seeing that case, we speculated that perineural cell differentiation can be more frequent in ganglioneuromas, and this prompted us to collect and examine additional cases. Now, we would like to present our series of 8 ganglioneuromas. Examining these tumors, we have found that perineural cell differentiation appears to be a common feature of ganglioneuroma, and that it can be demonstrated by currently used perineural cell markers – EMA, claudin-1 and GLUT-1 (5–7).
MATERIAL AND METHODS
Eight cases with typical morphological and immunohistochemical features of ganglioneuroma were retrieved from our routine files. In all cases, the tumor tissue was fixed in 10% formalin and processed routinely. The sections were stained with hematoxylin and eosin. In all cases, available immunohistochemical slides showed typical immunophenotype of ganglioneuroma. The spindle cell component expressed the S100 protein, and the ganglion cells were positive for neurofilament protein and/or calretinin.
In all cases, we performed immunohistochemical examination for perineural cell markers on selected tissue blocks. The following primary antibodies were used: GLUT-1 (polyclonal, 1 : 200), EMA (clone E29, 1 : 700) (both from DAKO, Glostrup, Denmark), and claudin-1 (polyclonal, 1 : 50, Zymed). Immunostaining was performed according to standard protocols using avidin-biotin complex labeled with peroxidase or alkaline phosphatase. Appropriate positive and negative controls were applied.
RESULTS
Main clinical and gross features of the tumors are listed in table (Table 1). Mean age of the patients was 42.3 years (range 26–68 years), six patients were females and two were males. Five tumors were located in the adrenal gland and 3 tumors in the retroperitoneum.
1. Clinical data and immunohistochemical expression of perineural cell markers in 8 ganglioneuromas. 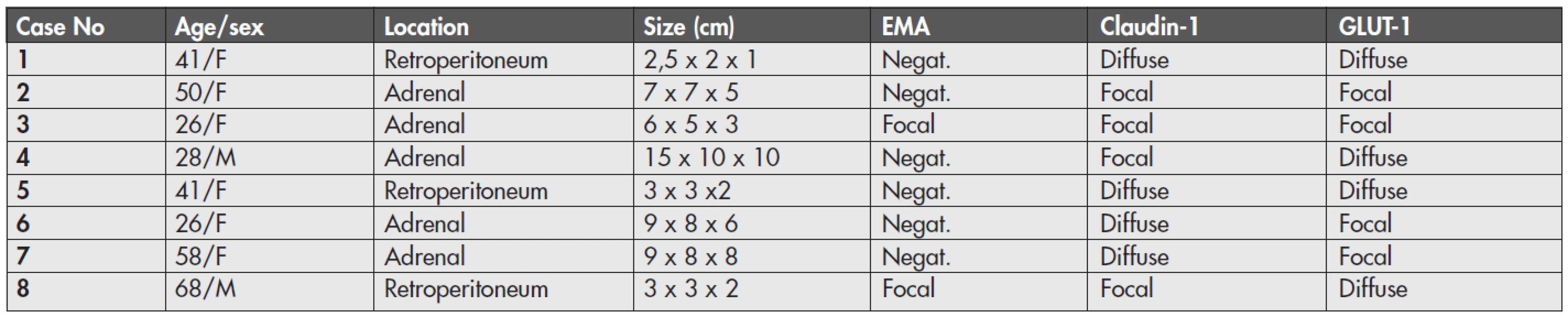
F – female; M – male; EMA – epithelial membrane antigen; Negat. – negative; Focal – positive cells seen in less than 30 % of the tumor volume Histologically (Fig. 1), the tumors were typical ganglioneuromas composed of mature ganglion cells and surrounding bland-appearing neuroid spindle cells. Ganglion cells were seen as isolated cells or in small cell clusters. They had abundant cytoplasm, round to oval nuclei, and some of them showed degenerative vacuolization. The spindle-shaped cells had moderate cytoplasm and wavy spindle shaped nuclei. They were arranged haphazardly or as intersecting fascicles. These fascicles were usually compact in appearance, and, focally, they were separated by a loose myxoid stroma. Calcifications were seen in two tumors (cases 3 and 4), and adipose metaplasia in two lesions (cases 2 and 6). No mitotic activity or necrosis were present in any case.
Fig. 1. Typical ganglioneuroma showing neuroid stroma and ganglion cells. HE, magnification x 60 (A), and x 160 (B). 
Immunohistochemically (Fig. 2, Table 1), EMA was positive in spindle cells in two of 8 cases, and this positivity was only focal. Nevertheless, the reactivity was unequivocal. The positive cells were often arranged in organoid fashion around the schwannoid bundles or around intratumoral vessels. Claudin-1 and GLUT-1 were expressed in all lesions. The arrangement of the positive cells appeared similar to that of EMA, but was more apparent and well visible. In all tumors, these cells were arranged either as scattered cells or in the above-mentioned organoid fashion. The cells positive for at least one of these two markers were found in more than 70 % of the tumor volume in 6 tumors, whereas they were only focally seen in 2 cases, i.e., conversely, every lesion contained at least some areas without positivity for claudin-1 and/or GLUT-1 (where the spindle cell population was composed exclusively of S100-positive cells).
Fig. 2. Perineural cell differentiation in ganglioneuromas. Immunohistochemical findings: (A) claudin-1 in scattered spindle cells; (B) claudin-1 positive cells arranged in organoid fashion around the schwannoid bundles; (C) GLUT-1 in scattered stromal cells; (D) focal EMA positivity (seen in 2 cases). ABC technique, magnifications x100 (A-C), x160 (D). 
DISCUSSION
We have found that all cases in our study of ganglioneuroma expressed perineural cell markers claudin-1 and GLUT-1, whereas EMA positivity was seen in two of 8 cases and was only focal. Thus, it seems that perineural cell differentiation is a constant feature of ganglioneuroma. Some ultrastructural studies found among spindle cells of ganglioneuroma cells with some perineural cell features, such as bipolar thin cell processes, external lamina and pinocytotic vesicles (2,3). However, perineural cell differentiation was not found immunohistochemically in studies using EMA, and therefore ganglioneuroma is regarded traditionally as an EMA-negative tumor (1,8). In our series, EMA-positive cells were seen in 2 of 8 cases. This expression was, however, focal and not very conspicuous. The perineural cells have extremely thin cell processes, and EMA antibody bound to cell membranes of these processes produces only less visible, fine and fiber-like staining. Such weak EMA positivity or even EMA negativity has already been observed and discussed in various studies of perineural cell neoplasms (9–12). It explains why EMA represents a less sensitive marker for perineural cell differentiation in tumors, including ganglioneuromas, as was seen in our series. In contrast, recently discovered perineural cell markers claudin-1 and GLUT-1 are more sensitive – in our series, they showed expression in all cases.
The histogenesis of cells with perineural differentiation in ganglioneuroma is unclear. As for all spindle cells in this tumor, the following three possible origins can be considered (3): /1/ neuroblastoma cells can differentiate along neuronal, Schwann cell and perineural cell lines; /2/ spindle cells including Schwann cells and perineural cells are formed by differentiation of ubiquitous (non-neoplastic) mesenchymal cells in response to the formation of neuritic processes; /3/ non-neoplastic Schwann cells and perineural cells in the surrounding tissues are induced to proliferate and migrate into the tumor.
Our observation of perineural cell differentiation in ganglioneuromas cannot answer definitively the question of their histogenesis. We believe that perineural cell differentiation represents one of the features of neoplastic cell maturation. This impression is strong especially in organoid areas with positivity of perineural cell markers seen in the periphery of the fascicles, resembling the normal microscopic anatomy of the nerve sheath. Stromal cells of ganglioneuroma, including cells of the perineural type, could arise from an immature neoplastic cell that is capable of differentiating terminally toward various cell types of the nervous system. Such histogenesis is analogical with histogenesis of “mixed“ nerve sheath tumors in which various types of nerve sheath cells were described, for example neurofibroma-schwannoma (13), schwannoma-perineurioma, neurofibroma-perineurioma (14), nerve sheath myxoma (9), neurofibroma with perineural cells (15,16).
In conclusion, our findings indicate that ganglioneuromas show perineural cell differentiation with immunohistochemical expression of claudin-1, GLUT-1 or (less often) of EMA. The spindle cell population in these tumors appears to be heterogeneous, and, in this respect, similar to the cell population of neurofibromas. In practice, the perineural cell markers may assist in diagnosis of ganglioneuroma.
Correspondence address:
M. Zamecnik, MD
Medicyt, s.r.o., lab. Trencin
Legionarska 28, 91171 Trencin, Slovak Republic
tel.: +421-907-156629
e-mail: zamecnikm@seznam.cz
Sources
1. Weiss SW, Goldblum JR. Ewing’s sarcoma/PNET tumor family and related lesions. In: Weiss SW, Goldblum JR, eds. Enzinger and Weiss s Soft Tissue Tumors (4th ed). Philadelphia, PA, USA: Mosby Inc.; 2001 : 1265–1322.
2. Shimada H. Transmission and scanning electron microscopic studies on the tumors of neuroblastoma group. Acta Pathol Jpn 1982; 32(3): 415–426.
3. Ricci A Jr, Parham DM, Woodruff JM, Callihan T, Green A, Erlandson RA. Malignant peripheral nerve sheath tumors arising from ganglioneuromas. Am J Surg Pathol 1984; 8(1): 19–29.
4. Zámečník M, Chlumská A, Ondriaš F. Ganglioneuroma with perineural cell differentiation. Report of a case. Cesk Patol. 2012; 48(2): 94–96.
5. Theaker JM, Gillett MB, Fleming KA, Gatter KC. Epithelial membrane antigen expression by meningiomas, and the perineurium of peripheral nerve. Arch Pathol Lab Med 1987; 111(5): 409.
6. Folpe AL, Billings SD, McKenney JK, Walsh SV, Nusrat A, Weiss SW. Expression of claudin-1, a recently described tight junction-associated protein, distinguishes soft tissue perineurioma from potential mimics. Am J Surg Pathol 2002; 26(12): 1620–1626.
7. Ahrens WA, Ridenour RV 3rd, Caron BL, Miller DV, Folpe AL. GLUT-1 expression in mesenchymal tumors: an immunohistochemical study of 247 soft tissue and bone neoplasms. Hum Pathol 2008; 39(10): 1519–1526.
8. Parham DM. Small round cell tumors. In: Miettinen M. ed. Modern Soft Tissue Pathology. Tumors and Non-Neoplastic Conditions (1st ed.), New York: Cambridge University Press, 2010 : 896–929.
9. Zámečník M, Sedláček T. Nerve sheath myxoma with bidirectional schwannomatous and perineural differentiation. Cesk Pathol 2010; 46(3): 73–76.
10. Zámečník M, Mukenšnabl P, Sokol L, Michal M. Perineurial cells and nerve axons in gastrointestinal schwannomas: a similarity with neurofibromas. An immunohistochemical study of eight cases. Cesk Patol 2004; 40(4): 150–153.
11. Groisman GM, Polak-Charcon S. Fibroblastic polyp of the colon and colonic perineurioma: 2 names for a single entity? Am J Surg Pathol 2008; 32(7): 1088–1094.
12. Giannini C, Scheithauer BW, Jenkins RB, et al. Soft-tissue perineurioma. Evidence for an abnormality of chromosome 22, criteria for diagnosis, and review of the literature. Am J Surg Pathol 1997; 21(2): 164–173.
13. Feany MB, Anthony DC, Fletcher CD. Nerve sheath tumours with hybrid features of neurofibroma and schwannoma: a conceptual challenge. Histopathology 1998; 32(5): 405–410.
14. Kazakov DV, Pitha J, Šíma R, et al. Hybrid peripheral nerve sheath tumors: Schwannoma-perineurioma and neurofibroma-perineurioma. A report of three cases in extradigital locations. Ann Diagn Pathol 2005; 9(1): 16–23.
15. Erlandson RA. The enigmatic perineurial cell and its participation in tumors and in tumorlike entities. Ultrastruct Pathol 1991; 15(4-5): 335–351.
16. Zámečník M, Michal M. Perineurial cell differentiation in neurofibromas. Report of eight cases including a case with composite perineurioma-neurofibroma features. Pathol Res Pract 2001; 197(8): 537–544.
Labels
Anatomical pathology Forensic medical examiner Toxicology
Article was published inCzecho-Slovak Pathology

2012 Issue 4-
All articles in this issue
- Pleomorphic adenoma of salivary glands: diagnostic pitfalls and mimickers of malignancy
- Pseudotumors of the central nervous system
- Histiocytic necrotizing lymphadenitis / Kikuchi-Fujimoto disease (HNL/K-F) and its differential diagnosis: analysis of 19 patients
- Expression of p57 marker in differential diagnosis of complete and partial mole – correlation with DNA analysis
- Pseudotumors and mimickers of malignancy of the head and neck pathology
- Gliosarcoma with alveolar rhabdomyosarcoma-like component: Report of a case with a hitherto undescribed sarcomatous component
- Perineural cell differentiation in ganglioneuromas. Report of 8 cases with immunohistochemical expression of perineural cell markers
- Czecho-Slovak Pathology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Pleomorphic adenoma of salivary glands: diagnostic pitfalls and mimickers of malignancy
- Histiocytic necrotizing lymphadenitis / Kikuchi-Fujimoto disease (HNL/K-F) and its differential diagnosis: analysis of 19 patients
- Pseudotumors of the central nervous system
- Expression of p57 marker in differential diagnosis of complete and partial mole – correlation with DNA analysis
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

