-
Medical journals
- Career
Concurrent weekly cisplatin and simultaneous integrated boost intensity-modulated radiotherapy of locally advanced squamous cell carcinoma of the head and neck
Authors: P. Dubinský 1,2; B. Jeremic 3; M. Švajdová 4,5; G. Barilíková 1; P. Matula 1; D. Nadzonová 1; V. Vojtek 1
Authors‘ workplace: Department of Radiation Oncology, East Slovakia Institute of Oncology, Košice, Slovakia 1; Faculty of Health, Catholic University, Ružomberok, Slovakia 2; School of Medicine, University of Kragujevac, Kragujevac, Serbia 3; Department of Radiation and Clinical Oncology, Central Military Hospital – Teaching Hospital Ružomberok, Slovakia 4; Department of Radiation Oncology, Faculty of Medicine, Masaryk University, Masaryk Memorial Cancer Institute, Brno, Czech Republic 5
Published in: Klin Onkol 2022; 35(4): 307-314
Category: Original Articles
doi: https://doi.org/10.48095/ccko2022307Overview
Background: Radiotherapy of locally advanced head and neck cancer represents a major clinical challenge. Any treatment intensification aiming at improved treatment outcomes potentially results in a higher toxicity. The search for optimal treatment schedule involving conventional or altered fractionation of radiotherapy and the frequency and dose of concomitant cisplatin or other systemic agents has been spanning over several decades. Purpose: To evaluate long-term outcomes and toxicity of accelerated chemoradiotherapy of locally advanced squamous cell carcinoma of the head and neck (LA SCCHN). Patients and methods: Forty patients with stage III and IVA (TNM, 7th Ed.) LA SCCHN were treated with accelerated radiotherapy with a total dose of 67.5 Gy in 6 weeks delivered with simultaneous integrated boost intensity-modulated radiotherapy (SIB IMRT) and concomitant weekly cisplatin 40 mg/m2. Five-year outcomes and early and late toxicity were evaluated. Results: With the median follow-up of 47.8 months, a 5-year locoregional control rate (LCR) was 56.5%, distant control rate (DCR) was 87% and 5-year progression-free survival (PFS) and overall survival (OS) were 37 and 45%, respectively. Cisplatin cumulative dose of ≥ 200 mg/m2 was administered in 83% of patients. Grade ≥ 2 late toxicity with dietary change was observed in 21 (53%) patients. Human papillomavirus (HPV) status determined by p16 immunohistochemistry was the only significant factor in 5-year treatment outcomes analysis with LCR 100 vs. 41% (P < 0.01), DCR 100 vs. 78% (P = 0.154), PFS 80 vs. 23% (P = 0.01) and OS 80 vs. 34% (P = 0.03) for HPV positive oropharyngeal cancer (OPC) and other HPV negative LA SCCHN. Conclusion: High proportion of patients with LA SCCHN received an adequate cumulative dose of concurrent cisplatin with accelerated radiotherapy with SIB IMRT. This study demonstrated that chemoradiotherapy with weekly cisplatin resulted in favorable local control rate and survival in patients with HPV+ OPC.
Keywords:
cisplatin – human papillomavirus – oropharyngeal cancer – radiotherapy – head and neck cancer
Introduction
Radiotherapy of locally advanced head and neck cancer represents a major clinical challenge. Any treatment intensification aiming at improved treatment outcomes potentially results in a higher toxicity. The search for optimal treatment schedule involving conventional or altered fractionation of radiotherapy and the frequency and dose of concomitant cisplatin or other systemic agents has been spanning over several decades. All this endeavor is complicated by the establishment of human papillomavirus-associated oropharyngeal cancer (HPV+ OPC), as a new entity with distinct biology.
We evaluated long-term treatment outcomes and toxicity of accelerated fractionation based on simultaneous integrated boost (SIB) delivered by intensity-modulated radiotherapy (IMRT), which may require a lower cumulative dose of concurrent cisplatin [1,2], in a cohort of prospectively treated patients. Radiotherapy was combined with weekly administration of cisplatin, a schedule that needs further testing in HPV positive and HPV negative LA SCCHN.
Materials and methods
A retrospective analysis of acute and late toxicity and long-term treatment outcomes was conducted for the cohort of consecutive patients with LA SCCHN treated with accelerated SIB IMRT with concurrent weekly cisplatin at the Department of Radiation Oncology of the East Slovakia Institute of Oncology (ESIO). The treatment protocol was approved by the institutional Ethics Committee of the ESIO and the study was conducted in compliance with recognized international standards including the Declaration of Helsinki.
Diagnostic workup and treatment
We identified 40 consecutive patients with LA SCCHN between January 2013 and October 2014. All patients included into the study met the following criteria: squamous cell carcinoma (SCC) of oral cavity, oropharynx, hypopharynx and larynx in stages III and IVA (TNM, 7th Ed.), World Health Organization performance status 0 – 1 and no contraindication for cisplatin. All patients were seen by members of the multidisciplinary head and neck cancer team. Routine pretreatment workup consisted of medical history, physical examination of the head and neck, direct endoscopy under general anesthesia, dental and nutritional evaluation, CT imaging of the head and neck and chest X-ray. The HPV status in oropharynx carcinoma patients was assessed by p16 immunohistochemistry [3]. All patients signed the informed consent.
In all patients, step-and-shoot IMRT with accelerated SIB was used. The prescribed total radiation doses were 67.5 Gy for the gross tumor planning target volume (PTV_High), 60 Gy for the clinical target volume (PTV_Mid) and 54 Gy for the prophylactic neck irradiation (PTV_Low) in 30 fractions of 2.25/2.0/1.8 Gy per fraction over 6 weeks. The planning target volumes were defined as follows:
- PTV_High: all CT visible tumor and clinically visible mucosal spread with an isotropic 7 mm margin;
- PTV_Mid: gross tumor volume with a 10-mm edited margin for a primary and the whole involved nodal area for nodal metastases with a 5 mm margin;
- PTV_Low: nodal areas according to consensus recommendations [4] with a 5 mm margin.
The patients were treated daily, 5 times a week, with no compensation of missing days. Portal imaging or megavoltage cone-beam CT was used for weekly setup verification.
Intravenous cisplatin 40 mg/m2 was administered before radiotherapy weekly with a maximum of 6 courses unless pre-specified criteria for chemotherapy stopping were met.
Prophylactic feeding tubes were not utilized. Reactive nasogastric tube was inserted in the case of > 10% weight loss. Examinations by a dietician and a dentist were scheduled before the start of the therapy and when necessary.
The patients were seen by a radiation oncologist weekly during the treatment with documentation of acute side effects, oral intake, weight loss, whole blood count and biochemistry profile. Common Terminology Criteria of Adverse Events (CTCAE), version 4.0, were used for acute and late toxicity assessment [5].
After treatment completion, follow-up visits were scheduled in 3-month intervals in year 1, in 4-month intervals in year 2, and in 6-month intervals afterwards. Each visit consisted of a history of symptoms and physical examination with endoscopic evaluation when needed. In the case of suspected recurrence, patients were referred for radiologic evaluation and examination under anesthesia with biopsies.
Statistical analysis
The endpoints of analysis included locoregional control rate (LCR), distant control rate (DCR), progression-free survival (PFS), overall survival (OS) and toxicity. All survival data were calculated from the date of the first fraction of radiotherapy. The closeout date for survival was December 1, 2019. Cumulative survival data were calculated using the Kaplan – Meier method. Univariate and multivariate analyses using the Cox regression model were performed for the total cohort patients to determine the prognostic significance of the following factors: HPV status, cisplatin cumulative dose, overall treatment time (OTT) prolongation, stage and tumor site. Univariate analysis using the Cox regression model was subsequently performed for the subgroup of HPV negative SCCHN patients. The analyses were performed by the statistical program SPSS for Windows version 18.0 (IBM SPSS Statistics for Windows, Armonk, NY).
Results
We included 40 patients (median age 54 years, range 34–64 years) with oral cavity, laryngeal, hypopharyngeal and oropharyngeal cancers in stages III and IVA. The HPV status was assessed by p16 immunohistochemistry and was positive in 10 (25%) patients and negative in 30 (75%) patients. The characteristics of all patients and the HPV+ OPC subgroup are in Tab. 1.
1. Patient and treatment characteristics. 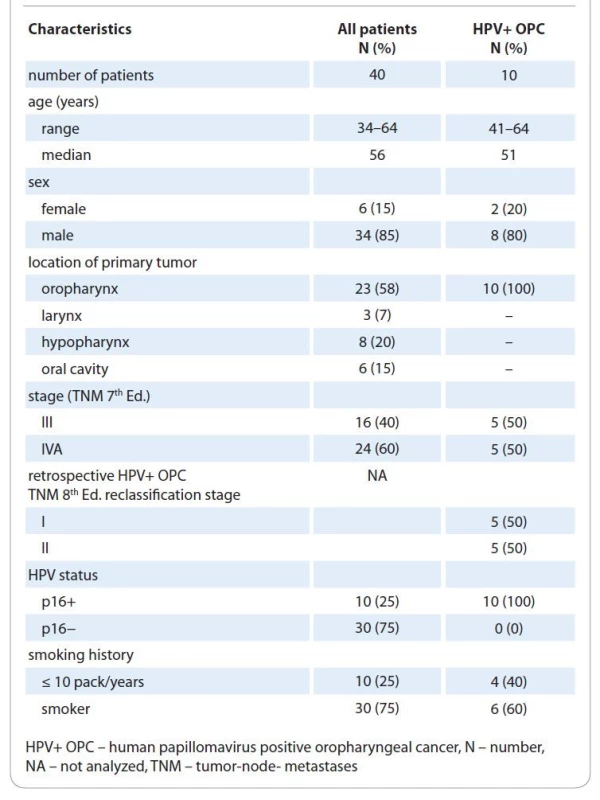
Median OTT prolongation was 7 days (0–13 days) mostly due to holidays and machine service. No measures were taken to compensate for treatment breaks. Cisplatin cumulative dose of at least 80% of the planned dose, i. e. ≥ 200 mg/m2, was delivered in 34 (85%) and < 200 mg/m2 in 6 (15%) patients. The reasons for not completing 6 courses included hematologic toxicity in 4 patients, renal toxicity in 1 patient and general condition deterioration in 1 patient. In HPV − and HPV+ OPC subgroups, 50 and 80% of patients received both ≥ 200 mg/m2 cisplatin doses with OTT prolongation less than one week. Salvage and upfront neck dissection were performed in 1 and 2 cases, respectively.
Treatment outcomes
With the median follow-up of 47.8 months (range 5–82 months), locoregional failure was identified in 12 (30%) patients out of 40. Six patients (15%) developed distant metastases, with concurrent 1 local and 1 locoregional failure. The 5-year LCR and DCR were 56.5 and 87%, respectively (Graphs 1, 2).
Graph 1. Five-year locoregional control rate for all 40 study patients. 
Graph 2. Five-year distant control rate for all 40 study patients. 
At the time of evaluation, 25 patients had died; none of treatment-related toxicity, 17 of disease progression, 5 of second primary malignancy (4 of lung cancer and 1 of stomach cancer) and 3 of other causes. The 5-year PFS and OS were 37 and 45%, respectively (Graph 3).
Graph 3. Five-year overall survival for all 40 study patients. 
We performed an analysis of known prognostic factors in all patients and in the p16 negative subgroup to exclude HPV association as the most prominent confounding factor. The HPV status, cisplatin cumulative dose (< 200 mg/m2 vs. ≥ 200 mg/m2) and OTT prolongation (≤ 7 days vs. > 7 days) were significant for the 5-year LCR, PFS and OS in univariate analysis, while the stage, tumor site, gender and smoking status were not. The multivariate analysis confirmed HPV status as the only significant factor in the 5-year treatment outcomes analysis for HPV+ OPC and HPV - LA SCCHN with LCR 100 vs. 41% (P < 0.01), DCR 100 vs. 78% (P = 0.154), PFS 80 vs. 23% (P = 0.01) and OS 80 vs. 34% (P = 0.03), respectively (Graphs 4, 5).
Graph 4. Five-year locoregional control rate for patients with human papillomavirus positive oropharyngeal cancer (solid line) and other locally advanced squamous cell head and neck carcinoma patients (dotted line). 
Graph 5. Five-year overall survival for human papillomavirus positive oropharyngeal cancer patients (solid line) and other locally advanced squamous cell head and neck carcinoma patients (dotted line). 
The site of the primary tumor, stage, age, gender, smoking status and OTT prolongation were not significant in OS, PFS or LCR; neither for all patients nor for the p16 negative subgroup.
Toxicity
Twenty-one patients (53%) developed G2 mucositis and in 15 patients (38%) G3 mucositis was observed. Reactive feeding tubes were placed on treatment in 8 (20%) patients with a median duration of placement of 6 weeks (2–17 weeks).
Clinically significant G3 hematologic toxicity was observed in 5 (13%) patients (2x anemia, 2x neutropenia and 1x thrombocytopenia) which led to cisplatin dose reduction or hospital stay prolongation. Other G3 toxicities involved nausea and vomiting in 3 (8%) patients, acute kidney injury in 1 (2.5%) patient and dermatitis in 1 (2.5%) patient.
At least one grade ≥ 2 late toxicity was observed in 21 patients (53%) (Tab. 2). Dietary change was caused mostly by dysphagia in 11 (28%) patients, xerostomia in 6 (15%) patients and mandibular osteoradionecrosis in 1 (2.5%) patient, trismus in 1 (2.5%) patient and peripheral neuropathy in 1 (2.5%) patient. One patient (2.5%) suffered from G3 dysphagia one year after chemoradiotherapy and had nasogastric tube placement with some oral intake for 8 months. No patient with a controlled tumor suffered from malnutrition. Severe peripheral motor and sensory neuropathy was diagnosed in 1 (2.5%) patient with p16 − OPC, with gradual onset of 6 months after the treatment resulting in severe symptoms limiting self-care.
2. Results of late toxicity evaluation of all 40 study patients. 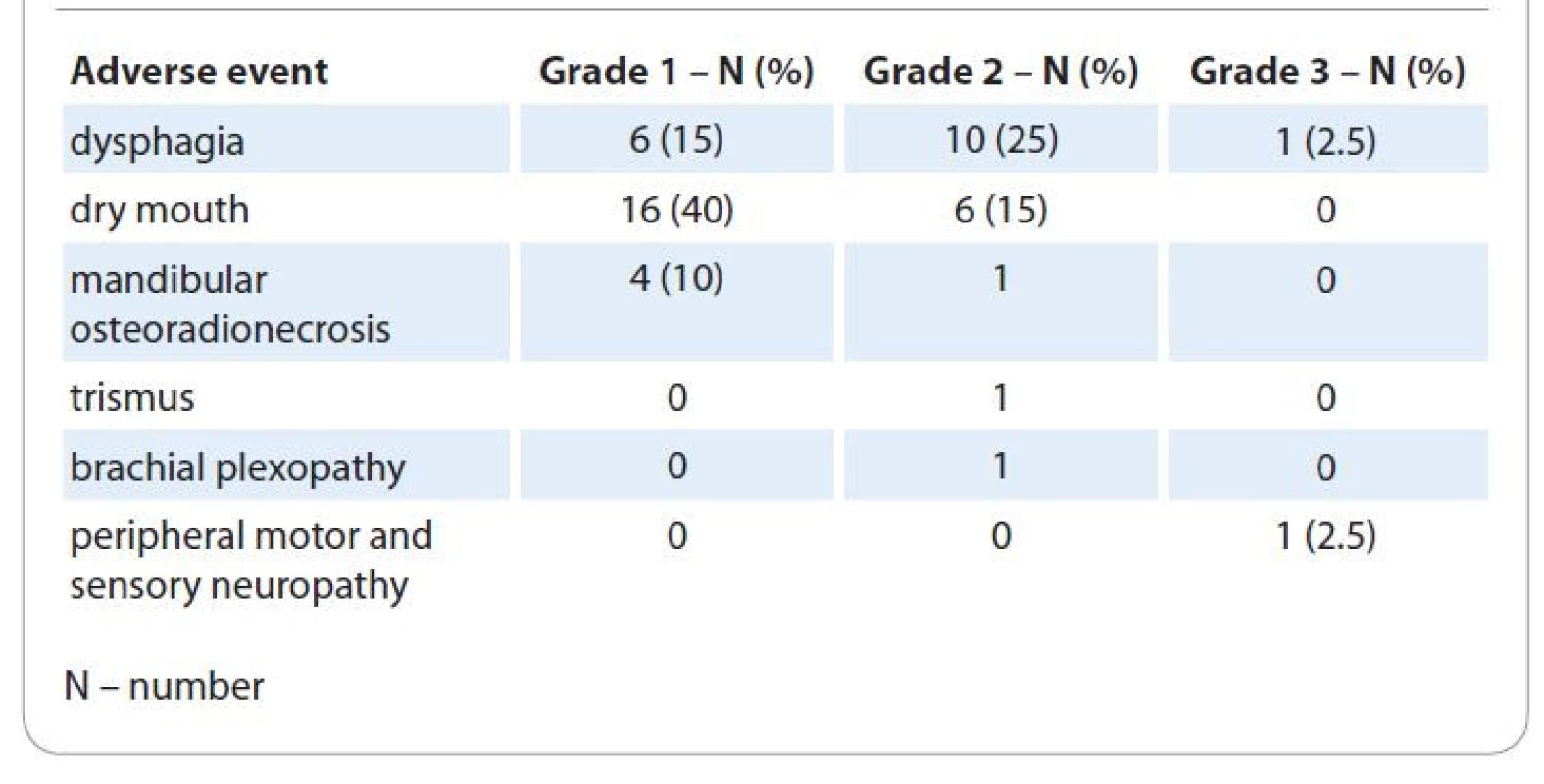
Discussion
Historically, efforts in treatment outcomes improvement and toxicity reduction have been focused on three principal areas: technology development, altered fractionation and optimization of systemic treatment. We incorporated all these aspects into the treatment protocol. We involved IMRT, a rather new technology at that time with treatment acceleration by delivery of SIB and administration of concomitant weekly cisplatin.
Dosimetric and planning studies have mostly documented the superiority of various IMRT techniques over 2D or 3D techniques, both in conformity and dose distribution [6–8], as well as sparing organs at risk (OARs).
We may expect significant reduction of grade 2–4 xerostomia in IMRT treated patients as has been shown by Gupta et al [9] in the analysis of 7 prospective randomized controlled trials including 1,155 patients. Intensity-modulated radiotherapy led to a risk reduction of 36% in grade > 2 acute xerostomia and a reduction of 56% in grade > 2 late xerostomia. We observed a 15% cumulative incidence of late G2 and none G3 xerostomia. The recorded proportion of patients suffering from parotid glands damage (15%) was also low in comparison to the benchmark study of Nutting et al (38% at 12 months and 29% at 14 months) [10]. This observation might have been due to the true effect of IMRT parotid sparing but also due to low concordance between late toxicity grading by CTCAE in our population and LENT SOMA scale in the study of Nutting at al [11]. In general, the occurrence of clinically significant local late effects other than dysphagia and xerostomia was low with only 3 grade 2 and no grade ≥ 3 events recorded.
Dosimetric comparison of SIB IMRT with sequential boost IMRT is equivocal with studies suggesting better dose conformity and OAR sparing [12,13], leading to fewer side effects [14,15] with SIB IMRT, while others showed superiority of sequential boost IMRT [16,17] due to a better coverage of the high dose regions, conformity and homogeneity, with fewer monitor units being used. Recent metaanalysis [18] compared sequential boost IMRT with SIB IMRT in head and neck cancer including 7 studies with a total of 1,049 patients. Interestingly, there was no difference in any of the endpoints used: OS (P = 0.71), PFS (P = 0.79), local recurrence-free survival (P = 0.91) and distant metastasis-free survival (P = 0.63) including no difference in side effects. We chose SIB IMRT planning as it was more practical using a single plan from the start and allowed irradiation of three clearly different (risk-wise) areas at the same time. Moreover, this technique enables acceleration of the treatment by shortening the OTT to 6 weeks by moderate hypofractionation in high risk PTV. Acceleration without reduction of the total dose has been shown to offer significant benefit on locoregional control over conventional fractionation [19,20].
There is no consensus on dose per fraction neither in high risk nor in prophylactic PTVs for acceleration with SIB. We used radiobiological considerations summarized by Mohan et al [21]. Calculations had been made for 42 days of OTT which we were unable to achieve. With the median of one week of radiotherapy prolongation, the potential benefit of acceleration on local control might have been lost. Conversely, undesirable OTT prolongation might have decreased the rate of acute G3 mucositis, G3 dermatitis and feeding tubes placements in comparison to a similar series of patients [22–24].
Optimal administration of radiotherapy and cisplatin in the definitive treatment remains unsolved, despite the fact that doses of 100 mg/m2 applied every 3 weeks were both suggested and largely practiced in the past 3 decades [25,26]. Common clinical practice of a weekly administration of cisplatin, mostly at a dose of 40 mg/m2 is based on the expected lower toxicity and potentially better radiosensitization, theoretically leading to a better therapeutic ratio. Unfortunately, high quality and multiple prospective randomized trials investigating the issue of concurrent scheduling of cisplatin are strikingly lacking. We opted for weekly cisplatin in our protocol, anticipating lower toxicity. The treatment adherence was good with cisplatin cumulative dose ≥ 200 mg/m2 delivered in the high proportion of patients, similarly to the study by Noronha et al [27]. Grade 3 acute hematologic and non-hematologic toxicity was low, comparable to weekly cisplatin arms in recent meta-analyses [28–30] which points to a better systemic toxicity profile of weekly administration in comparison to a 3-weekly schedule [31].
We observed one case of irreversible peripheral neuropathy, both motoric and sensory, limiting the patient’s daily activities. Neuropathy is rare at cumulative doses of ≤ 300 mg/m2 and cisplatin dose intensity does not appear to enhance the severity of the neuropathy [32].
The HPV status assessed by surrogate p16 expression was the only risk factor determining treatment outcomes. Better compliance of HPV+ OPC in patients with both radiotherapy and chemotherapy and no case in stage III (TNM, 8th Ed.) might have been contributed to striking differences in LCR, PFS and OS between the subgroups with HPV+ OPC and other LA SCCHN. No local or distal recurrence was observed during a long-term follow-up in HPV+ OPC patients despite a history of smoking in a half of them.
Recently, 3-weekly cisplatin has been established as a standard concomitant schedule in HPV+ OPC [33,34]. A weekly cisplatin schedule may represent a reasonable alternative for this subgroup of patients in stages I and II as a component of treatment de-escalation strategies. Very likely, a weekly dose of cisplatin may be reduced to the cumulative dose of ≤ 200 mg/m2 [35].
With no IVB patients included, we considered outcomes of our protocol in HPV − LA SCCHN suboptimal. We believe that weekly cisplatin would not compensate for significant OTT prolongation in this group of patients.
Optimized treatment of an individual patient should be thoroughly considered to provide an appropriate balance between various factors in the decision-making process. This applies especially in the case when treatment optimization is still facing challenges, in both HPV − and HPV+ patients [36,37].
We understand that there are considerable limitations in interpretation of our rather small, single-arm observational study. Nevertheless, the excellent 5-year treatment outcomes observed in a subgroup of HPV+ OPC patients included in this study (5-year LCR, DCR, PFS and OS of 100%, 100%, 80% and 80%, respectively) emphasize the need for further refinement of cytoreduction strategies as a part of treatment de-intensification protocols in HPV+ OPC patients. In 157 HPV+ OPC patients treated with primary chemoradiotherapy to a dose of 60 Gy with concurrent weekly cisplatin 40 mg/m2 within NRG-HN002, 80.9% had ≥ 5 cycles of cisplatin and the observed 2-year PFS and OS were 90.5 and 96.7%, respectively [38]. Similarly, in two prospective trials, Chera et al reduced primary chemoradiation dose to 60 and 54 Gy at high-risk areas and regions of subclinical microscopic spread in combination with weekly cisplatin 30 mg/m2, respectively. In these trials, the 2 - and 3-year locoregional control, distant metastases-free survival and OS were 95–100%, 91–100% and 95% with no grade ≥ 3 late adverse event observed in either of the studies, respectively [39, 40]. More randomized controlled trials and long-term follow-up is undoubtedly necessary to refine the use of weekly cisplatin in patients with HPV+ OPC.
We believe that the choice of cisplatin schedule may be based on the HPV status. Currently, we continue to treat patients with stages I and II HPV+ OPC with weekly cisplatin in a de-escalation protocol while other patients with LA SCCHN receive SIB IMRT accelerated radiotherapy for 6 weeks with three-weekly cisplatin.
Conclusion
A high proportion of patients with LA SCCHN received an adequate cumulative dose of concurrent cisplatin with accelerated radiotherapy with SIB IMRT. This study demonstrated that chemoradiotherapy with weekly cisplatin resulted in a favorable local control rate and survival in patients with HPV+ OPC. Despite limitations in the size and the design of our study, the results suggest that weekly cisplatin administration may be considered an appropriate option in primary concomitant chemoradiotherapy of stages I and II HPV+ OPC.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
The Editorial Board declares that the manuscript met the ICMJE recommendation for biomedical papers.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.Michaela Švajdová, MD, PhD
Department of Radiation
and Clinical Oncology
Central Military Hospital – Teaching
Hospital Ružomberok
Gen. Miloša Vesela 43
034 26 Ružomberok
Slovakia
e-mail:
michaela.svajdova@svetzdravia.com
Submitted/Obdrženo: 10. 11. 2021
Accepted/Přijato: 15. 12. 2021
Sources
1. Nguyen-Tan PF, Zhang Q, Ang KK et al. Randomized phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the Radiation Therapy Oncology Group 0129 trial: long-term report of efficacy and toxicity. J Clin Oncol 2014; 32 (34): 3858–3866. doi: 10.1200/JCO.2014.55.3925.
2. Bourhis J, Sire C, Graff P et al. Concomitant chemoradiotherapy versus acceleration of radiotherapy with or without concomitant chemotherapy in locally advanced head and neck carcinoma (GORTEC 99-02): an open-label phase 3 randomised trial. Lancet Oncol 2012; 13 (2): 145–153. doi: 10.1016/S1470-2045 (11) 70346-1.
3. Fakhry C, Lacchetti C, Rooper LM et al. Human papillomavirus testing in head and neck carcinomas: ASCO Clinical Practice Guideline Endorsement of the College of American Pathologists Guideline. J Clin Oncol 2018; 36 (31): 3152–3161. doi: 10.1200/JCO.18.00684.
4. Grégoire V, Ang K, Budach W et al. Delineation of the neck node levels for head and neck tumors: a 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother Oncol 2014; 110 (1): 172–181. doi: 10.1016/j.radonc.2013.10.010.
5. Common terminology criteria for adverse events (CTCAE). [online]. Available from: https: //evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf.
6. Clark CH, Bidmead AM, Mubata CD et al. Intensity-modulated radiotherapy improves target coverage, spinal cord sparing and allows dose escalation in patients with locally advanced cancer of the larynx. Radiother Oncol 2004; 70 (2): 189–198. doi: 10.1016/j.radonc.2003.10.012.
7. Grégoire V, De Neve W, Eisbruch A et al. Intensity-modulated radiation therapy for head and neck carcinoma. Oncologist 2007; 12 (5): 555–564. doi: 10.1634/theoncologist.12-5-555.
8. Mendenhall WM, Amdur RJ, Palta JR. Intensity-modulated radiotherapy in the standard management of head and neck cancer: promises and pitfalls. J Clin Oncol 2006; 24 (17): 2618–2623. doi: 10.1200/JCO.2005.04.7225.
9. Gupta T, Kannan S, Ghosh-Laskar S et al. Systematic review and meta-analyses of intensity-modulated radiation therapy versus conventional two-dimensional and/or or three-dimensional radiotherapy in curative-intent management of head and neck squamous cell carcinoma. PLoS One 2018; 13 (7): e0200137. doi: 10.1371/journal.pone.0200137.
10. Nutting CM, Morden JP, Harrington KJ et al. Parotid-sparing intensity-modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol 2011; 12 (2): 127–136. doi: 10.1016/S1470-2045 (10) 70290-4.
11. Denis F, Garaud P, Bardet E et al. Late toxicity results of the GORTEC 94-01 randomized trial comparing radiotherapy with concomitant radiochemotherapy for advanced-stage oropharynx carcinoma: comparison of LENT/SOMA, RTOG/EORTC, and NCI-CTC scoring systems. Int J Radiat Oncol Biol Phys 2003; 55 (1): 93–98. doi: 10.1016/s0360-3016 (02) 03819-1.
12. Franceschini D, Paiar F, Meattini I et al. Simultaneous integrated boost intensity-modulated radiotherapy in head and neck cancer. Laryngoscope 2013; 123 (12): E97–E103. doi: 10.1002/lary.24257.
13. Ho KF, Fowler JF, Sykes AJ et al. IMRT dose fractionation for head and neck cancer: variation in current approaches will make standardization difficult. Acta Oncol 2009; 48 (3): 431–439. doi: 10.1080/02841860802372272.
14. Stromberger C, Ghadjar P, Marnitz S et al. Comparative treatment planning study on sequential vs. simultaneous integrated boost in head and neck cancer patients: differences in dose distributions and potential implications for clinical practice. Strahlenther Onkol 2016; 192 (1): 17–24. doi: 10.1007/s00066-015-0913-4.
15. Spiotto MT, Weichselbaum RR. Comparison of 3D conformal radiotherapy and intensity modulated radiotherapy with or without simultaneous integrated boost during concurrent chemoradiation for locally advanced head and neck cancers. PloS One 2014; 9 (4): e94456. doi: 10.1371/journal.pone.0094456.
16. Miyazaki M, Nishiyama K, Ueda Y et al. Preliminary analysis of the sequential simultaneous integrated boost technique for intensity-modulated radiotherapy for head and neck cancers. J Radiat Res 2016; 57 (4): 406–411. doi: 10.1093/jrr/rrw010.
17. Vlacich G, Stavas MJ, Pendyala P et al. A comparative analysis between sequential boost and integrated boost intensity-modulated radiation therapy with concurrent chemotherapy for locally-advanced head and neck cancer. Radiat Oncol 2017; 12 (1): 13. doi: 10.1186/s13014-016-0756-x.
18. Jiang L, Zhang Y, Yang Z et al. A comparison of clinical outcomes between simultaneous integrated boost (SIB) versus sequential boost (SEQ) intensity modulated radiation therapy (IMRT) for head and neck cancer: a meta-analysis. Medicine 2019; 98 (34): e16942. doi: 10.1097/MD.0000000000016942.
19. Bourhis J, Overgaard J, Audry H et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet 2006; 368 (9538): 843–854. doi: 10.1016/S0140-6736 (06) 69121-6.
20. Lacas B, Bourhis J, Overgaard J et al. Role of radiotherapy fractionation in head and neck cancers (MARCH): an updated meta-analysis. Lancet Oncol 2017; 18 (9): 1221–1237. doi: 10.1016/S1470-2045 (17) 30458-8.
21. Mohan R, Wu Q, Manning M et al. Radiobiological considerations in the design of fractionation strategies for intensity-modulated radiation therapy of head and neck cancers. Int J Rad Oncol Biol Phys 2000; 26 (3): 619–630. doi: 10.1016/s0360-3016 (99) 00438-1.
22. Rütten H, Pop LA, Janssens GO et al. Long-term outcome and morbidity after treatment with accelerated radiotherapy and weekly cisplatin for locally advanced head-and-neck cancer: results of a multidisciplinary late morbidity clinic. Int J Rad Oncol Biol Phys 2011; 81 (4): 923–929. doi: 10.1016/j.ijrobp.2010.07. 013.
23. Jacinto AA, Batalha Filho ES, Viana LS et al. Feasibility of concomitant cisplatin with hypofractionated radiotherapy for locally advanced head and neck squamous cell carcinoma. BMC Cancer 2018; 18 (1): 1026. doi: 10.1186/s12885-018-4893-5.
24. Montejo ME, Shrieve DC, Bentz BG et al. IMRT with simultaneous integrated boost and concurrent chemotherapy for locoregionally advanced squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 2011; 81 (5): e845–852. doi: 10.1016/j.ijrobp.2010.10.021.
25. Pfister DG, Spencer S, Adelstein D et al. Head and neck cancers, version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2020; 18 (7): 873–898. doi: 10.6004/jnccn.2020.0031.
26. Ang KK, Chen A, Curran WJ Jr et al. Head and neck carcinoma in the United States: first comprehensive report of the Longitudinal Oncology Registry of Head and Neck Carcinoma (LORHAN). Cancer 2012; 118 (23): 5783–5792. doi: 10.1002/cncr.27609.
27. Noronha V, Joshi A, Patil VM et al. Once-a-week versus once-every-3-weeks cisplatin chemoradiation for locally advanced head and neck cancer: a phase III randomized noninferiority trial. J Clin Oncol 2018; 36 (11): 1064–1072. doi: 10.1200/JCO.2017.74.9457.
28. Szturz P, Wouters K, Kiyota N et al. Weekly low-dose versus three-weekly high-dose cisplatin for concurrent chemoradiation in locoregionally advanced non-nasopharyngeal head and neck cancer: a systematic review and meta-analysis of aggregate data. Oncologist 2017; 22 (9): 1056–1066. doi: 10.1634/theoncologist.2017 - 0015.
29. Jacinto JK, Co J, Mejia MB et al. The evidence on effectiveness of weekly vs triweekly cisplatin concurrent with radiotherapy in locally advanced head and neck squamous cell carcinoma (HNSCC): a systematic review and meta-analysis. Br J Radiol 2017; 90 (1079): 20170442. doi: 10.1259/bjr.20170442.
30. Mohamed A, Twardy B, Zordok MA et al. Concurrent chemoradiotherapy with weekly versus triweekly cisplatin in locally advanced squamous cell carcinoma of the head and neck: comparative analysis. Head Neck 2019; 41 (5): 1490–1498. doi: 10.1002/hed.25379.
31. Jeremic B, Dubinsky P, Filipovic N et al. Optimal administration frequency of cisplatin concurrently with radical radiotherapy in the definitive treatment of locally advanced, inoperable squamous cell cancer of the head and neck still obscured by clouds? Turk J Oncol 2019; 34 (2): 133–136. doi: 10.5505/tjo.2019.2015.
32. Hilkens PH, van der Burg ME, Moll JW et al. Neurotoxicity is not enhanced by increased dose intensities of cisplatin administration Eur J Cancer 1995; 31A (5): 678–681. doi: 10.1016/0959-8049 (94) 00497-s.
33. Gillison ML, Trotti AM, Harris J et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet 2019; 393 (10166): 40–50. doi: 10.1016/S0140-6736 (18) 32779-X.
34. Mehanna H, Robinson M, Hartley A et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet 2019; 393 (10166): 51–60. doi: 10.1016/S0140-6736 (18) 32752-1.
35. Spreafico A, Huang SH, Xu W et al. Impact of cisplatin dose intensity on human papillomavirus-related and -unrelated locally advanced head and neck squamous cell carcinoma. Eur J Cancer 2016; 67 : 174–182. doi: 10.1016/j.ejca.2016.08.013.
36. Jeremic B, Ozyigit G, Dubinsky P et al. Importance of HPV positivity in squamous cell head and neck cancer. Turk J Oncol 2019; 34 (3): 204–214. doi: 10.5505/tjo.2019.2079.
37. Slavik M, Kazda T, Selingerová I et al. Effect of tumor size and p16 status on treatment outcomes – achievement of complete remission in prospectively followed patients with oropharyngeal tumors. Klin Onkol 2019; 32 (1): 58–65.
38. Yom SS, Torres-Saavedra P, Caudell JJ et al. A randomized phase II trial for patients with p16-positive, non-smoking-associated, locoregionally advanced oropharyngeal cancer. Int J Radiat Oncol Biol Phys 2019; 105 (3): 684–685. doi: 10.1016/j.ijrobp.2019.08.038.
39. Chera, BS, Amdur RJ, Tepper JE et al. Mature results of a prospective study of deintensified chemoradiotherapy for low-risk human papillomavirus-associated oropharyngeal squamous cell carcinoma. Cancer 2018; 124 (11): 2347–2354. doi: 10.1002/cncr.31338.
40. Chera BS, Amdur RJ, Green R et al. Phase II trial of de-intensified chemoradiotherapy for human papillomavirus-associated oropharyngeal squamous cell carcinoma. J Clin Oncol 2019; 37 (29): 2661–2669. doi: 10.1200/ JCO.19.01007.
Labels
Paediatric clinical oncology Surgery Clinical oncology
Article was published inClinical Oncology

2022 Issue 4-
All articles in this issue
- Onkologická léčba 21. století a Kaplan-Meierovy křivky
- Rosai-Dorfman-Destombes disease – histiocytic disorder with infl ammatory manifestation
- Patients with neuregulin 1 (NRG1) rearranged cancer are suitable for the theranostic approach and targeted therapy
- Olanzapine in oncology palliative care
- Immunotherapy for cancer treatment
- How fatigue affects return to work in breast cancer patients
- Informace z České onkologické společnosti
- Slow increase of bilirubin concentration during administration of lenalidomide, bortezomib and dexamethasone for multiple myeloma (unmasking previously undiagnosed Gilbert syndrome) and disappearance of necrobiotic xanthogranuloma after complete remission of multiple myeloma
- Regulatory network of competitively interacting RNAs and effectiveness of rectal tumors radiotherapy
- Concurrent weekly cisplatin and simultaneous integrated boost intensity-modulated radiotherapy of locally advanced squamous cell carcinoma of the head and neck
- Microwave ablation of a solitary colorectal liver metastasis complicated by stomach perforation and gastrocutaneous fistula – a case report
- Clinical Oncology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Olanzapine in oncology palliative care
- Immunotherapy for cancer treatment
- Rosai-Dorfman-Destombes disease – histiocytic disorder with infl ammatory manifestation
- How fatigue affects return to work in breast cancer patients
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

