-
Medical journals
- Career
Multimodal Imaging of Choroidal Nodules in Neurofibromatosis Type I
Authors: Filip Kecer; Ana Sharashidze; Veronika Popová; Beáta Bušányová; Anton Gerinec; Dana Tomčíková
Authors‘ workplace: Klinika detskej oftalmológie Národného ústavu detských chorôb a Lekárskej fakulty Univerzity Komenského v Bratislave
Published in: Čes. a slov. Oftal., 80, 2024, No. 2, p. 86-92
Category: Original Article
doi: https://doi.org/10.31348/2024/9Overview
Aim: To clarify the possibilities and role of posterior segment imaging in patients with neurofibromatosis type I (NF1), and to show the prevalence of this disease in the pediatric population in Slovakia.
Material and methods: Until recently, ophthalmologic consultations in patients with NF1 were limited mainly to the observation of Lisch nodules of the iris and the presence of optic nerve glioma. However, advances in imaging capabilities have made it possible to investigate and describe new f indings concerning the ocular manifestations of this disease. Between October 2020 and November 2021, we examined the anterior and posterior segment of 76 eyes (38 children – 12 boys and 26 girls) with genetically confirmed NF1 gene mutation at our clinic. The age of the patients ranged from 4 to 18 years. The anterior segment was checked for the presence of Lisch nodules biomicroscopically with a slit lamp. On the posterior segment, the presence of choroidal nodules was checked by various imaging methods – fundus camera, infrared confocal selective laser ophthalmoscopy, MultiColor imaging, OCT, and OCT angiography. All the patients had magnetic resonance imaging performed in order to detect potential optic nerve gliomas for the purpose of diagnosis. We observed the correlation between the patients’ age, presence of Lisch nodules and the presence of choroidal nodules. Eight patients also had other manifestations of the disease – optic nerve gliomas or microvascular changes (so-called “corkscrew” vessels).
Results: Out of 38 patients, Lisch iris nodules were present in 20 patients (53%) and choroidal nodules in 24 patients (63%). There was no positive correlation between the presence of these two manifestations within the same patient or eye, but there is a clear correlation between the presence of choroidal nodules and patient age.
Conclusion: The results suggest that a previously unknown ocular manifestation of neurofibromatosis type I, namely choroidal nodules, has a higher prevalence than Lisch nodules also in the pediatric population and can be easily visualized using various imaging modalities. It will be important to include follow-up observation of this finding among the standard controls for ocular findings in NF1, and it will be very interesting to correlate this f inding with the exact NF1 mutation
Keywords:
Neurofibromatosis type 1 – von Recklinghausen’s disease – choroidea – choroidal nodules – imaging modalities
INTRODUCTION
Neurofibromatosis type I is one of the most common autosomal dominant disorders (incidence 1 : 2500–3000), with potentially severe complications, a progressive character and variable clinical manifestation [1,2]. It is caused by pathogenic variants in the NF1 gene in chromosome 17q11.2, and is characterized by skin pigment anomalies such as café-au-lait spots, freckling in the axillary and inguinal regions, or cutaneous neurofibromas. Furthermore, these patients may suffer from learning or concentration disorders, hyperactivity, skeletal abnormalities or plexiform neurofibromas [3]. The first internationally recognized classification of neurofibromatosis originates from the National Institute of Health Consensus Conference from 1987, where the diagnostic criteria for neurofibromatosis type I were also defined as follows [4]:
- Six or more café-au-lait spots, > 15 mm in adults, > 5 mm in children
- Two or more neurofibromas or one plexiform neurofibroma
- Freckling in the axillary and inguinal regions
- Two or more Lisch nodules of the iris
- Glioma of the optic nerve
- First-degree relative with diagnosed NF1 disorder
- Characteristic skeletal dysplasia (e.g. tibial pseudoarthrosis or anterolateral bowing of the tibia [4]
For determination of the diagnosis it is necessary for the individual to meet at least two of the above seven criteria [5]. With regard to ocular manifestations this concerns Lisch nodules of the iris (Fig. 1) and optic nerve glioma. Lisch nodules are hamartomas of the iris which are well visible with the use of a slit lamp. Initially they are pale, with age they grow darker and increase in number. They appear in childhood (though only in a small percentage of children aged under 6 years), but in adults with NF1 they are present in as many as 95% of cases. They can be distinguished from iris nevi by their three-dimensional appearance, resembling nodules. Lisch nodules of the iris do not cause disorders of vision [5,6]. Optic nerve glioma in individuals with NF1 is mostly asymptomatic, and remains so throughout the patient’s entire life [6]. The clinical course of optic nerve glioma linked to NF1 is usually milder than in isolated form, it is stable or with only slight progression [7]. Symptomatic optic nerve gliomas linked to NF1 are usually present before the sixth year of age, and cause a deterioration of central visual acuity, exophthalmos or strabismus [8].
The development of new imaging modalities has brought new and extended options for the diagnosis of ocular manifestations of this pathology. The first to describe choroidal abnormalities in patients with NF1, referred to at the time as “choroidal neurofibromatosis”, was Takahuru Yasunari in the year 2000. By examining the posterior segment of the eye of adult patients with NF1 with the aid of infrared (IR) imaging with a confocal scanning laser ophthalmoscope (cSLO), he determined the presence of multiple pale spots on the posterior pole and its surrounding area in all of the examined patients. In those patients on whom indocyanine green angiography (ICGA) was also performed, hyperfluorescent areas were found, corresponding to the finding of the pale flecks on IR imaging. By contrast, no abnormalities corresponding to this finding were determined in the patients examined by fluorescein angiography (FA) or standard fundoscopic examination [9].
Relatively new imaging modalities that we have used and shall describe later in the article also include MultiColor and OCT angiography. The MultiColor imaging modality uses simultaneously emitted laser beams in three different wavelengths (488 nm, 518 nm and 815 nm) for the purpose of imaging the fundus in various depths. The result is three monochromatic and one consolidated MultiColor image. OCT angiography is a non-invasive method, serving for imaging of the vascular system of the retina and choroidea.
Based on several publications focusing on this subject, in May 2021 an update of the diagnostic criteria for neurofibromatosis type I was published (Table 1), which supplements the criterion “two or more Lisch nodules of the iris” with the addition of “identified by a slit lamp, or two or more choroidal abnormalities defined as pale, spotted nodules displayed with the aid of optical coherence tomography or infrared imaging” [10]. The reason for classification of choroidal abnormalities as an ophthalmological criterion is their high sensitivity and specificity for NF1, and their large (60–70%) presence of manifestation in pediatric patients [11], which even exceeds the presence of Lisch nodules of the iris in this group of patients [12].
MATERIAL AND METHOD
In the period from October 2020 to November 2021, a total of 38 children (76 eyes) were examined at our clinic, of whom 12 were boys and 26 were girls, referred from another center for the purpose of conducting an ophthalmological consultation. All these patients had genetically confirmed mutation of the NF1 gene. They were examined with the use of a slit lamp for the purpose of determining the presence of Lisch nodules of the iris, and subsequently, following the application of mydriatic agents, the ocular fundus was also examined. Since patient cooperation was important, the youngest patient included in the group was aged 4 years, the oldest 18 years. The posterior segment was scanned with cSLO (IR and MultiColor imaging), optical coherence tomography and OCT angiography (Spectralis, Heidelberg Engineering). The aim of these examinations was to determine the presence and frequency of ocular manifestations of neurofibromatosis type 1 in the pediatric population in Slovakia with reference to choroidal nodules. We intended to identify whether a correlation exists between the presence of these two manifestations within the individual or eye, or between their presence and the age of the individual. At the same time, relatively new imaging methods were used – MultiColor and OCT angiography.
On each patient we used infrared (815 nm) cSLO imaging with a standard 30° and wide-angle 55° lens, with simultaneous SD-OCT scanning of the posterior pole. If hyperreflectivity resembling a choroidal nodule was present, we proceeded to a closer examination with the use of the MultiColor modality, which enables us to obtain a view of various different levels of the retina with the use of different wavelengths during scanning, helping us to achieve rapid localization of the origin of the hyperreflective signal into the retina or choroidea (Fig. 2). The region of the signal was then scanned with a dense OCT pattern, most frequently with dimensions of 25° x 5° and a maximum distance of 60 µm between the B-scans. At the same time, EDI (Enhanced Depth Imaging) was used in each OCT scan in order to attain increased sensitivity of imaging of deep structures (Fig. 3). OCT angiography was performed in various scan sizes according to necessity, in order to obtain additional information about vascular changes within the choroidea (Fig. 4).
In order to enable timely identification of structural changes of the optic nerve disc due to the potential incidence of gliomas in these patients, each of them was examined as standard with regard to the BMO-MRW parameter (Bruchs Membrane Opening – Minimal Rim Width) and retinal nerve fiber layer thickness with a 12° peripapillary RNFL (Retinal Nerve Fiber Layer) scan.
We divided our patients into three age groups – (A) from 4 to 8 years (n = 8), (B) from 9 to 13 years (n = 18) and (C) from 14 to 18 years (n = 13). We used basic statistical methods for the data processing.
1. Lisch nodules of the iris can be distinguished from nevi by their three-dimensional appearance 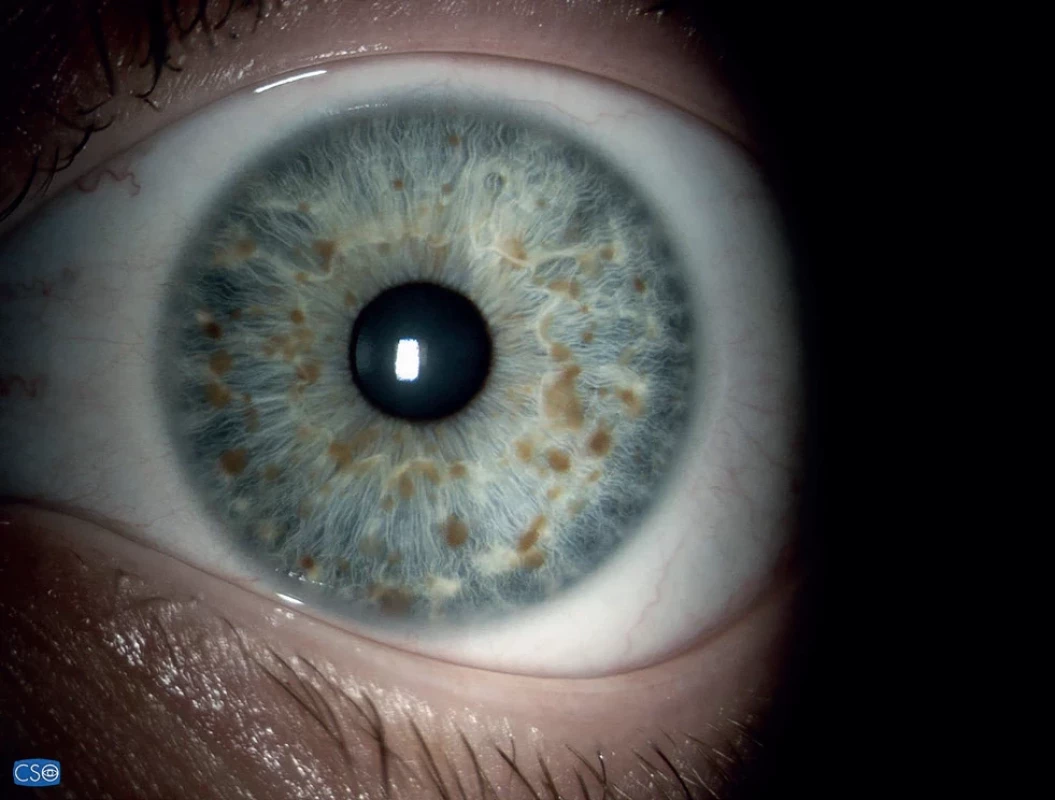
Table 1. Revised diagnostic criteria for neurofibromatosis type I
A. The diagnostic criteria for NF1 are met in an individual who does not have a parent diagnosed with NF1 if two or more of the following are present:
• six or more café-au-lait macules over 5 mm in greatest diameter in prepubertal individuals and over 15 mm in greatest diameter in postpubertal individuals
• freckling in the axillary or inguinal region
• two or more neurofibromas of any type or one plexiform neurofibroma
• optic pathway glioma
• two or more iris Lisch nodules identified by slit lamp examination or two or more choroidal abnormalities defined as bright, patchy nodules imaged by optical coherence tomography/near-infrared reflectance imaging
• a distinctive osseous lesion such as sphenoid dysplasia, anterolateral bowing of the tibia, or pseudoarthrosis of a long bone
• a heterozygous pathogenic NF1 variant with a variant allele fraction of 50% in apparently normal tissue such as white blood cells
B. A child of a parent who meets the diagnostic criterias specified in A merits a diagnosis of NF1 if one or more of the criteria in A are present2. Comparison of visualization of choroidal nodules on pseudocolor MultiColor image 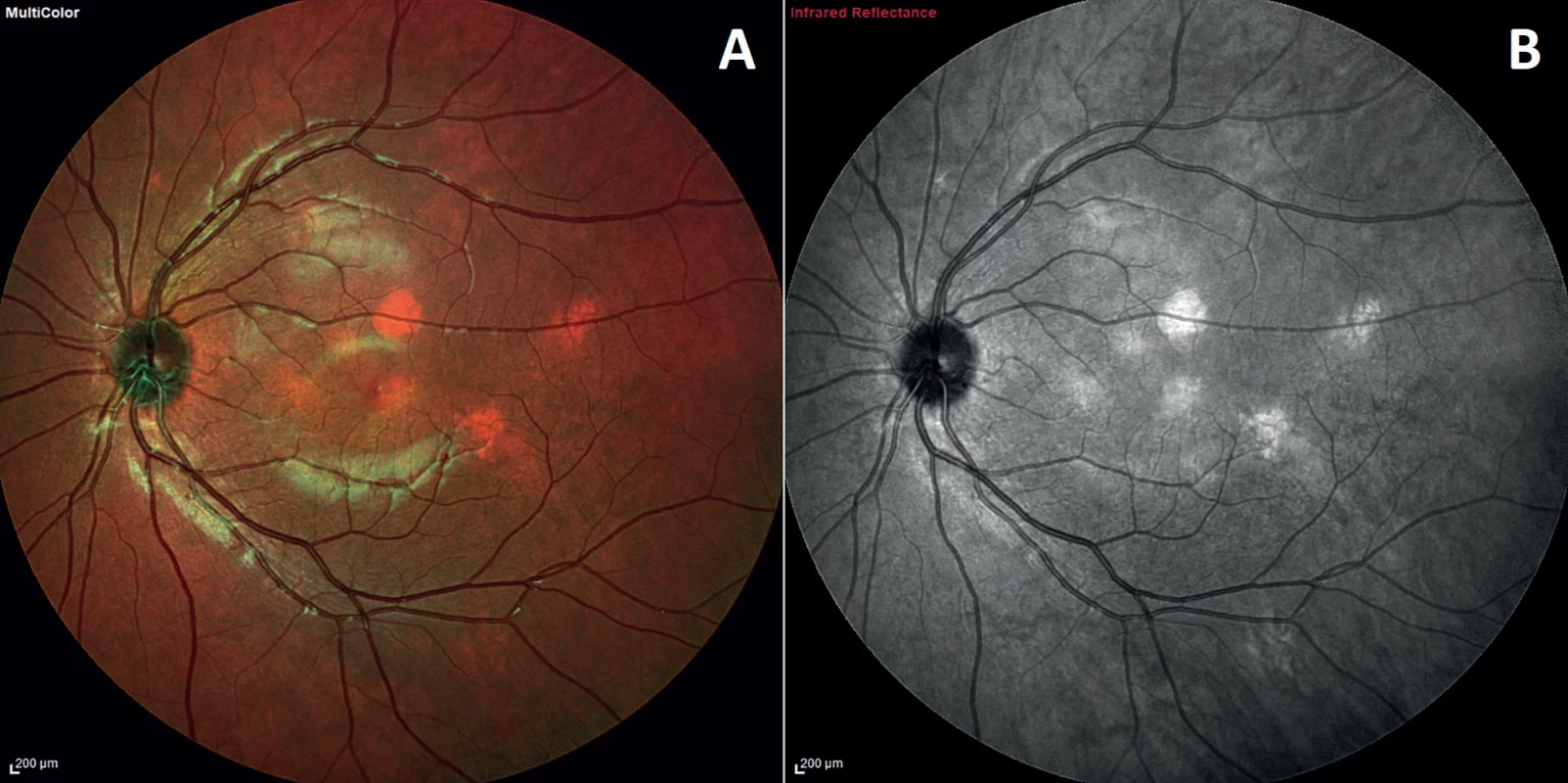
(A) and using infrared wavelength (B). Choroidal nodule can vary in shape or size, both bounded or diffuse, but occur exclusively on the posterior pole and around the optic nerve head 3. 55° IR image with OCT section through two choroidal nodules shows a hyperreflective lesion touching the choriocapillaris layer (between the blue arrows) and the other, embedded in the deep choroid (between yellow arrows) 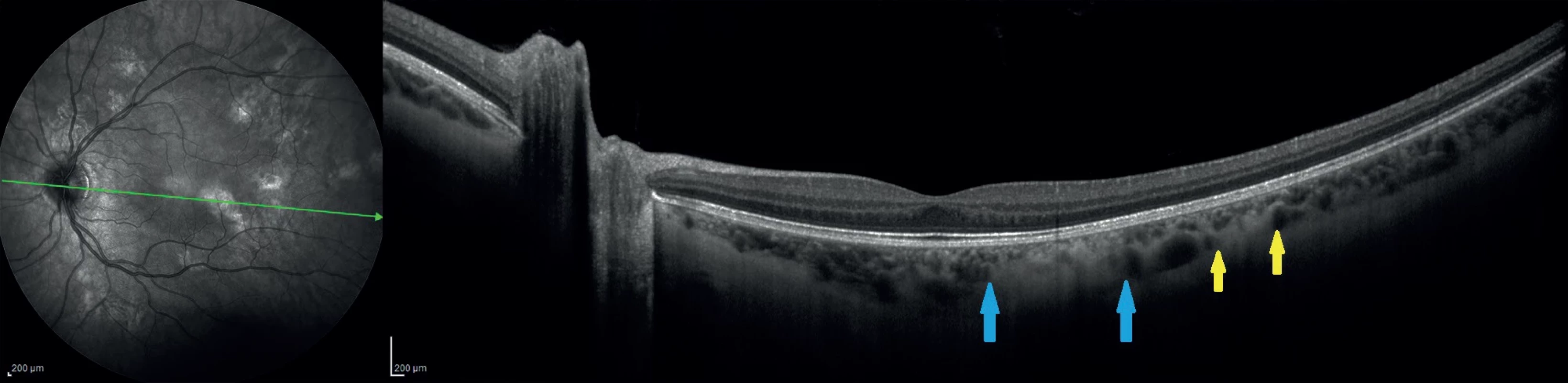
4. En-face image of the deep choroid derived from structural OCT 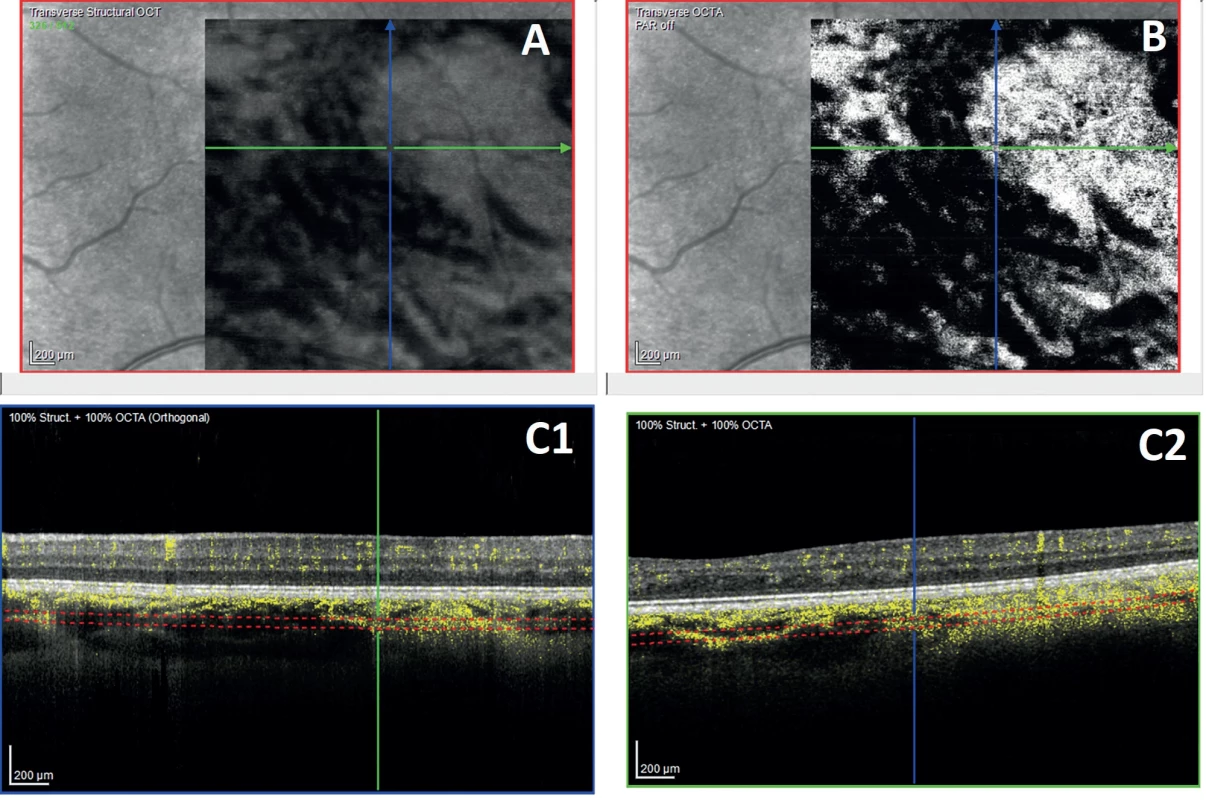
(A) and en-face image of OCTA (B) show hyperperfused areas corresponding to the area of the choroidal nodule. Areas marked in yellow on the vertical and horizontal B scans (C1, C2) indicate the signal originated from the perfused structures 1. Results of a comparison of the incidence of choroidal nodules and Lisch nodules in the pediatric population in Slovakia 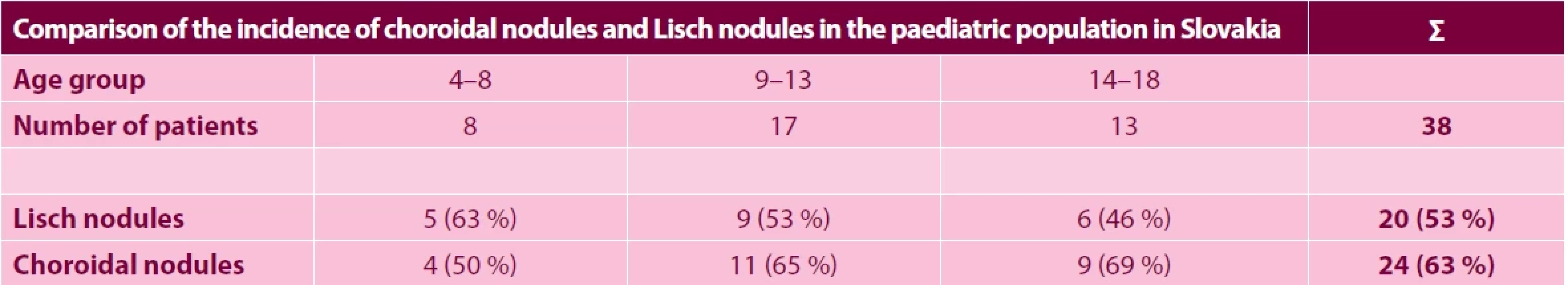
RESULTS
In the group with the youngest patients, both choroidal nodules and Lisch nodules of the iris were detected at the earliest at 6 years of age. Lisch nodules, as a long-term diagnostic criterion for NF1, were present in 5 children (63%) in group A, in 9 children (53%) in group B, and in 6 children (46%) in group C. Contrastingly, choroidal nodules as a new diagnostic criterion were present in 4 children (50%) in group A, 11 children (65%) in group B and 9 children (69%) in group C. In total, in our cohort of 38 this means 20 children (53%) with Lisch nodules and as many as 24 children (63%) with choroidal nodules (Table 2). No positive correlation was determined between the presence of these two manifestations within a single eye or patient, though there is evidently a correlation between choroidal nodules and patient age.
Secondarily, we were also interested in the presence of other ocular manifestations of NF1 in the pediatric population, namely optic never glioma, as well as microvascular changes on the retina (tortuosity) presented in a number of publications, referred to as corkscrew vessels. Glioma diagnosed on the basis of an MR examination was present in 5 children, of whom one was affected bilaterally. Tortuosity detected fundoscopically or on an SLO image was present in 6 children. However, its precise nature in connection with neurofibromatosis type I has not yet been clarified.
DISCUSSION
The currently available diagnostic instruments enable high-quality non-invasive imaging of various structures and processes within the retina, and enable the further study of hitherto unknown manifestations of a broad range of pathologies. One of these is choroidal abnormalities, so-called nodules, which were first described by Yasunari in the year 2000 [9], who was able to identify them with the aid of IR imaging and ICG angiography in all the examined adult patients with a diagnosis of NF1. This work has been followed up by further authors, who have described their presence in 82–100% of cases in adult patients [9,12], whereas in pediatric patients aged up to 12 years the ratio is 60.5–78.9% [11–13]. The first more significant publication focusing on the presence of choroidal manifestations of neurofibromatosis type I in the pediatric population was the study by Vagge et al. [11], which was conducted on a cohort of 78 pediatric patients with NF1 and compared with a control group of 96 healthy children. Choroidal nodules were present in 69.2% of children, whereas Lisch nodules were present in 48.7% of children. The youngest patient with a finding of choroidal nodules was only 2 years of age. This study also illustrated that the topographic distribution of choroidal nodules develops in connection with age.
Further interesting findings were presented by Cassiman et. al. in 2017 [14], and also by Tucci in the same year [15], who described that at least two choroidal nodules were always present in NF1 patients, and in 56% of cases more than four were present. This is important in differential diagnostics with Legius syndrome, which also has café-au-lait spots as an important diagnostic marker, and in childhood age the clinical picture may be impossible to differentiate. However, in Legius syndrome choroidal nodules appear in only 18% of cases, and never in a number greater than one.
CONCLUSION
Our results indicate that the presence of choroidal nodules increases with age and exceeds the incidence of Lisch nodules of the iris in the pediatric population, which corresponds with the findings in foreign publications. An advantage is that it is possible to display them with the aid of what are now commonly available non-invasive and safe imaging methods. However, at present we do not have knowledge of any available data on their incidence in toddlers and children up to two years of age, though for this purpose specific equipment is required which is not available as standard at ophthalmological centers. It would also be interesting to correlate this finding with the precise mutation of the NF1 gene.
In our results we did not succeed in demonstrating any progression in the finding of choroidal nodules, although such an endeavor would require a far larger cohort of patients and their long-term observation. We are prevented from conducting such a study by the irregularity of their visits to our eye clinic, since these patients are referred to us for consultancy examinations by dermatovenereologists or oncologists, with whom they are under observation. As a pediatric ophthalmology clinic we are limited by patient age, and as a result patients are removed from our records after reaching adulthood.
Inasmuch as these nodules are not of a uniform nature in terms of their shape, intensity, density or bordering, we consider it necessary to determine their classification, which may aid ophthalmologists or other specialists in describing findings more precisely, or to correlate them with other findings and better predict the development of the disease.
Ing. Filip Kecer
Klinika detskej oftalmológie NÚDCH a LF UK
Limbová 1
831 01 Bratislava
filip.kecer@gmail.comThe authors of the study declare that no conflict of interests exists in the compilation, theme and subsequent publication of this professional communication, and that it is not supported by any pharmaceuticals company. The authors further declare that study has not been submitted to any other journal or printed elsewhere, with the exception of congress abstracts.
Presented at the 16th Pediatric Ophthalmology Symposium in June 2023 (Bratislava) and at the 28th Congress of the Slovak Ophthalmology Society in October 2023 (High Tatras)
Submitted to the editorial board: November 18, 2023
Accepted for publication: December 13, 2023
Sources
- Bolcekova A, Nemethova M, Zatkova A, et al. Clustering of mutations in the 5' tertile of the NF1 gene in Slovakia patients with optic pathway glioma. Neoplasma. 2013;60(6):655-665. doi: 10.4149/neo_2013_084 PMID: 23906300
- Rybárová A, Ilenčíková D, Hlavatá A, Kovács L. Juraj s hnedými škvrnami a podkožnými uzlíkmi mal náhle bolesti brucha. Pediatr. Prax, 2011;12(1):33-34. Slovak.
- Kehrer-Sawatzki H, Cooper DN. Challenges in the diagnosis of neurofibromatosis type 1 (NF1) in young children facilitated by means of revised diagnostic criteria including genetic testing for pathogenic NF1 gene variants. Hum Genet. 2022 Feb;141(2):177-191. doi: 10.1007/s00439-021-02410-z
- Neurofibromatosis: Conference Statement. Arch Neurol. 1988;45(5):575-578. doi: 10.1001/archneur.1988.00520290115023
- Humhejová D, Petrák B. Neurofibromatóza z pohledu dermatologa. Čes-slov Derm. 2015;90(3):95-110. Czech.
- Friedman JM. Neurofibromatosis 1 [internet]. 1998 Oct 2 [Updated 2022 Apr 21]. In: Adam MP, Mirzaa GM, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1109
- Sellmer L, Marangoni M, Farschtschi S, et al. Serial MRIs provide novel insight into natural history of optic pathway gliomas in patients with neurofibromatosis 1. Orphanet J Rare Dis. 2018;13 : 62
- Friedrich RE, Nuding MA. Optic pathway glioma and cerebral focal abnormal signal intensity in patients with neurofibromatosis type 1: characteristics, treatment choices and follow-up in 134 affected individuals and a brief review of the literature. Anticancer Res. 2016 Aug;36(8):4095-4121.
- Yasunari T, Shiraki K, Hattori H, Miki T. Frequency of choroidal abnormalities in neurofibromatosis type 1. Lancet. 2000 Sep 16;356(9234):988-992. doi:10.1016/S0140-6736(00)02716-1
- Legius E, Messiaen L, Wolkenstein P, et al. International Consensus Group on Neurofibromatosis Diagnostic Criteria (I-NF-DC); Huson SM, Evans DG, Plotkin SR. Revised diagnostic criteria for neurofibromatosis type 1 and Legius syndrome: an international consensus recommendation. Genet Med. 2021 Aug;23(8):1506-1513. doi: 10.1038/s41436-021-01170-5
- Vagge A, Camicione P, Capris C, et al. Choroidal abnormalities in neurofibromatosis type 1 detected by near-infrared reflectance imaging in paediatric population. Acta Ophthalmol. 2015 Dec;93(8):e667-71. doi: 10.1111/aos.12750
- Viola F, Villani E, Natacci F, et al. Choroidal abnormalities detected by near-infrared reflectance imaging as a new diagnostic criterion for neurofibromatosis 1. Ophthalmology. 2012 Feb;119(2):369-375. doi: 10.1016/j.ophtha.2011.07.046
- Goktas S, Sakarya Y, Ozcimen M, et al. Frequency of choroidal abnormalities in pediatric patients with neurofibromatosis type 1. J Pediatr Ophthalmol Strabismus. 2014 Jul 1;51(4):204-208. doi: 10.3928/01913913-20140513-02
- Cassiman C, Casteels I, Jacob J, et al. Choroidal abnormalities in café-au-lait syndromes: a new diferential diagnostic tool? Clin Genet. 2017 Apr;91(4):529-535. doi: 10.1111/cge.12873
- Tucci A, Saletti V, Menni F, et al. The absence that makes the diference: choroidal abnormalities in Legius syndrome. J Hum Genet. 2017 Nov;62(11):1001-1004. doi: 10.1038/jhg.2017.78
Labels
Ophthalmology
Article was published inCzech and Slovak Ophthalmology
2024 Issue 2-
All articles in this issue
- Multimodal Imaging of Choroidal Nodules in Neurofibromatosis Type I
- Diagnostic Challenges of Ocular Rosacea
- Central Serous Chorioretinopathy. A Review
- Comparison of Early Vision Quality of SBL-2 and SBL-3 Segmented Refractive Lens
- The Impact of the COVID-19 Pandemic on the Quality of Examination in Eye Clinics in the Czech Republic – Questionnaire Study
- Kaposi’s Sarcoma. A Case Report
- Czech and Slovak Ophthalmology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Diagnostic Challenges of Ocular Rosacea
- Central Serous Chorioretinopathy. A Review
- Multimodal Imaging of Choroidal Nodules in Neurofibromatosis Type I
- Kaposi’s Sarcoma. A Case Report
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

