-
Medical journals
- Career
Antimycobacterial activity of novel derivatives of arylcarbonyloxyaminopropanols
Authors: S. Kečkéšová 1; E. Sedlárová 1; J. Čižmárik 1; V. Garaj 1; J. Csöllei 2; P. Mokrý 2; F. Andriamainty 1; I. Malík 1; J. Kaustová 3
Authors‘ workplace: Comenius University, Faculty of Pharmacy, Department of Pharmaceutical Chemistry, Bratislava 1; University of Veterinary and Pharmaceutical Sciences, Faculty of Pharmacy, Institute of Chemical Drugs, Brno 2; Regional Institute of Public Health, Ostrava 3
Published in: Čes. slov. Farm., 2009; 58, 203-207
Category: Original Articles
Overview
The study examined the antimicrobial activity of 2-hydroxy-3-[4-(2-fluoro, 4-fluoro, and 2‑metoxyphenyl)-piperazin-1-yl]-propyl-4-[(alkoxycarbonyl)amino] benzoates, their salts with hydrochloric acid, with one to four carbon atoms in the alkoxy group of the carbamoyl group studied and described. The series of these substances were evaluated in vitro against Mycobacterium tuberculosis, Mycobacterium avium, Mycobacterium kansasii and against clinically isolated strains of Mycobacterium kansasii 6 509/96. The correlation of this activity with lipophilicity (log k’) was used to describe the structure-antimycobacterial activity relationships (QSARs). The tests have shown that practically all the substances under study possess antituberculotic activity. In vitro antimycobacterial activity increased with increasing lipophilicity. According to the obtained results, the substances can be considered as potential antituberculotics.
Key words:
antimycobacterial activity – antituberculotic activity – potential antituberculotics – arylcarbonyloxyaminopropanols – lipophilicity – phenylcarbamatesIntroduction
In consideration of minutely increasing counts of the multiresistant strains of Mycobacterium tuberculosis, the design, preparation and study of new potential antituberculotics with structures different from those used in clinical praxis nowadays, belongs to the topical tasks of the drug research and development.
The task is really topical because there is also another source of the threat for European inhabitants – the occasionally pathogenic mycobacterial strains, such as Mycobacterium avium, Mycobacterium kansasii and Mycobacterium intercellulare 1).
The paper 1) described the antimycobacterial activity of 2-(4-alkylpiperazin-yl)-ethyl esters of 2-heptyloxyphenylcarbamic acid. The series of these substances was evaluated in vitro against Mycobacterium tuberculosis, Mycobacterium kansasii, Mycobacterium avium and against clinically isolated strains of Mycobacterium kansasii 6 509/96. According to the obtained results, all the studied substances possessed antituberculotic activity. The substances under study have in their chemical structure a fragment of substituted ester of phenylcarbamic acid and 4-substituted piperazine. These two groups are present in the studied structures and participate in the formation of some common metabolites.
The aim of this paper was to verify whether the studied structures exerted an antimycobacterial effect against hereinbefore mentioned strains.
EXPERIMENTAL PART
Synthesis
All of the compounds under study were prepared according to 2) and published in the papers 3,4). The structure of the substances studied is given in Table 1.
1. Chemical structure of the compounds under study 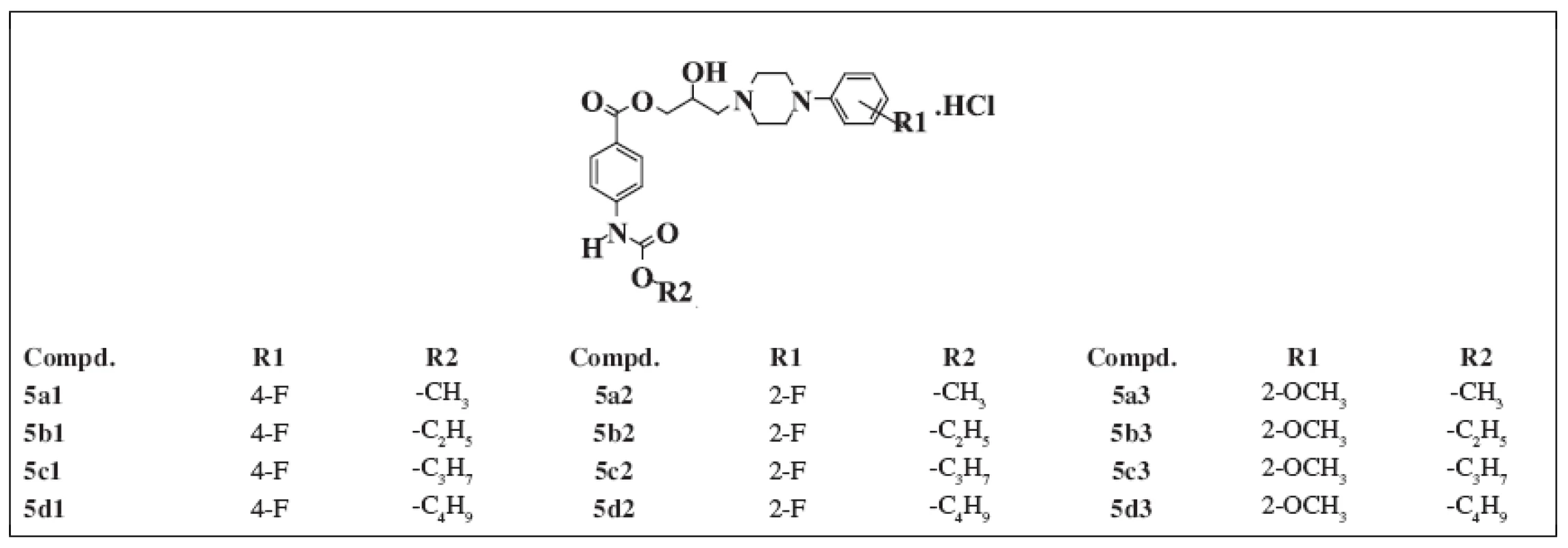
Microbiology
For the evaluation of the antimycobacterial activity in vitro, the following strains were used: Mycobacterium tuberculosis CNCTC My 331/88, Mycobacterium avium CNCTC My 330/88, Mycobacterium kansasii CNCTC My 235/80 obtained from the Czech National Collection of Type Cultures (CNCTC), National Institute of Public Health, Prague, and the clinically isolated strain of Mycobacterium kansasii 6 509/96. Antimycobacterial activity was determined in Šula semisynthetic medium (SEVAC, Prague). The substances were added to the medium in a Me2SO solution. The final concentrations of the compounds were 1–500 μmol/l. The minimum inhibitory concentration (MIC, i.e. the lowest concentration of a substance at which the inhibition of growth occurred) 5) was determined after incubation at 37°C for 14 and 21 days. The results are summarized in Table 2. The antituberculotic activity of isoniazide was used as the reference standard.
2. The minimum inhibitory concentration (MIC) and the logarithm of the retention factor k (log k) of the substances under study 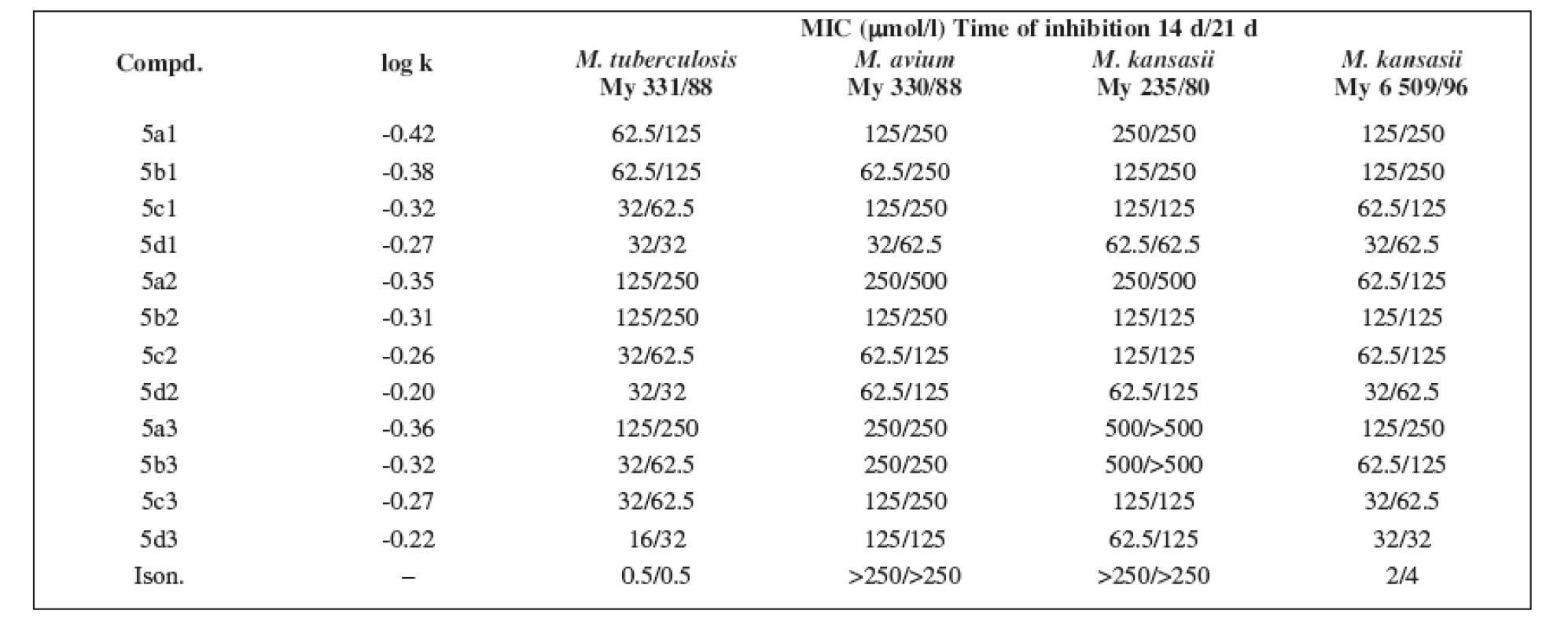
Determination of lipophilic properties
Since many years, lipophilicity is recognized as a meaningful parameter in many structure-activity and structure-ADME relationships. It is also the single most informative and successful physicochemical property in medicinal chemistry 6).
The chromatographic system for HPLC analysis for the retention factor k determination consisted of a pump Delta Chrom SDS (Watrex, Slovakia) with an injection valve and a UV detector Delta Chrom UVD 200 (Watrex, Slovakia). The analytical chromatography column was a Sepharon SGX C18 (254 × 4 mm, particle size 7 μm). The mobile phase was a mixture of 94 % methanol in an amount of 500 ml and 3.4 g of natrium acetate, the pH value was adjusted with acetic acid to pH = 6.0. The flow rate of the mobile phase was 0.6 ml.min-1, the injection volume was 1.10-2 ml, the chromatograms were scanned at the wavelength of 274 nm. The solution of NaNO2 (c = 0.1 mol.l-1) was used to determine the dead time t, and the solution of the analyzed compound in methanol medium (c = 0.4 . 10 3 mol.l 1) was used for the determination of tR.
The quantitative structure-activity relationships (QSARs)
The quantitative structure-activity relationships were determined according to two computing models of linear regression (log MIC = a log k’ + b) and parabolic regression (log MIC = a (log k’)2 + b (log k’)2 + c). Both types of the calculation were carried out using the Microsoft Excel program. The results are summarized in Tables 3–8.
3. Linear regressions (5a1–5d1 derivatives) 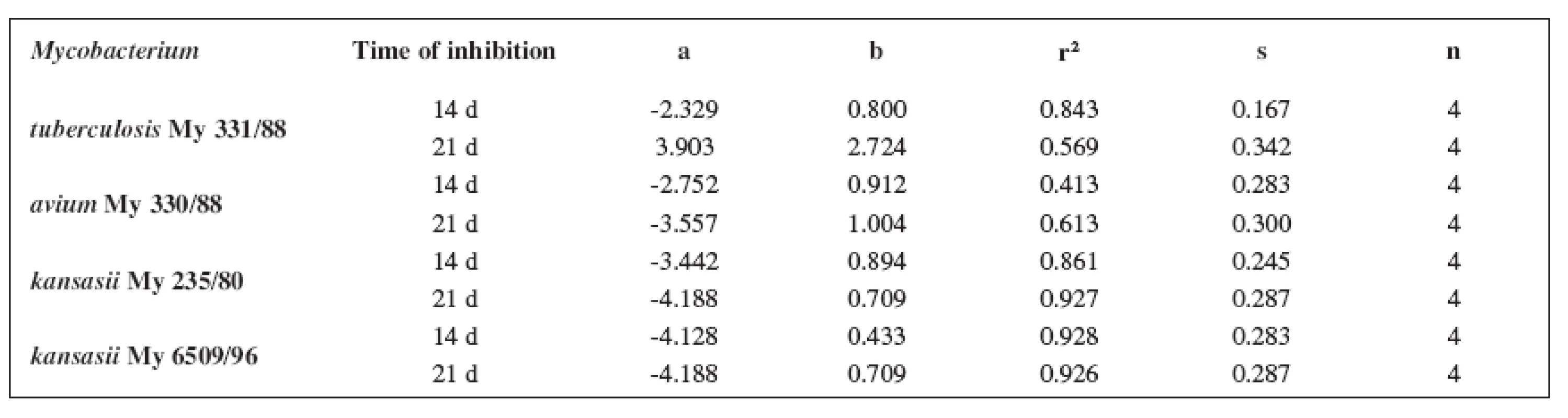
4. Parabolic regressions (5a1–5d1 derivatives) 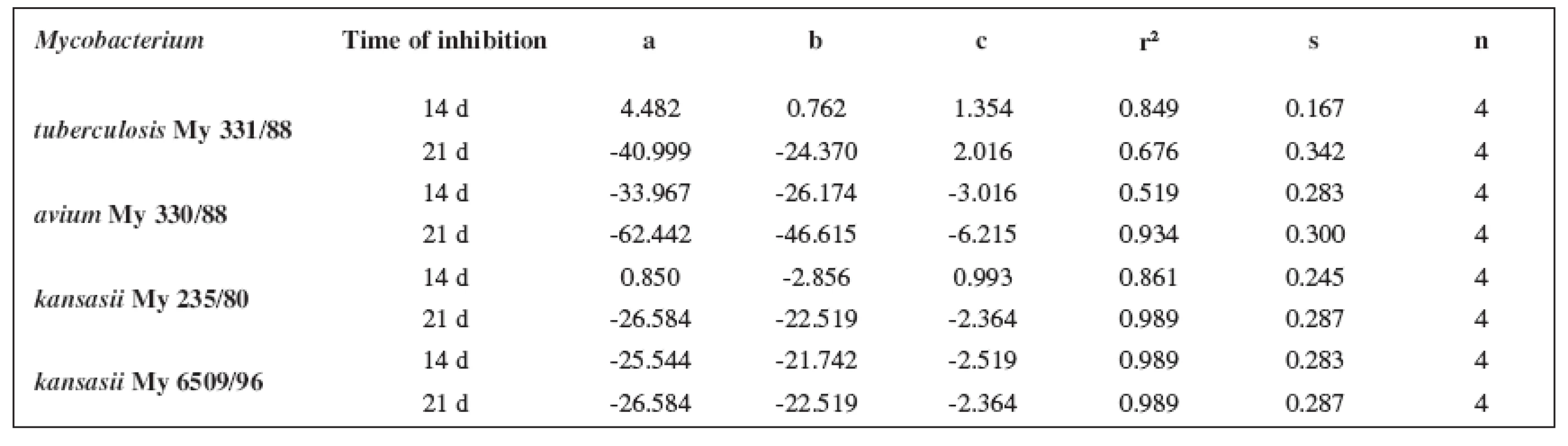
5. Linear regressions (5a2–5d2 derivatives) 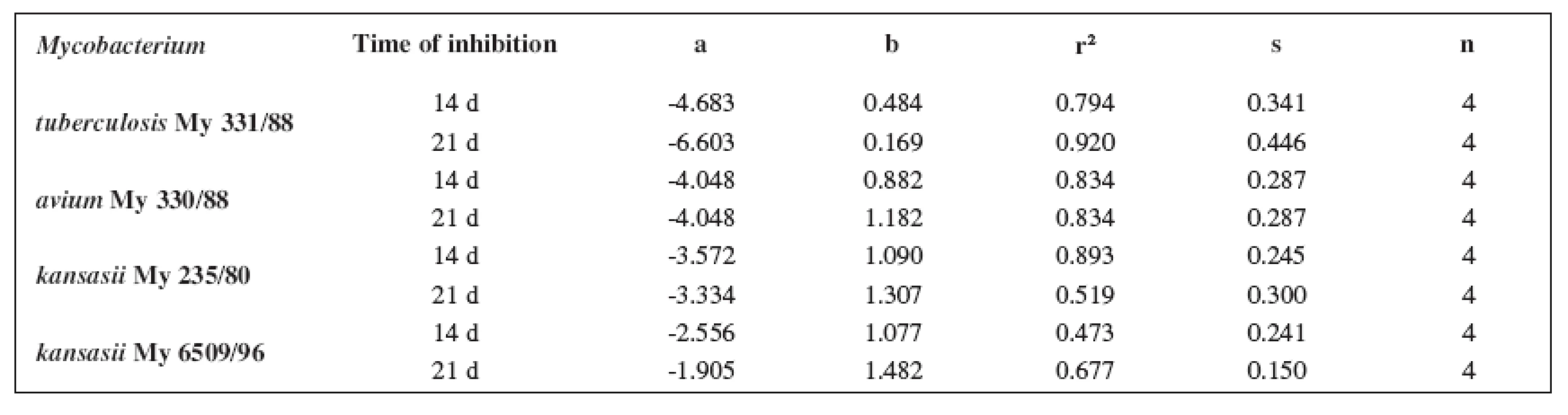
6. Parabolic regressions (5a2–5d2 derivatives) 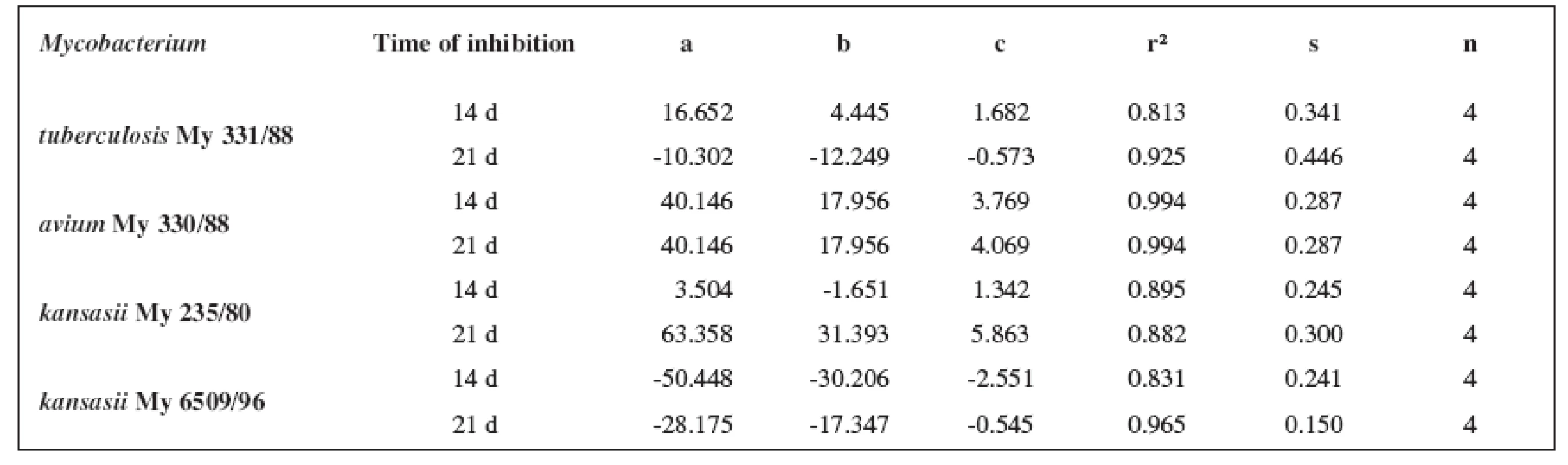
7. Linear regressions (5a3–5d3 derivatives) 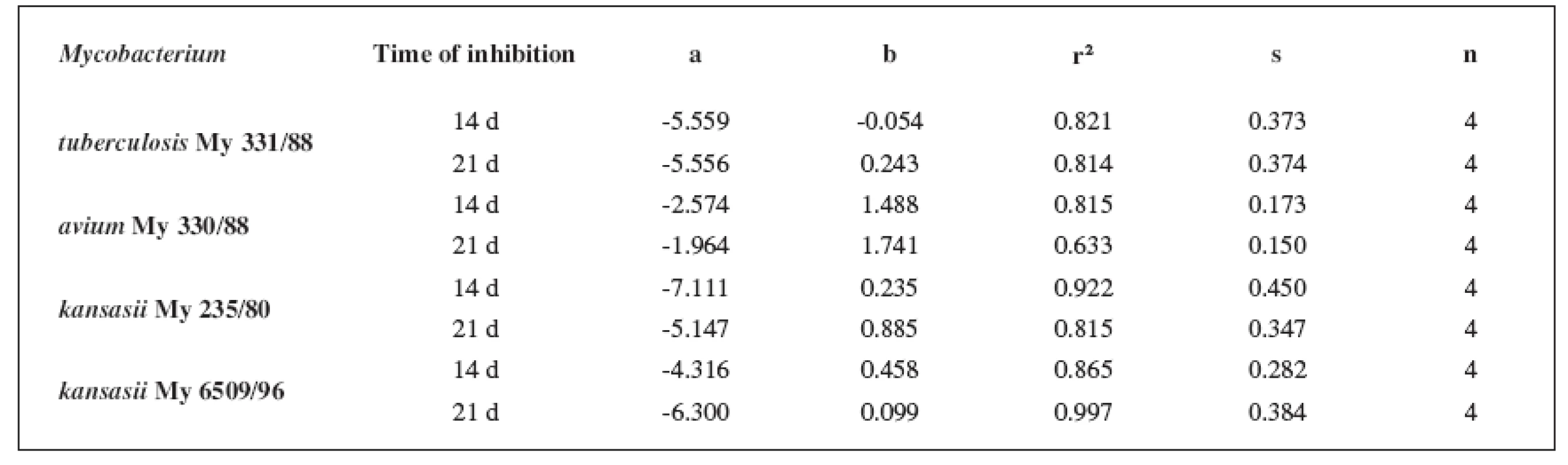
8. Parabolic regressions (5a3–5d3 derivatives) 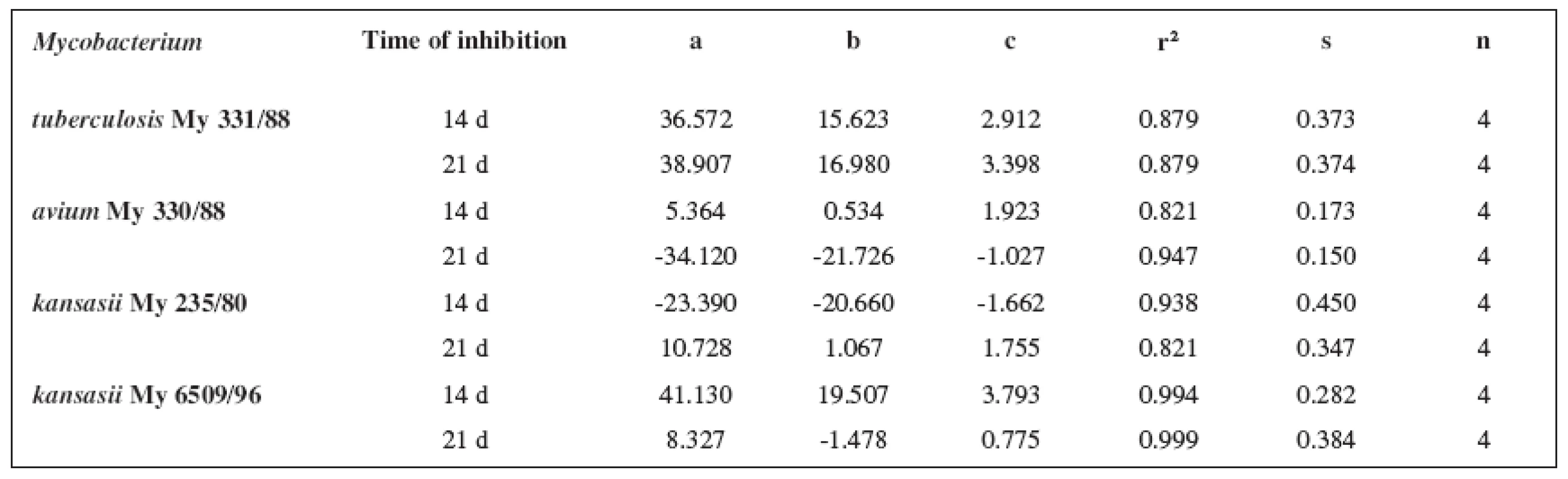
RESULTS AND DISCUSSION
The paper focused on the antimicrobial activity of the new compounds.
The logarithm of the retention factor k, like the lipophilicity parameter, was obtained by the HPLC method. The log k values of the substances under evaluation increased with the increasing number of carbon atoms in the alkoxy chain of the carbamoyl group. The results of the analysis confirm that the activity of the compounds increases with increasing lipophilicity.
The conclusions indicate that practically all of the derivatives show expected antimycobacterial activity in consideration of all of the strains studied, and some of them are more efficient than the standard isoniazide.
This study was supported by the VEGA grant No. 1/4291/07.
Received 5 October 2009 / Accepted 4 November 2009
Address for correspodence:
PharmDr. Simona Kečkéšová, PhD.
Comenius University, Faculty of Pharmacy, Department of Pharmaceutical Chemistry
Odbojárov 10, 832 32 Bratislava, SR
e-mail: skeckesova@yahoo.co.uk
Sources
1. Čižmárik, J., Waisser, K., Doležal, R., Kaustová, J.: Farm. Obzor, 2007; 76, 144–146.
2. Mokrý, P., Zemanová, M., Csöllei, J., Račanská, E., Tumová, I.: Pharmazie, 2003; 58, 18–21.
3. Kečkéšová, S., Sedlárová, E., Csöllei, J., Mokrý, P.: Čes. a Slov. Farm., 2008; 57, 160–164.
4. Kečkéšová, S., Sedlárová, E., Csöllei, J., Mokrý, P., Vančo, J., Vanko, M.: Acta Facult. Pharm. Univ. Com., 2008; 55, 122–128.
5. Waisser, K., Dražková, K., Čižmárik, J., Kaustová, J.: Folia Microbiol., 2003; 48, 45–50.
6. Malík, I., Sedlárová, E., Csöllei, J., Andriamainty, F., Čižmárik, J., Kečkéšová, S.: Acta Facult. Pharm. Univ. Com., 2007; 54, 136–145.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2009 Issue 5-6-
All articles in this issue
- Methods of preparation of microparticles in pharmaceutical technology
- Hypolipidemic effect of amaranth constituents
- Influence of formulation technology on theophylline release from chitosan-based pellets
- Monitoring of surface cytotoxic drugs in the environment of hospital pharmacies in the Czech Republic
- Contributions to the development of advertising in pharmacy I
- A contribution to the histories of the pharmacies of the Brethren of Mercy in the territory of contemporary Slovakia in the first decades of the 20th century
- Examination of selected quality parameters of the parenteral nutrition AIO – a pilot study
- Antimycobacterial activity of novel derivatives of arylcarbonyloxyaminopropanols
- Comparison of renal accumulation of [DOTA0, 1-Nal3]-octreotide labelled with selected radiometals
- Adhesivity of branched plasticized oligoesters
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Methods of preparation of microparticles in pharmaceutical technology
- Contributions to the development of advertising in pharmacy I
- Examination of selected quality parameters of the parenteral nutrition AIO – a pilot study
- Hypolipidemic effect of amaranth constituents
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

