-
Medical journals
- Career
Universal adhesives – a new direction in the development of adhesive systems
Authors: A. Tichý 1,2; K. Hosaka 2; J. Tagami 2
Authors‘ workplace: Stomatologická klinika, 1. lékařská fakulta Univerzity Karlovy a Všeobecná fakultní nemocnice, Praha 1; Department of Cariology and Operative Dentistry, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, Tokyo, Japan 2
Published in: Česká stomatologie / Praktické zubní lékařství, ročník 120, 2020, 1, s. 4-12
Category: Review Article
Overview
Objectives: In the past decade, many new adhesive systems labeled as universal were introduced. Their common characteristic is that they can be applied to hard dental tissues either in self-etch or etch-and-rinse mode, and they are also able to bond to various restorative materials. Moreover, universal adhesives are mostly one-step and user-friendly. Because numerous papers about their properties were published, the purpose of this review article is to critically discuss the available information about the properties of universal adhesives and their bonding to various materials.
Conclusions: The simplification of the application procedure is accompanied by several drawbacks associated with the necessity to mix all components into a single bottle. Due to the content of hydrophilic monomers, water and volatile solvents, adhesive layers of universal adhesives are more susceptible to water sorption. Consequently, they are more prone to hydrolytic and enzymatic degradation, thus exhibiting a lower durability. Universal adhesives also have limitations in bonding to some materials and require preceding surface treatments. To enhance the bond strength to enamel, selective enamel etching with phosphoric acid is recommended because the self-etching effect of universal adhesives may be insufficient. In contrast, more durable bonding to dentin was reported in self-etch mode compared to etch-and-rinse. For glass ceramics, a silane coupling agent should be applied in a separate step prior to the application of universal adhesives despite that some of them are silane-containing. The silanes are unstable in acidic conditions and their premature hydrolysis precludes the chemical interaction with glass. Lastly, zirconia ceramics require mechanical pre-treatment using air-abrasion because the chemical bond alone is not sufficient. In conclusion, universal adhesives can be used in various indications, however, it is necessary to be aware of their drawbacks and limitations.
Keywords:
Dentin – adhesion – universal adhesives – enamel – ceramics – resin composite – review
INTRODUCTION
Adhesive dentistry has developed substantially in recent decades and it has allowed highly aesthetic and minimally invasive treatment options in many indications [1, 2]. However, a laborious and technique-sensitive procedure is required to overcome the different properties of the hydrophilic dentin and hydrophobic restorative materials. Therefore, the effort to simplify and shorten the adhesives’ application procedure has become one of the trends in adhesive dentistry and lead to the development of adhesives labeled as universal. Although no formal definition exists, most universal adhesives are one-bottle systems which can be applied to hard dental tissues either in self-etch or etch-and-rinse mode. Universal adhesives are also characterized by the ability to bond to almost all restorative materials. As numerous scientific texts and commercial claims have been published about their properties over the past decade, it is difficult to follow the current state-of-the-art. Therefore, the aim of this review article is to critically discuss and summarize the available information about universal adhesives and their bonding to various materials.
CLASSIFICATION OF ADHESIVE SYSTEMS
The classification of adhesives has been extensively described in the literature [3]; therefore, it will be just briefly reviewed in this article. It seems to be most suitable to classify the adhesives as etch-and-rinse, which utilize phosphoric-acid etching, or self-etch which contain acidic methacrylate monomers. These two groups are further subdivided according to the number of application steps.
Etch-and-rinse adhesives
Conventionally, a 32–40% phosphoric acid gel is used as the etchant to remove the smear layer from enamel and dentin surfaces and to form irregular surface patterns suitable for micromechanical bonding (Fig. 1, 2). In the case of enamel, porosities created by the selective dissolution of minerals are infiltrated by the adhesive and the bond is created by its polymerization. In the case of dentin, the monomers of the adhesive penetrate exposed collagen fibers and after polymerization, the so-called hybrid layer [4] is formed and provides the bond to dentin. In addition, the adhesive flows into dentinal tubules exposed by the smear layer removal and forms resin tags which also contribute to the bond strength.
1. Enamel surface with exposed prisms after phosphoric acid etching. Scanning electron microscope, magnification 2000×. 
2. Dentin surface with open dentinal tubules after phosphoric acid etching. Remnants of silica particles from the etching gel were observed. Scanning electron microscope, magnification 2000×. 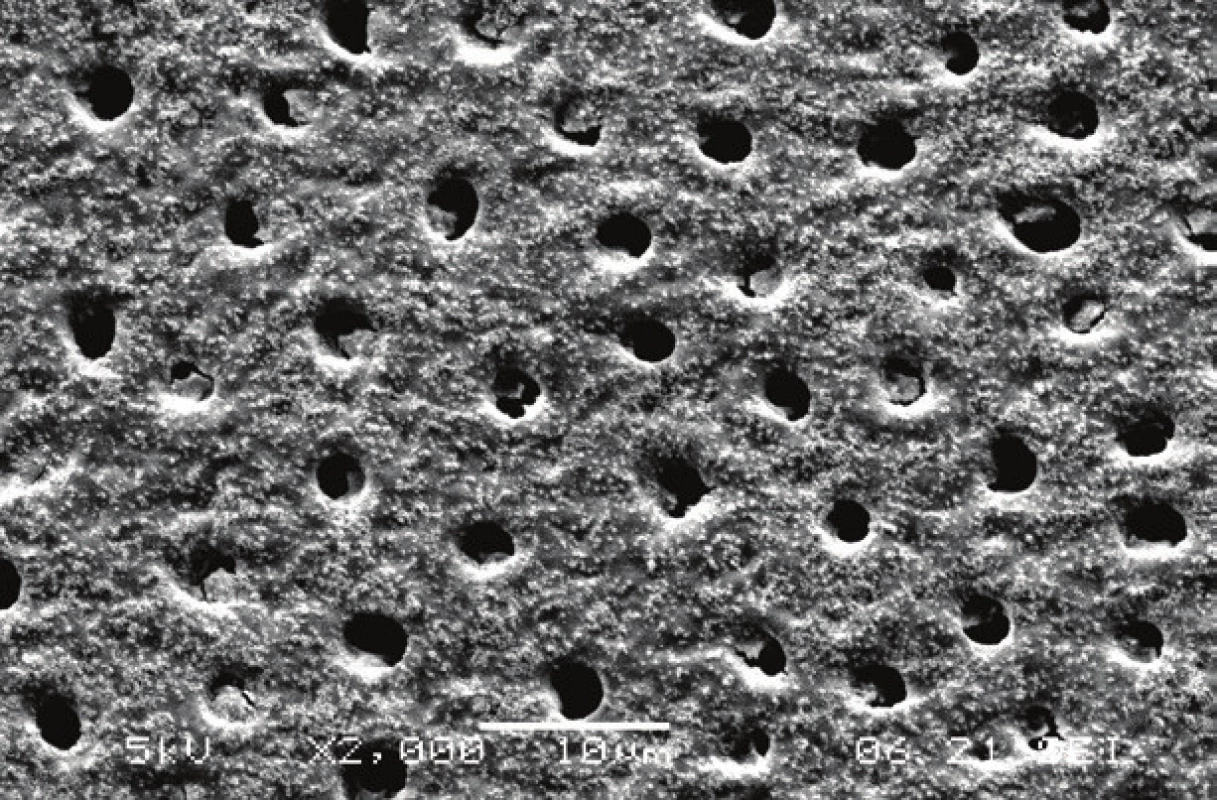
Etch-and-rinse adhesives are available as three-step or two-step. Three-step systems consist of 1) phosphoric acid etching, 2) priming with solvated hydrophilic methacrylate monomers, and 3) bonding with hydrophobic methacrylate monomers. Long-term results showed an excellent clinical reliability of these systems [5], they are therefore considered as the standard reference for new products. In two-step etch-and-rinse adhesives, primer and bond are combined into one bottle in order to simplify the application procedure. Nowadays, they are rarely used due to the increased hydrophilicity of the primer-bond mixture [6], which resulted in their inferior clinical performance [5].
Self-etch adhesives
The development of self-etching primers was a breakthrough in adhesive dentistry because the acidic methacrylate monomers [7, 8] enabled simultaneous etching and priming. Consequently, the risk of insufficient impregnation of collagen fibers and nanoleakage [9] within the hybrid layer decreased. The use of self-etching primers is also easier, it considerably shortens the adhesive application procedure, and it eliminates the critical step of dentin air-drying after phosphoric-acid etching and rinsing. Furthermore, lower post-operative sensitivity was observed with self-etch adhesives compared to etch-and-rinse, because phosphoric acid etching completely removes the smear layer and exposes the orifices of dentinal tubules [10].
The pH of self-etch systems has a crucial influence on their bonding performance [10, 11]. Strongly acidic (pH<1) and intermediately acidic (pH»1–2) adhesives exhibited an etching effect similar to phosphoric acid and achieved promising results on enamel. However, their bonding to dentin was not stable in the long term due to the increased diffusion of water into the hybrid layer. As a consequence of remaining deposits of hydrolytically unstable salts, an osmotic gradient between dentin and the hybrid layer was formed and resulted in the accelerated decomposition of the adhesive joint [12]. Therefore, mildly or very mildly acidic adhesives (pH»2–3) which hybridize the smear layer and interact with dentin to a depth of about 1 µm are mostly used at present. While this is much less compared with phosphoric acid etching, which demineralizes dentin to a depth of approximately 5–10 mm, the laboratory and clinical results of mild self-etch adhesives are comparable to the three-step etch-and-rinse systems [5]. However, for very mildly acidic adhesives whose demineralizing effect is insufficient on enamel, selective enamel etching with phosphoric acid is recommended, especially when bonding to unprepared enamel [13].
Self-etch adhesives are available as two-step and one-step. While the two-step systems consist of 1) a self-etching primer and 2) a hydrophobic bond, the two components are applied simultaneously in the one-step approach. Because one-step adhesives contain large amounts of solvents which must be thoroughly air-dried, these systems form very thin adhesive layers (Fig. 3). Although most of the current one-step adhesives are one-bottle, some are separated into two bottles and the solutions are mixed just before the application. This can solve issues associated with the long-term instability of all components mixed into one bottle but it cannot prevent the principal drawback of one-step adhesives – their higher hydrophilicity and water sorption, which may lead to a lower durability of the adhesive joint [10].
3. A cross-sectional view of the adhesive joint between dentin and a hybrid resin composite formed by a thin (~5 μm) adhesive layer of the universal adhesive G-Premio Bond (GC, Tokyo, Japan). Scanning electron microscope, magnification 2000×. 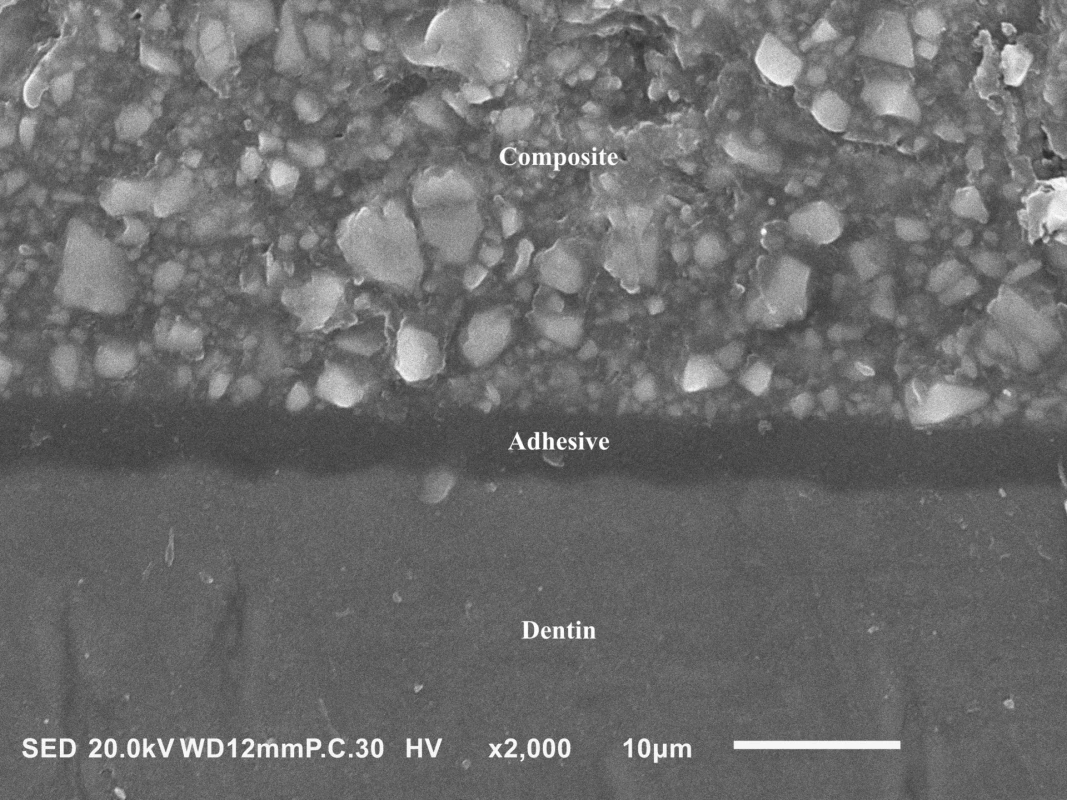
UNIVERSAL ADHESIVES
By their composition, universal adhesives are similar to one-step self-etch systems and their use is very fast and simple as well. The distinctive property of universal adhesives is that they can be applied either in self-etch or etch-and-rinse mode, which is the reason why they have also been labeled as multi-mode [14, 15]. Besides hard dental tissues, universal adhesives can bond to most restorative materials. The major advantage of their versatility is the option to adapt to many clinical situations without the need for more adhesive systems.
The versatility of universal adhesives is mainly provided by 10-MDP (10-methacryloyloxydecyl dihydrogenphosphate) which bonds chemically with calcium ions of hydroxyapatite. The calcium salts of 10-MDP are almost insoluble in water, thus the bond with hard dental tissues is stable over the long term [16]. Transmission electron microscopy also revealed nanolayering of 10-MDP applied to hard dental tissues, a regular arrangement in 4nm layers which apparently also contributes to the bond stability [17]. Another advantage of 10-MDP is its ability to adhere to restorative materials, such as zirconia ceramics [18, 19]. In order to promote the adhesion in other clinical situations, some universal adhesives also contain silanes for bonding to silica-based materials, and chemical polymerization initiators which enable polymerization even under insufficient light irradiation.
On the other hand, the need to mix all components into one bottle poses several disadvantages. Higher hydrophilicity of the adhesive layer resulting from the presence of hydrophilic monomers, water and solvent residues appears to be the most serious drawback. The most commonly used hydrophilic monomer is 2-hydroxyethyl methacrylate (HEMA), which is essential for the infiltration of wet dentin, but it also increases water sorption, impairs the mechanical properties and affects the polymerization of adhesives [20]. However, HEMA cannot be simply eliminated for its amphiphilic nature that keeps hydrophobic methacrylates in solution with water, which is necessary to ionize the acidic monomers. It was reported that HEMA-free adhesives have a lower water sorption [21] but phase separation was observed (Fig. 4), i.e. the formation of water droplets separated from hydrophobic methacrylates [22] which could negatively affect the adhesive’s properties. Therefore, some modern materials replace HEMA partially or completely by monomers with amide groups which are also able to prevent phase separation [23, 24], while being less hydrophilic [25] and exhibiting a higher degree of conversion [24] and durability [23, 24].
4. Phase separation observed in the adhesive layer of the HEMA-free universal adhesive G-Premio Bond (GC, Tokyo, Japan). The round-shaped voids were formed by water droplets separated from hydrophobic methacrylates. Scanning electron microscope, magnification 2000×. 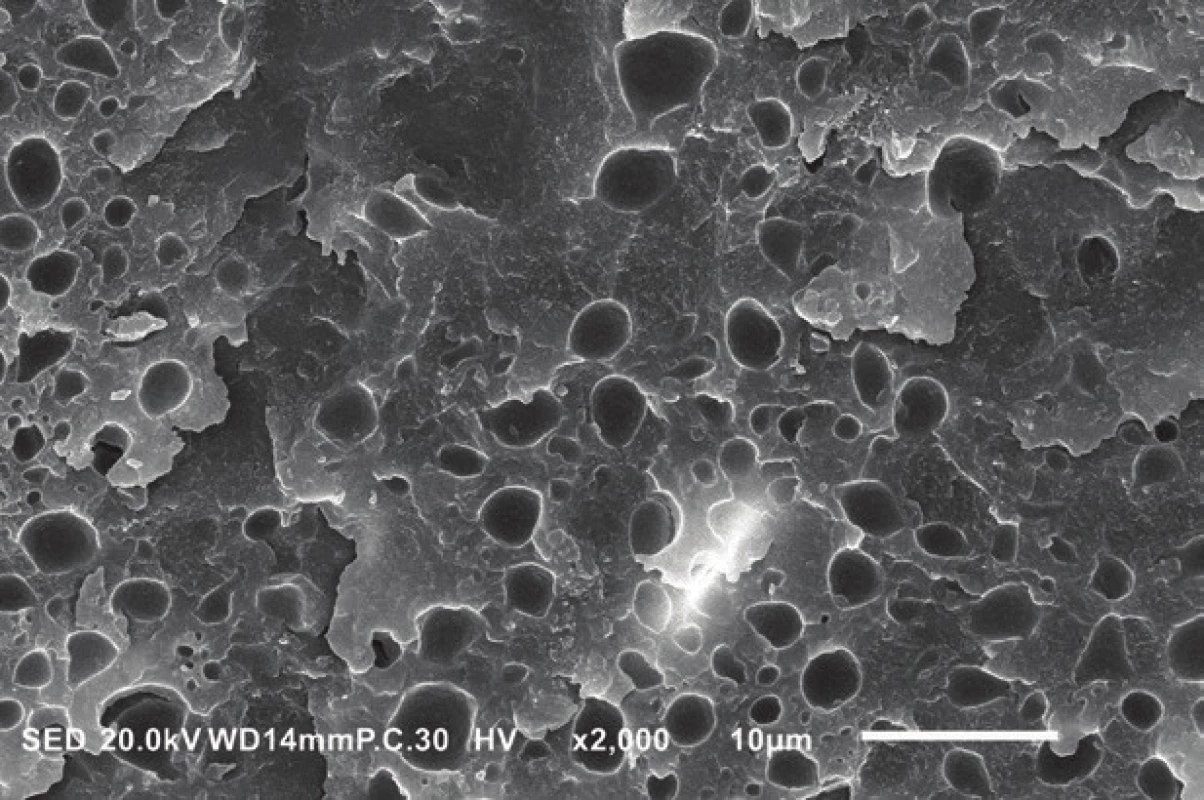
The presence of water and volatile organic solvents also plays an important role in the bonding performance of adhesives. The hydrophilicity and low viscosity of solvents improve the infiltration of hard dental tissues by monomers and solvents also facilitate the evaporation of water [26]. Alcohols (ethanol, less tert-butanol or isopropanol) and acetone are used as solvents in most adhesives and may account for up to 80% of their weight. However, the solvents cannot be completely evaporated under clinical conditions, and their residues may adversely affect the polymerization of the adhesive layer and increase its permeability [27–29]. This is one of the reasons why universal adhesives underperform multi-step systems, whose adhesive layers mainly constitute of hydrophobic monomers that prevent water sorption.
As a consequence of the hydrophilicity and permeability of the adhesive layer of universal adhesives, increased nanoleakage was observed [10]. Moreover, microscopic studies revealed water-treeing [30] described as a network of channels allowing fluid movement within the adhesive layer. All the aforementioned factors might accelerate the hydrolytic degradation of the adhesive joint and consequently impair its long-term resistance [12, 31, 32]. Besides that, combining all the adhesives’ components into one bottle might also reduce their shelf life, as the ester bond of the conventional methacrylates is susceptible to hydrolysis in acidic conditions [33]. In this aspect, hydrolytically more stable methacrylates with an ether or an amide bond appear to be more suitable [33]. The shelf life may also be affected by improper storage as a consequence of volatile solvent evaporation (acetone in particular), so it is necessary to close the bottles immediately after use, to avoid their exposure to high temperatures, and to store them according to the manufacturer’s recommendation.
BONDING OF UNIVERSAL ADHESIVES TO VARIOUS MATERIALS
Adhesive systems in dentistry serve to bond a wide range of materials. These include primarily hard dental tissues, but also composite resins, various types of ceramics, metal alloys and others. Because of the different characteristics of these materials, a truly universal adhesive procedure is very difficult to be achieved. Moreover, it is required that the adhesive joint is sufficiently strong and stable in the long term even in the aggressive environment of the oral cavity.
Adhesion to enamel
Compared to phosphoric acid, the acidity and hence the etching effect of universal adhesives is lower, which together with their shorter application time negatively affects the quality of adhesion to enamel. A meta-analysis of laboratory studies showed that the initial bond strength of universal adhesives to enamel and its durability were significantly improved if enamel was etched with phosphoric acid prior to the application of universal adhesives [14, 34–36]. Alternative strategies for the enhancement of the adhesion to enamel include a prolonged [37] or repeated [38] application of the universal adhesive.
Adhesion to dentin
One of the characteristics defining universal adhesives is the possibility of application in either self-etch or etch-and-rinse mode. The bond strength to dentin in both these modes was investigated in many studies and meta-analyses of short-term data, gathered mostly after 24 h, revealed no significant difference between the two modes [14, 39]. Only for the very mildly acidic All-Bond Universal (pH=3.1; Bisco, Schaumburg, IL, USA), phosphoric acid etching increased its bond strength to dentin significantly [14]. On the other hand, a negative effect of phosphoric acid etching on the durability of the adhesive joint was observed in some studies [32, 40–42]. This is probably due to insufficient sealing of the demineralized zone in the etch-and-rinse mode [43], which may result in an accelerated hydrolytic degradation of the resin polymer network and an enzymatic decomposition of unprotected collagen by matrix metalloproteinases and cysteine proteases [31]. In addition, the removal of smear layer unplugs dentinal tubules, which might increase the postoperative sensitivity and deteriorate the adhesive’s properties due to higher outflow of the tubular fluid [10, 44]. It is therefore appropriate to limit the phosphoric acid etching to enamel.
Several studies have also examined how to improve the adhesion of universal adhesives. It has been shown that active [45, 46] or prolonged [25] application can improve their bond strength, probably due to better adhesive infiltration of the treated dentin. This is in contrast to the “no-waiting” concept proposed by manufacturers of some recent universal adhesives, which recommends immediate solvent evaporation and polymerization of the adhesive. However, laboratory studies suggested that this strategy might have some limitations [47, 48]. Other studies aimed to reduce the negative effect of universal adhesives’ increased hydrophilicity by extended air-drying [27, 49] or the use of warm air [50] which improved the solvent evaporation. More demanding procedures include, for example, the deproteinization of the smear layer with agents such as NaOCl. The objective is to eliminate the organic component of the hybrid layer, which is susceptible to enzymatic degradation, and to improve chemical bonding to dentin [51]. The major drawback of this method are residual free radicals in the structure of dentin produced by the decomposition of NaOCl, which adversely affect the polymerization of the adhesives. In order to improve the bond strength, a subsequent application of a reducing agent is necessary to decompose the radicals [52]. It was also proposed to use hydrophobic bonding agents to coat universal adhesives applied in the first step. In most of the studies, this two-step approach led to an improved durability of the adhesive joint [23, 53–56] but its efficiency was material-dependent and influenced by whether the universal adhesive was light-cured prior to the application of the bonding agent [53, 54]. However, it must be noted that universal adhesives and bonding agents may be incompatible, especially their photoinitiating systems [53], so they cannot be combined arbitrarily.
Adhesion to glass ceramics
To improve the adhesion to glass ceramics, it is essential to create a suitable surface relief by hydrofluoric acid which etches the silicate structure of glass [57]. The concentration of hydrofluoric acid and its application time is dependent on the type of glass ceramics and manufacturer’s instructions for use should be followed. The application of trialkoxysilanes, usually 3-methacryloxypropyltrimethoxysilane (MPTS) [58], in the next step is recommended to achieve chemical bonding. By the hydrolysis of alkoxy groups and their reaction with silanol groups of the ceramic’s glass phase, MPTS is chemically bonded to the ceramic surface and by polymerization, its methacrylate group connects with the monomers of the adhesive [59]. Silanes are also contained in some universal adhesives, but their efficacy was doubted because they are unstable in acidic environment containing water. As a result, they prematurely hydrolyze and condensate into oligomers, by which they lose the ability to bond to the silicate structures [60, 61]. Several studies demonstrated that the bond strength of a silane-containing universal adhesive to glass ceramics was significantly improved if a silane coupling agent was applied in a separate preceding step [60–63]. Alternatively, MPTS can be added to the adhesive just before its application, but just a day of storage has deactivated the silane again [60]. This implies that it cannot be relied upon the silane content in the adhesive and that a silane coupling agent should be applied to glass ceramics in a separate step.
Adhesion to zirconia ceramics
Given its excellent mechanical properties, zirconia ceramics have been increasingly used in clinical practice. Unlike ceramic materials containing a glass phase, zirconia ceramics cannot be effectively etched by hydrofluoric acid [57]. Therefore, many alternative pretreatments have been tested to achieve optimal adhesion [64, 65]. Air-abrasion with Al2O3 particles followed by the application of 10-MDP-based primers is most used because 10-MDP can chemically bond with zirconia [18, 19]. Alternatively, tribochemical silica coating and a subsequent application of a silane coupling agent can be used as well. According to a meta-analysis of laboratory studies, these methods combining mechanical and chemical surface treatment provide the strongest and most durable adhesion to zirconia ceramics [65] . Since most universal adhesives contain 10-MDP, they can provide a chemical bond with zirconia and they have been successfully tested as an alternative to ceramic primers [66–70], provided that the surface was previously air-abraded. However, the stability of the adhesion of universal adhesives to zirconia ceramics has not yet been sufficiently investigated.
Adhesion to resin-based materials
Universal adhesives can be used for the repair of composite restorations as well. To date, there is no consensus on how to achieve a stable bond between repaired and freshly applied composites. Usually, air-abrasion of the restoration surface is recommended prior to adhesive application [71–74] . Although the effect of silane application is questionable, promising results were obtained when silanization was combined with tribochemical silica coating [75–77]. Using universal adhesives, a few studies showed that a good composite-to-composite bond strength can be achieved by their application to the air-abraded surface of the repaired composite [76–79] and that some systems may benefit from silanization [77, 78].
In combination with resin cements, universal adhesives are also used for cementing posts and indirect reconstructions. However, it is necessary to use the materials in accordance with the manufacturer's recommendations because the incompatibility of one-step adhesives with self-cured and dual-cured resin cements has been reported [80, 81]. It is caused by the reaction of unpolymerized acidic monomers with tertiary amines, which are used as co-initiators in the cements. The decreased activity of the initiating system leads to an insufficient polymerization of the cement, lower mechanical properties and a decreased durability of the adhesive joint [80, 81]. In contrast, so-called "touch and cure" systems can improve the quality of polymerization by the reaction of an initiator in the cement and co-initiators contained in the adhesive which promote chemical polymerization. It was reported that these systems can significantly improve bond strengths especially under insufficient light irradiation [82–84].
CONCLUSION
Universal adhesives offer dentists a simple and quick bonding procedure to various materials in many clinical situations. However, the results of laboratory studies suggest that the simplification of the workflow has several drawbacks and that it might lead to lower long-term durability of the adhesive joints. Nevertheless, the development and optimization of universal adhesives are still ongoing, and some new products show promising results in laboratory studies. However, it is necessary to wait for more clinical data which are scarce to date.
MDDr. Antonin Tichy
Institute of Dental Medicine, First Faculty of Medicine of the Charles University and General University Hospital in Prague, Karlovo namesti 32
121 11 Prague
Czech Republic
E-mail: antonin.tichy@lf1.cuni.cz
Sources
1. Tyas MJ, Anusavice KJ, Frencken JE, Mount GJ. Minimal intervention dentistry – a review. Int Dent J. 2000; 50(1): 1–12.
2. Frencken JE, Peters MC, Manton DJ, Leal SC, Gordan VV, Eden E. Minimal intervention dentistry for managing dental caries – a review. Int Dent J. 2012; 62(5): 223–243.
3. Sofan E, Sofan A, Palaia G, Tenore G, Romeo U, Migliau G. Classification review of dental adhesive systems: from the IV generation to the universal type. Ann Stomatol (Roma). 2017; 8(1): 1–17.
4. Nakabayashi N, Nakamura M, Yasuda N. Hybrid layer as a dentin-bonding mechanism. J Esthet Restor Dent. 1991; 3(4): 133–138.
5. Peumans M, De Munck J, Mine A, Van Meerbeek B. Clinical effectiveness of contemporary adhesives for the restoration of non-carious cervical lesions. A systematic review. Dent Mater. 2014; 30(10): 1089–1103.
6. De Munck J, Van Meerbeek B, Yoshida Y, Inoue S, Vargas M, Suzuki K, Lambrechts P, Vanherle G. Four-year water degradation of total-etch adhesives bonded to dentin. J Dent Res. 2003; 82(2): 136–140.
7. Watanabe I, Nakabayashi N, Pashley DH. Bonding to ground dentin by a phenyl-P self-etching primer. J Dent Res. 1994; 73(6): 1212–1220.
8. Van Landuyt KL, Snauwaert J, De Munck J, Peumans M, Yoshida Y, Poitevin A, Coutinho E, Suzuki K, Lambrechts P, Van Meerbeek B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials. 2007; 28(26): 3757–3785.
9. Sano H, Takatsu T, Ciucchi B, Horner JA, Matthews WG, Pashley DH. Nanoleakage: leakage within the hybrid layer. Oper Dent. 1995; 20(1): 18–25.
10. Van Meerbeek B, Yoshihara K, Yoshida Y, Mine A, De Munck J, Van Landuyt KL. State of the art of self-etch adhesives. Dent Mater. 2011; 27(1): 17–28.
11. Giannini M, Makishi P, Ayres AP, Vermelho PM, Fronza BM, Nikaido T, Tagami J. Self-etch adhesive systems: a literature review. Braz Dent J. 2015; 26(1): 3–10.
12. Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Lenarda R, De Stefano Dorigo E. Dental adhesion review: aging and stability of the bonded interface. Dent Mater. 2008; 24(1): 90–101.
13. Perdigao J, Geraldeli S. Bonding characteristics of self-etching adhesives to intact versus prepared enamel. J Esthet Restor Dent. 2003; 15(1): 32–41.
14. Rosa WLdOd, Piva E, Silva AFd. Bond strength of universal adhesives: A systematic review and meta-analysis. J Dent. 2015; 43(7): 765–776.
15. Nagarkar S, Theis-Mahon N, Perdigão J. Universal dental adhesives: Current status, laboratory testing, and clinical performance. J Biomed Mater Res B. 2019; 107(6): 2121–2131.
16. Yoshida Y, Nagakane K, Fukuda R, Nakayama Y, Okazaki M, Shintani H, Inoue S, Tagawa Y, Suzuki K, De Munck J, Van Meerbeek B. Comparative study on adhesive performance of functional monomers. J Dent Res. 2004; 83(6): 454–458.
17. Yoshida Y, Yoshihara K, Nagaoka N, Hayakawa S, Torii Y, Ogawa T, Osaka A, Van Meerbeek B. Self-assembled nano-layering at the adhesive interface. J Dent Res. 2012; 91(4): 376–381.
18. Kern M, Wegner SM. Bonding to zirconia ceramic: adhesion methods and their durability. Dent Mater. 1998; 14(1): 64–71.
19. Nagaoka N, Yoshihara K, Feitosa VP, Tamada Y, Irie M, Yoshida Y, Van Meerbeek B, Hayakawa S. Chemical interaction mechanism of 10-MDP with zirconia. Sci Rep. 2017; 7(1): 455–463.
20. Van Landuyt KL, Snauwaert J, Peumans M, De Munck J, Lambrechts P, Van Meerbeek B. The role of HEMA in one-step self-etch adhesives. Dent Mater. 2008; 24(10): 1412–1419.
21. Takahashi M, Nakajima M, Hosaka K, Ikeda M, Foxton RM, Tagami J. Long-term evaluation of water sorption and ultimate tensile strength of HEMA-containing/-free one-step self-etch adhesives. J Dent. 2011; 39(7): 506–512.
22. Van Landuyt KL, De Munck J, Snauwaert J, Coutinho E, Poitevin A, Yoshida Y, Inoue S, Peumans M, Suzuki K, Lambrechts P, Van Meerbeek B. Monomer-solvent phase separation in one-step self-etch adhesives. J Dent Res. 2005; 84(2): 183–188.
23. Ahmed MH, De Munck J, Van Landuyt K, Peumans M, Yoshihara K, Van Meerbeek B. Do universal adhesives benefit from an extrabonding layer? J Adhes Dent. 2019; 21(2): 117–132.
24. Tichy A, Hosaka K, Abdou A, Nakajima M, Tagami J. Degree of conversion contributes to dentin bonding durability of contemporary universal adhesives. Oper Dent. 2019; Accepted for publication.
25. Kuno Y, Hosaka K, Nakajima M, Ikeda M, Klein Junior CA, Foxton RM, Tagami J. Incorporation of a hydrophilic amide monomer into a one-step self-etch adhesive to increase dentin bond strength: Effect of application time. Dent Mater J. 2019; 38(6): 892–899.
26. Ekambaram M, Yiu CKY, Matinlinna JP. An overview of solvents in resin–dentin bonding. Int J Adhes Adhes. 2015; 57 : 22–33.
27. Luque-Martinez IV, Perdigão J, Muñoz MA, Sezinando A, Reis A, Loguercio AD. Effects of solvent evaporation time on immediate adhesive properties of universal adhesives to dentin. Dent Mater. 2014; 30(10): 1126–1135.
28. Pucci CR, Gu L-S, Zhang H-y, Song Q, Xia VW, Davis LB, de Souza Andrade D, Mazzoni A, Breschi L, Pashley DH, Tay FR, Niu LN. Water-associated attributes in the contemporary dentin bonding milieu. J Dent. 2018; 74 : 79–89.
29. Nawareg MMA, Zidan AZ, Zhou J, Chiba A, Tagami J, Pashley DH. Adhesive sealing of dentin surfaces in vitro: A review. Am J Dent. 2015; 28(6): 321–332.
30. Tay FR, Pashley DH, Hiraishi N, Imazato S, Rueggeberg FA, Salz U,
Zimmermann J, King NM. Tubular occlusion prevents water-treeing and through-and-through fluid movement in a single-bottle, one-step self-etch adhesive model. J Dent Res. 2005; 84(10): 891–896.
31. Frassetto A, Breschi L, Turco G, Marchesi G, Di Lenarda R, Tay FR, Pashley DH, Cadenaro M. Mechanisms of degradation of the hybrid layer in adhesive dentistry and therapeutic agents to improve bond durability – A literature review. Dent Mater. 2016; 32(2): e41–e53.
32. Zhang ZY, Tian FC, Niu LN, Ochala K, Chen C, Fu BP, Wang XY, Pashley DH, Tay FR.Defying ageing: An expectation for dentine bonding with universal adhesives? J Dent. 2016; 45 : 43–52.
33. Salz U, Zimmermann J, Zeuner F, Moszner N. Hydrolytic stability of self-etching adhesive systems. J Adhes Dent. 2005; 7(2): 107–116.
34. Tsujimoto A, Barkmeier WW, Takamizawa T, Watanabe H, Johnson WW, Latta MA, Miyazaki M. Influence of duration of phosphoric acid pre-etching on bond durability of universal adhesives and surface free-energy characteristics of enamel. Eur J Oral Sci. 2016; 124(4): 377–386.
35. Suda S, Tsujimoto A, Barkmeier WW, Nojiri K, Nagura Y, Takamizawa T, Latta MA, Miyazaki M. Comparison of enamel bond fatigue durability between universal adhesives and two-step self-etch adhesives: Effect of phosphoric acid pre-etching. Dent Mater J. 2018; 37(2): 244–255.
36. Suzuki T, Takamizawa T, Barkmeier WW, Tsujimoto A, Endo H, Erickson RL, Latta MA, Miyazaki M. Influence of etching mode on enamel bond durability of universal adhesive systems. Oper Dent. 2016; 41(5): 520–530.
37. Cardenas AM, Siqueira F, Rocha J, Szesz AL, Anwar M, El-Askary F, Reis A, Loguercio A. Influence of conditioning time of universal adhesives on adhesive properties and enamel-etching pattern. Oper Dent. 2016; 41(5): 481–490.
38. Loguercio AD, Muñoz MA, Luque-Martinez I, Hass V, Reis A, Perdigão J. Does active application of universal adhesives to enamel in self-etch mode improve their performance? J Dent. 2015; 43(9): 1060–1070.
39. Elkaffas AA, Hamama HHH, Mahmoud SH. Do universal adhesives promote bonding to dentin? A systematic review and meta-analysis. Restor Dent Endod. 2018; 43(3): e29.
40. Manfroi FB, Marcondes ML, Somacal DC, Borges GA, Júnior LHB, Spohr AM. Bond strength of a novel one bottle multi-mode adhesive to human dentin after six months of storage. Open Dent J. 2016; 10 : 268–277.
41. Marchesi G, Frassetto A, Mazzoni A, Apolonio F, Diolosà M, Cadenaro M, Di Lenarda R, Pashley DH, Tay F, Breschi L. Adhesive performance of a multi-mode adhesive system: 1-year in vitro study. J Dent. 2014; 42(5): 603–612.
42. Lezaja Zebic M, Dzeletovic B, Miletic V. Microtensile bond strength of universal adhesives to flat versus Class I cavity dentin with pulpal pressure simulation. J Esthet Restor Dent. 2018; 30(3): 240–248.
43. Hanabusa M, Mine A, Kuboki T, Momoi Y, Van Ende A, Van Meerbeek B, De Munck J. Bonding effectiveness of a new ‘multi-mode’ adhesive to enamel and dentine. J Dent. 2012; 40(6): 475–484.
44. Perdigao J. Dentin bonding-variables related to the clinical situation and the substrate treatment. Dent Mater. 2010; 26(2): e24–e37.
45. Thanatvarakorn O, Prasansuttiporn T, Takahashi M, Thittaweerat S, Foxton RM, Ichinose S, Tagami J, Nakajima M. Effect of scrubbing technique with mild self-etching adhesives on dentin bond strengths and nanoleakage expression. J Adhes Dent. 2016; 18(3): 197–204.
46. Moritake N, Takamizawa T, Ishii R, Tsujimoto A, Barkmeier WW, Latta MA, Miyazaki M. Effect of active application on bond durability of universal adhesives. Oper Dent. 2019; 44(2): 188–199.
47. Huang XQ, Pucci CR, Luo T, Breschi L, Pashley DH, Niu LN, Tay FR. No-waiting dentine self-etch concept-Merit or hype. J Dent. 2017; 62 : 54–63.
48. Saikaew P, Matsumoto M, Chowdhury A, Carvalho RM, Sano H. Does shortened application time affect long-term bond strength of universal adhesives to dentin? Oper Dent. 2018; 43(5): 549–558.
49. Fu J, Saikaew P, Kawano S, Carvalho RM, Hannig M, Sano H, Selimovic D. Effect of air-blowing duration on the bond strength of current one-step adhesives to dentin. Dent Mater. 2017; 33(8): 895–903.
50. Taguchi K, Hosaka K, Ikeda M, Kishikawa R, Foxton R, Nakajima M, Tagami J. The effect of warm air-blowing on the microtensile bond strength of one-step self-etch adhesives to root canal dentin. J Prosthodont Res. 2018; 62(3): 330–336.
51. Hosaka K, Prasansuttiporn T, Thanatvarakorn O, Kunawarote S, Takahashi M, Foxton RM, Tagami J, Nakajima M. Smear layer-deproteinization: Improving the adhesion of self-etch adhesive systems to caries-affected dentin. Curr Oral Health Rep. 2018; 5(3): 169–177.
52. Prasansuttiporn T, Nakajima M, Kunawarote S, Foxton RM, Tagami J. Effect of reducing agents on bond strength to NaOCl-treated dentin. Dent Mater. 2011; 27(3): 229–234.
53. Tichy A, Hosaka K, Bradna P, Ikeda M, Abdou A, Nakajima M, Tagami J. Subsequent application of bonding agents to a one-step self-etch adhesive – Its effect with/without previous light-curing. Dent Mater. 2019; 35(12): e299–e309.
54. Ermis RB, Ugurlu M, Ahmed MH, Van Meerbeek B. Universal adhesives benefit from an extra hydrophobic adhesive layer when light cured beforehand. J Adhes Dent. 2019; 21(2): 179–188.
55. Sezinando A, Luque-Martinez I, Muñoz MA, Reis A, Loguercio AD, Perdigão J. Influence of a hydrophobic resin coating on the immediate and 6-month dentin bonding of three universal adhesives. Dent Mater. 2015; 31(10): e236–e246.
56. Reis A, Albuquerque M, Pegoraro M, Mattei G, Bauer JRdO, Grande RHM, Klein-Junior CA, Baumhardt-Neto R, Loguercio AD. Can the durability of one-step self-etch adhesives be improved by double application or by an extra layer of hydrophobic resin? J Dent. 2008; 36(5): 309–315.
57. Özcan M, Vallittu PK. Effect of surface conditioning methods on the bond strength of luting cement to ceramics. Dent Mater. 2003; 19(8): 725–731.
58. Tian T, Tsoi JKH, Matinlinna JP, Burrow MF. Aspects of bonding between resin luting cements and glass ceramic materials. Dent Mater. 2014; 30(7): e147–e162.
59. Matinlinna JP, Lung CYK, Tsoi JKH. Silane adhesion mechanism in dental applications and surface treatments: A review. Dent Mater. 2018; 34(1): 13–28.
60. Yoshihara K, Nagaoka N, Sonoda A, Maruo Y, Makita Y, Okihara T, Irie M, Yoshida Y, Van Meerbeek B. Effectiveness and stability of silane coupling agent incorporated in ‘universal’ adhesives. Dent Mater. 2016; 32(10): 1218–1225.
61. Yao C, Yu J, Wang Y, Tang C, Huang C. Acidic pH weakens the bonding effectiveness of silane contained in universal adhesives. Dent Mater. 2018; 34(5): 809–818.
62. Moro AFV, Ramos AB, Rocha GM, Perez CDR. Effect of prior silane application on the bond strength of a universal adhesive to a lithium disilicate ceramic. J Prosthet Dent. 2017; 118(5): 666–671.
63. Cardenas AM, Siqueira F, Hass V, Malaquias P, Gutierrez MF, Reis A, Perdigão J, Loguercio A. Effect of MDP-containing silane and adhesive used alone or in combination on the long-term bond strength and chemical interaction with lithium disilicate ceramics. J Adhes Dent. 2017; 19(3): 203–212.
64. Tzanakakis EGC, Tzoutzas IG, Koidis PT. Is there a potential for durable adhesion to zirconia restorations? A systematic review. J Prosthet Dent. 2016; 115(1): 9–19.
65. Inokoshi M, De Munck J, Minakuchi S, Van Meerbeek B. Meta-analysis of bonding effectiveness to zirconia ceramics. J Dent Res. 2014; 93(4): 329–334.
66. Seabra B, Arantes-Oliveira S, Portugal J.Influence of multimode universal adhesives and zirconia primer application techniques on zirconia repair. J Prosthet Dent. 2014; 112(2): 182–187.
67. Xie H, Li Q, Zhang F, Lu Y, Tay FR, Qian M, Chen C. Comparison of resin bonding improvements to zirconia between one-bottle universal adhesives and tribochemical silica coating, which is better? Dent Mater. 2016; 32(3): 403–411.
68. Sharafeddin F, Shoale S. Effects of universal and conventional MDP primers on the shear bond strength of zirconia ceramic and nanofilled composite resin. J Dent (Shiraz). 2018; 19(1): 48–56.
69. Yang X, Liu Y. Influence of different surface treatments on zirconia/resin shear bond strength using one-bottle universal adhesive. Adv Appl Ceram. 2019; 118(1–2): 70–77.
70. Amaral M, Belli R, Cesar PF, Valandro LF, Petschelt A, Lohbauer U. The potential of novel primers and universal adhesives to bond to zirconia. J Dent. 2014; 42(1): 90–98.
71. Ferracane JL. Resin composite – State of the art. Dental Materials. 2011; 27(1): 29–38.
72. Özcan M, Koc-Dundar B. Composite-composite adhesion in dentistry: a systematic review and meta-analysis. J Adhes Sci Technol. 2014; 28(21): 2209–2229.
73. Valente LL, Sarkis-Onofre R, Gonçalves AP, Fernández E, Loomans B, Moraes RR. Repair bond strength of dental composites: systematic review and meta-analysis. Int J Adhes Adhes. 2016; 69 : 15–26.
74. Comba L, Bradna P, Dudek M, Fialova V, Duskova J, Housova D. The effect of surface treatment on composite repair bond strength longevity. Czech Dent J. 2012; 112(2): 36–46.
75. Rinastiti M, Özcan M, Siswomihardjo W, Busscher HJ. Immediate repair bond strengths of microhybrid, nanohybrid and nanofilled composites after different surface treatments. J Dent. 2010; 38(1): 29–38.
76. Tantbirojn D, Fernando C, Versluis A. Failure strengths of composite additions and repairs. Oper Dent. 2014; 40(4): 364–371.
77. Altinci P, Mutluay M, Tezvergil-Mutluay A. Repair bond strength of nanohybrid composite resins with a universal adhesive. Acta Odontol Scand. 2018; 4(1): 10–19.
78. Çakir NN, Demirbuga S, Balkaya H, Karadaş M. Bonding performance of universal adhesives on composite repairs, with or without silane application. J Conserv Dent. 2018; 21(3): 263–268.
79. Fornazari I, Wille I, Meda E, Brum R, Souza E. Effect of surface treatment, silane, and universal adhesive on microshear bond strength of nanofilled composite repairs. Oper Dent. 2017; 42(4): 367–374.
80. Tay FR, Pashley DH, Yiu CK, Sanares AM, Wei SH. Factors contributing to the incompatibility between simplified-step adhesives and chemically-cured or dual-cured composites. Part I. Single-step self-etching adhesive. J Adhes Dent. 2003; 5(1): 27–40.
81. Michaud PL, MacKenzie A. Compatibility between dental adhesive systems and dual-polymerizing composite resins. J Prosthet Dent. 2016; 116(4): 597–602.
82. Kawano S, Fu J, Saikaew P, Chowdhury AFMA, Fukuzawa N, Kadowaki Y, Kakuda S, Hoshika S, Nakaoki Y, Ikeda T, Tanaka T, Sano H. Microtensile bond strength of a newly developed resin cement to dentin. Dent Mater J. 2015; 34(1): 61–69.
83. Kadowaki Y, Kakuda S, Kawano S, Katsumata A, Ting S, Hoshika S, Ikeda T, Tanaka T, Carvalho RM, Sano H. Bond performance of “Touch and Cure” adhesives on resin core systems. Dent Mater J. 2016; 35(3): 386–391.
84. Tagami A, Takahashi R, Nikaido T, Tagami J. The effect of curing conditions on the dentin bond strength of two dual-cure resin cements. J Prosthodont Res. 2017; 61(4): 412–418.
Labels
Maxillofacial surgery Orthodontics Dental medicine
Article was published inCzech Dental Journal

2020 Issue 1
Most read in this issue- The impact of neglect and failed treatment of early childhood caries
- Universal adhesives – a new direction in the development of adhesive systems
- Obstructive sleep apnea in relation to orthodontic treatment in children
- Profesor Zdeněk Broukal zemřel
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

