-
Medical journals
- Career
Repeated Multilevel Botulinum Toxin A Treatment Maintains Long-Term Walking Ability in Children with Cerebral Palsy
Authors: M. Svehlik 1,2; T. Kraus 1; G. Steinwender 1; E. B. Zwick 1; M. Ladecky 3; Z. Szabo 3; W. E. Linhart 1
Authors‘ workplace: Peadiatric Orthopaedic, Department of Paediatric Surgery, Medical University of Graz, Austria 1; Department of Paediatric and Adult Orthopaedics and Traumalology, 2nd Faculty of Medicine, Charles University Prague 2; Faculty of Biomedical Engineering, Czech Technical University in Prague, Kladno 3
Published in: Cesk Slov Neurol N 2012; 75/108(6): 737-741
Category: Short Communication
Overview
Aims:
Botulinum toxin type A (BoNT-A) offers a targeted form of therapy to reduce spasticity and has become a standard form of treatment in children with cerebral palsy (CP). However, the effects of repeated BoNT-A treatment over a longer periods of time are not well known. We hypothesized that children treated with multi-level, high-dose, repeated BoNT-A applications would not deteriorate in their gait function over a minimum period of four years.Materials and methodology:
Gait in 18 children with spastic diplegic CP, mean age 6 years 9 months, Gross Motor Function Classification System I–III, treated according to the concept of integrated BoNT-A application over a minimum of four years was evaluated using the kinematic and kinetic gait analysis. The main outcome measure was the Gait Deviation Index (GDI).Results:
The mean follow-up time was 5 years and 9 months (SD 1 year 5 months). Each child received a mean of 1.02 (SD 0.37) applications of BoNT-A per year. The gait function assessed by GDI remained unchanged. However, gait kinematic and kinetic parameters revealed some intra-individual changes and documented an evolution of gait pattern. The majority of improvements occurred at the level of ankle, while hip function was deteriorating despite the therapy.Conclusions:
The study demonstrated that integrated multilevel BoNT-A treatment can be effective in maintaining the same level of walking in children with spastic diplegic cerebral palsy over a period of more than five years. The described integrated approach has limitations in the management of hip flexor and hamstrings tightness.Klíčová slova:
cerebral palsy – botulinum toxin A – gaitIntroduction
Cerebral Palsy (CP) is one of the most common causes of chronic physical disability in childhood. Spastic movement disorders in children with cerebral palsy result from involvement of the brain, central motor pathways, spinal circuits and musculoskeletal system [1]. Very often, children with cerebral palsy experience functional limitations in ambulation. Botulinum toxin type A (BoNT-A) offers a targeted form of therapy to reduce spasticity in specific muscle groups and has become one of the standard treatments. An application of the toxin leads to dose-related reversible chemo-denervation of the injected muscles by impairing the release of acetylcholine at the neuromuscular junction [2]. BoNT-A thereby reduces muscle tone and allows stretch to be applied that in itself stimulates muscle growth [1]. The benefit of BoNT-A therapy for lower-limb spasticity in children with CP has been established in randomized, placebo controlled trials and open-label studies [3–5]. Scholtes et al proved that multilevel BoNT-A application together with comprehensive physiotherapy significantly improves mobility in children with CP [6]. Although a multi-level application of BoNT-A seems to be a well-accepted procedure in children with bilateral CP [7,8], most of the studies investigated effects of single-level applications. Here, treatment of spastic equinus was the most frequently evaluated.
Also, only a few studies investigated the effects of repeated BoNT-A treatment. Two papers reported the effects of repeated BoNT-A application in calf spasticity [9,10]. Even if both studies showed a beneficial effect of equinus treatment with repeated BoNT-A application, they evaluated single-level treatment only, the follow-up time was short and BoNT-A was not applied more than 3-times. An exciting finding was reported by Tedroff et al who assessed long--term effect of BoNT-A treatment in CP. They found that BoNT-A was effective in reducing muscle tone over longer periods of time but was less effective in preventing development of contractures in spastic muscles [11]. Another group of researchers investigated long-term efficacy and tolerability of 1 year BoNT-A treatment of equinus in children with CP versus 4-month course of treatment [5]. However, their research did not allow for a clear conclusion on an injection regimen. A recent report on long-term use of multilevel BoNT-A treatment in children with CP focused on dosage consistency and treatment frequency [2]. They concluded that long-term, high-dosage, multi-level BoNT-A applications might be considered a safe and stable treatment option for children with cerebral palsy.
The aim of the present study was to evaluate the effects of multi-level, high--dose, repeated BoNT-A treatment on the gait pattern of children with CP. As the gait in children with cerebral palsy naturally deteriorates [12], we hypothesized that children who underwent multi-level, high-dose, repeated BoNT-A treatment would not deteriorate in their gait over a minimum of four years.
Methods
All children were assessed and treated at the specialized center for children with cerebral palsy that provides gait analysis as well as conservative and operative management. At this center, to document treatment outcome, children are followed up with repeated gait analysis irrespective of the applied form of treatment. To ensure consistent data collection, all the gait analyses were performed by the same experienced team over the entire study period. The inclusion criteria for the present retrospective study were:
- independently ambulating child with spastic diplegic cerebral palsy;
- conservative management over a minimum of four years;
- treatment forms: repeated multilevel BoNT-A applications, serial casting, physiotherapy;
- full set of gait analysis data before the start of the BoNT-A treatment, at follow-up examination after 2–3 years and at the follow-up after a minimum of 4 years (gait analysis data consisted of: time-distance, kinematic and kinetic data);
- no previous surgery at lower limbs;
- no oral or intrathecal antispastic medication.
Based on these inclusion and exclusion criteria, data on 18 children (36 limbs) were selected for further analysis. According to the Gross Motor Function Classification System (GMFCS), six children were classified as Level I, eight as Level II and four as Level III [13]. The study group consisted of twelve boys and six girls with the mean age of 6 years and 9 months (SD 1 year 4 months, range 5 years to 9 years 7 months) at the start of the study. The ethics committee of the Medical University approved the retrospective study.
Treatment concept
The integrated approach to BoNT-A treatment described by Molenaers et al was applied in all patients [2]. As per this approach and in agreement with the Updated European Consensus of 2009 on the use of botulinum toxin in children with cerebral palsy [1], reduction in muscle tone induced by BoNT-A injections was intended to provide an opportunity to optimize the effects of casting and orthotic management and to enhance both motor ability and functional skills. All patients in the study were treated with repeated multilevel injections of BoNT-A. To enhance the effect of BoNT-A, serial casts were applied for a period of 3-weeks in patients with equinus deformity. Outpatient physiotherapy was included in the treatment regimen in all patients. The goal of physiotherapy was always set individually but was generally aimed at muscle stretching, learning and training of new motor skills (e.g. gait with the heel initial contact instead of forefoot landing) and muscle strengthening. Additionally, all children were provided with orthopedic shoes and night splints.
BoNT-A (Botox®, Allergan, Irvine, CA) was administered by using multiple injection sites and endplate targeting technique [14]. The maximum dose per injection site was 25 units. Dosages were defined for each patient taking into account body weight, clinical examination, evaluation under general anesthesia and individual treatment goals. The interval between the BoNT-A applications correlated with the regular (every 4–6 months) clinical follow-up examinations. Repeated multilevel BTX-A application was indicated in patients with recurrent or deteriorating dynamic muscle tightness as selectively tested at different levels. The most frequently used dosages for specific muscles denoted as units per kilogram body weight are listed in Tab. 1. Each vial of BoNT-A was diluted with 2 ml of sterile sodium chloride solution. The needle placement was confirmed by stretching the targeted muscle and checking for the related motion of the needle during the stretching maneuver.
1. Dosing scheme for botulinum toxin A. 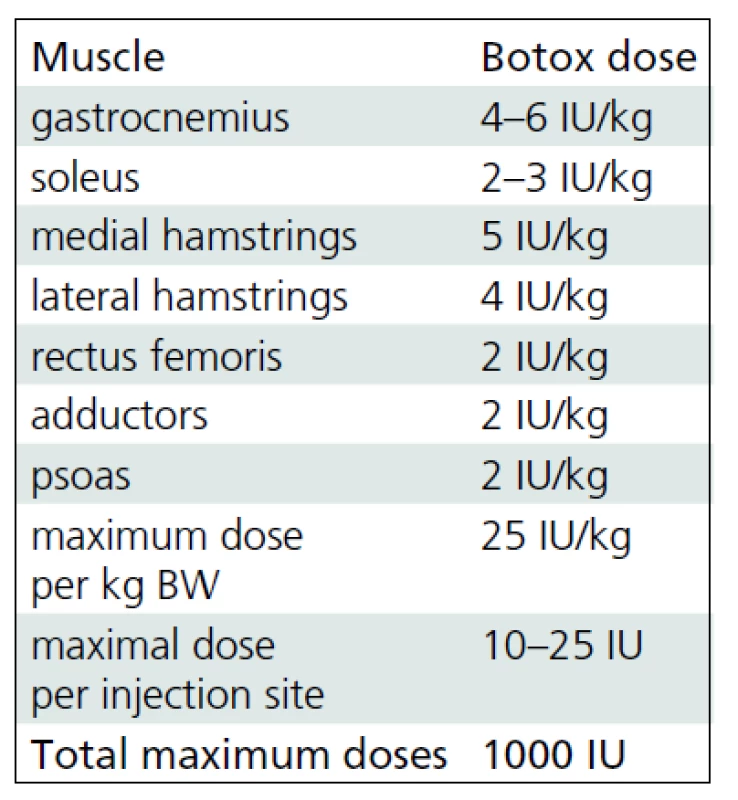
Usual botulinum toxin A (Botox®, Allergan, Irvine, CA) dose regimens for specific muscles of lower extremities at our institute. Gait analysis
Computerized gait analysis was performed using a video-based motion capturing system (Vicon, Oxford Metrics, Oxford, U.K.). Vicon Clinical Manager software (Vicon, Oxford Metrics, Oxford, U.K.) was used for data processing. Moment and power parameters were normalized to the weight of the children. Power generation and absorption patterns in the sagittal plane were calculated and labeled according to the method described by Winter [15]. All children walked at self-selected speed along a 10-meter walkway. A minimum of five trials were captured and averaged for each child. Data were collected at three separate occasions as described above. The Gait Deviation Index (GDI) was calculated [16] as a reliable summary measure of overall gait pathology taking into account nine independent kinematic variables. This parameter facilitated a comparison of gait abnormality documented by instrumented gait analysis over the study period. The GDI scales with Functional Assessment Questionnaire Walking Scale, topographic classification within the diagnosis of CP and is also strongly correlated with Gillette Gait Index [16]. Furthermore, the GDI demonstrated capacity to distinguish between different Gross Motor Classification System levels, further confirming its clinical usability [17]. The strength of the GDI is in its ability to illustrate overall change in pathological gait.
Statistical analysis
Repeated measures ANOVA with Fischer post-hoc test (Statistica 6.0, StatSoft, Tulsa, OK, USA) was used for comparisons of the three consecutive follow-up data sets. The significance level was set at p <0.05.
Results
The mean follow-up time was 5 years and 9 months (SD 1 year 5 months; range 4 years 2 months to 8 years 6 months). During this period, each study group child received 5.47 (SD 1.46; range 3 to 8) applications of BoNT-A, i.e. a mean of 1.02 (SD 0.37) applications per year. The mean dose of BoNT-A (Botox®, Allergan, Irvine, CA) was 17.7 (SD 2.8) units per kilogram body weight. Muscles most frequently treated were gastrocnemius followed by the medial hamstrings, soleus, lateral hamstrings, iliopsoas and adductors. The muscles injected least were tibialis anterior and rectus femoris.
Gait analysis allowed for an objective documentation of changes to gait over the entire study period. Although some improvement was observed, the Gait Deviation Index (GDI), as the main outcome measure, revealed no significant change at first follow-up when compared to pre--treatment values (Tab. 2). Similarly, the last gait analysis showed no deterioration in the GDI. Thus, the GDI and gait function remained stable over 5 years and 9 months of the follow-up. The unchanged GDI does not imply that the gait pattern remained the same. Instead, an improvement in maximum ankle dorsiflexion in stance and a substantial increase in the maximum ankle power generation was observed. The maximum knee extension during the stance phase of gait did not change significantly over the time (Fig. 1a). However, we observed a functional deterioration at the level of the hip. Here, the maximal hip extension decreased over time and the children were not able to extend their hips at the end of stance phase at the last follow-up. In addition, internal rotation of the hip increased over the study period (Fig. 1b).
1. The evolution of gait parameters of the ankle, knee (Fig. 1a) and hip (Fig. 1b) joints over 5 years and 9 months of follow-up. 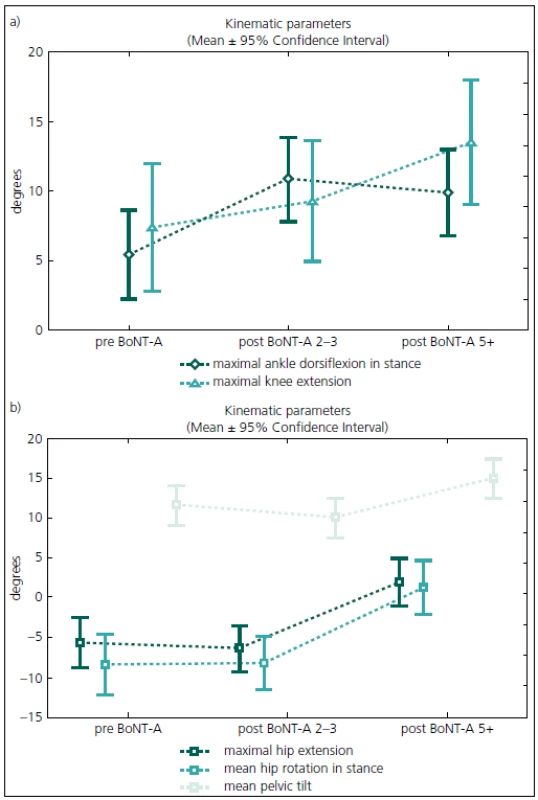
preBoNT-A – gait parameters prior to the first BoNT-A application; postBoNT-A 2–3 – gait parameters at the first follow-up after 2–3 years of treatment; postBoNT-A 5+ – gait parameters at the last follow-up after 5 years and 9 months of treatment 2. Summary of the main ANOVA results. 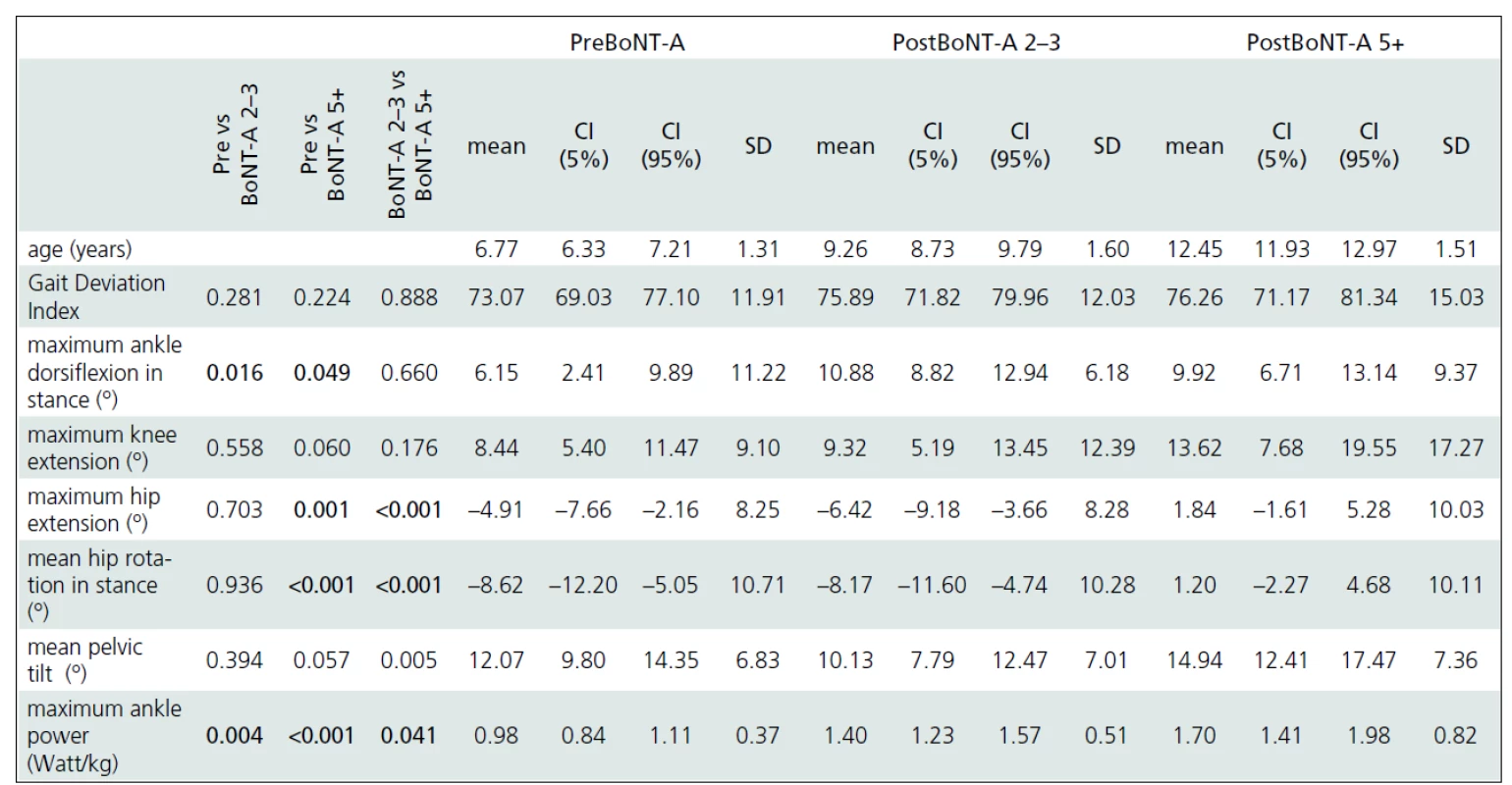
Detailed analysis of changes in important gait parameters over the mean follow-up period of 5 years and 9 months. Statistically significant results (One-way ANOVA, Fisher post-hoc test, P-level ≤0.05) are in bold. preBoNT-A – gait parameters prior to the first BoNT-A application; post 2–3 BoNT-A – gait parameters at the first follow-up after 2–3 years of treatment; Post 5+ BoNT-A – gait parameters at the last follow-up after 5 years and 9 months of treatment; CI – Confidence Interval; SD – Standard Deviation Discussion
The aim of treatment in children with CP is to minimize secondary problems such as contractures and bone deformities. Any reduction in muscle tone can help in providing adequate stretch to muscles, and can facilitate attempts to increase active range of motion and muscle power. Thereby any improvement in function might delay the need for surgery. As a child with diplegic CP rarely presents with an isolated problem at one joint or segment level, a multilevel approach needs to be advocated. Multilevel BoNT-A application provides a tool to reduce muscle tone in children with CP. The treatment is reversible, selective and safe and allows combined treatment with other conservative treatment options, such as physiotherapy, orthotic management or casting [18].
The five-year results of our study show that it is possible to maintain gait in children with spastic CP during growth using an integrated approach to BoNT-A application. The most unfavourable changes over time occurred at the level of the hip and pelvis. At the hips, children experienced a reduction in hip extension at the end of the stance and an increased internal rotation over the study period. The pelvic tilt increased and the maximum knee extension insignificantly decreased. A continuously increasing tightness of the hip flexors and hamstrings could explain these losses in function. In contrast, the function of the ankle joint improved as documented by kinematic as well as kinetic parameters. This proximal to distal deterioration in function is in contrast to what is usually observed during the natural development of children with CP. We argue that the more aggressive treatment of equinus deformity could play a role in these changes. The gastrocnemius was the most frequently treated muscle in this study. Furthermore, serial casting was used routinely after each BoNT-A application into the triceps surae muscle. However, casting is not suitable for treatment of hip problems and the stretching techniques might not be powerful enough to enhance the effect of BoNT-A application. On the other hand, Graham et al treated “hips at risk” in children with diplegic cerebral palsy with repeated BoNT-A applications to the adductors and hamstrings every 6 months over a 3 years period combined with an application of hip abduction braces. Because progressive hip displacement continued to occur in the treatment group, our data do not support this approach [19].
Some clinicians might be concerned about the safety of multi-level BoNT-A treatment procedure. Since several muscles are injected in one treatment session, multilevel treatment requires higher total dose compared to single-level treatments. Therefore, safety of higher doses and repeated applications of BoNT-A had been questioned. In their study of different doses of BoNT-A, Willis et al described the safety profile of higher dose BoNT-A and proved that adverse reactions are randomly distributed across groups with different doses [20]. Moreover, Molenaers et al studied long--term use of BoNT-A in children with CP and concluded that repeated, multi-level, high-dose BoNT-A applications may be considered as a safe and stable treatment option and the production of antibodies, responsible for secondary non-response, can be precluded [2].
Study limitations
This study focuses on the effects of repeated multilevel BoNT-A treatments on gait over a mean period of 5 years and 9 months. To simplify the comparison of multiple measures over the study period, the GDI was used as a descriptive parameter of overall gait function. The strength of the GDI is seen in its ability to detect overall changes in pathologic gait resulting from any kind of intervention [3]. Due to its nature, GDI cannot document changes at individual joints or body segments. To provide a deeper insight into gait changes following repeated multilevel BoNT-A applications, we also reported on selected relevant gait parameters and their changes over time. However, the study has further limitations. The number of children included into the study is rather low. The minimum follow-up time for this retrospective evaluation was 4 years and, therefore, data on many children could not be included. We report on a group of children with spastic diplegic CP and moderate to good function and our results should not be generalized to other children with different functionality or other types of CP. The retrospective design of the study has to also be taken into account.
Conclusions
In conclusion, the study demonstrates that integrated multilevel BoNT-A treatment is effective in maintaining walking ability in children with spastic diplegic cerebral palsy over a period of more than five years. Although the integrated approach to BoNT-A treatment has some limitations in the management of hip flexor and hamstrings tightness, it allows for the period between two BoNT-A applications to be extended to approximately 1 year.
Martin Svehlik, MD, PhD
Peadiatric Orthopaedic
Department of Paediatric Surgery
Medical University of Graz
Auenbruggerplatz 34
A-8036 Graz, Austria
e-mail: martin.svehlik@medunigraz.at
Accepted for review: 15. 11. 2011
Accepted for print: 17. 4. 2012
Sources
1. Heinen F, Desloovere K, Schroeder AS, Berweck S, Borggraefe I, van Campenhout A et al. The updated European Consensus 2009 on the use of Botulinum toxin for children with cerebral palsy. Eur J Paediatr Neurol 2009; 14(1): 45–66.
2. Molenaers G, Schörkhuber V, Fagard K, van Campenhout A, De Cat J, Pauwels P et al. Long-term use of botulinum toxin type A in children with cerebral palsy: treatment consistency. Eur J Paediatr Neurol 2009; 13(5): 421–429.
3. Koman LA, Brashear A, Rosenfeld S, Chambers H, Russman B, Rang M et al. Botulinum toxin type a neuromuscular blockade in the treatment of equinus foot deformity in cerebral palsy: a multicenter, open-label clinical trial. Pediatrics 2001; 108(5): 1062–1071.
4. Koog YH, Min BI. Effects of botulinum toxin A on calf muscles in children with cerebral palsy: a systematic review. Clin Rehabil 2010; 24(8): 685–700.
5. Kanovsky P, Bares M, Severa S, Richardson A. Long--term efficacy and tolerability of 4-monthly versus yearly botulinum toxin type A treatment for lower-limb spasticity in children with cerebral palsy. Dev Med Child Neurol 2009; 51(6): 436–445.
6. Scholtes VA, Dallmeijer AJ, Knol DL, Speth LA, Maathuis CG, Jongerius PH et al. The combined effect of lower-limb multilevel botulinum toxin type a and comprehensive rehabilitation on mobility in children with cerebral palsy: a randomized clinical trial. Arch Phys Med Rehabil 2006; 87(12): 1551–1558.
7. Desloovere K, Molenaers G, De Cat J, Pauwels P, van Campenhout A, Ortibus E et al. Motor function following multilevel botulinum toxin type A treatment in children with cerebral palsy. Dev Med Child Neurol 2007; 49(1): 56–61.
8. Molenaers G, Desloovere K, De Cat J, Jonkers I, De Borre L, Pauwels P et al. Single event multilevel botulinum toxin type A treatment and surgery: similarities and differences. Eur J Neurol 2001; 8 (Suppl 5): 88–97.
9. Metaxiotis D, Siebel A, Doederlein L. Repeated botulinum toxin A injections in the treatment of spastic equinus foot. Clin Orthop Relat Res 2002; 394 : 177–185.
10. Hawamdeh ZM, Ibrahim AI, Al-Qudah AA. Long--term effect of botulinum toxin (A) in the management of calf spasticity in children with diplegic cerebral palsy. Eura Medicophys 2007; 43(3): 311–318.
11. Tedroff K, Granath F, Forssberg H, Haglund-Akerlind Y. Long-term effects of botulinum toxin A in children with cerebral palsy. Dev Med Child Neurol 2009; 51(2): 120–127.
12. Bell KJ, Ounpuu S, DeLuca PA, Romness MJ. Natural progression of gait in children with cerebral palsy. J Pediatr Orthop 2002; 22(5): 677–682.
13. Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 1997; 39(4): 214–223.
14. Gracies JM, Lugassy M, Weisz DJ, Vecchio M, Flanagan S, Simpson DM. Botulinum toxin dilution and endplate targeting in spasticity: a double-blind controlled study. Arch Phys Med Rehabil 2009; 90(1): 9–16.
15. Winter DA. Energy generation and absorption at the ankle and knee during fast, natural, and slow cadences. Clin Orthop Relat Res 1983; 175 : 147–154.
16. Schwartz MH, Rozumalski A. The Gait Deviation Index: a new comprehensive index of gait pathology. Gait Posture 2008; 28(3): 351–357.
17. Molloy M, McDowell BC, Kerr C, Cosgrove AP. Further evidence of validity of the Gait Deviation Index. Gait Posture 2010; 31(4): 479–482.
18. Molenaers G, van Campenhout A, Fagard K, De Cat J, Desloovere K. The use of botulinum toxin A in children with cerebral palsy, with a focus on the lower limb. J Child Orhop 2010; 4(3): 183–195.
19. Graham HK, Boyd R, Carlin JB, Dobson F, Lowe K, Nattrass G et al. Does botulinum toxin a combined with bracing prevent hip displacement in children with cerebral palsy and ”hips at risk”? A randomized, controlled trial. J Bone Joint Surg Am 2008; 90(1): 23–33.
20. Willis AW, Crowner B, Brunstrom JE, Kissel A, Racette BA. High dose botulinum toxin A for the treatment of lower extremity hypertonicity in children with cerebral palsy. Dev Med Child Neurol 2007; 49(11): 818–822.
Labels
Paediatric neurology Neurosurgery Neurology
Article was published inCzech and Slovak Neurology and Neurosurgery

2012 Issue 6-
All articles in this issue
- Endovascular Treatment of an Ischemic Cerebrovascular Event
- Cortical Pathology in Multiple Sclerosis – Morphology, Immunopathology and Clinical Context
- Structure of Care in Neurorehabilitation
- Vascular Risk Factors and Alzheimer’s Disease
- A Global Epidemic of Multiple Sclerosis?
- Congenital Myasthenia as a Cause of Respiratory Failure in two Infants and a Toddler – Case Reports
- Laboratory Pathway Dissection from Medial Approach to Brain Hemisphere
- Papillary Tumor of the Pineal Region in a Child – a Case Report
- Predictors of Symptomatic Intracerebral Haemorrhage after Systemic Thrombolysis for Cerebral Infarction
- Occurence of Epileptic Seizures during Intraoperative Brain Stimulation – Our Experience
- Recurrence Quantification Analysis of Heart Rate Variability in Early Diagnosis of Diabetic Autonomic Neuropathy
- Measurement of Corpus Callosum and Comparison of MRI Techniques for Monitoring of Multiple Sclerosis
- Molecular Genetic Analysis of Fetal Tissues from a Family Affected by Myotonic Dystrophy
- Repeated Multilevel Botulinum Toxin A Treatment Maintains Long-Term Walking Ability in Children with Cerebral Palsy
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- A Global Epidemic of Multiple Sclerosis?
- Cortical Pathology in Multiple Sclerosis – Morphology, Immunopathology and Clinical Context
- Structure of Care in Neurorehabilitation
- Endovascular Treatment of an Ischemic Cerebrovascular Event
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

