-
Medical journals
- Career
The Relationship of FOXR2 Gene Expression Profile with Epithelial-Mesenchymal Transition Related Markers in Epithelial Ovarian Cancer
Authors: Samira Asadollahi 1,2; Mahta Naeini Mazaheri 1,3; Mojgan Karimi-Zarchi 4,5; Fesahat; Farzaneh 6,7
Authors‘ workplace: Department of Medical Genetics, School of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran 1; Diabetes Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran 2; Mother and Newborn Health Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran 3; Department of Obstetrics and Gynecology, Iran University of Medical Sciences, Tehran, Iran 4; Endometriosis Research Center, Iran University of Medical Sciences, Tehran, Iran 5; Reproductive Immunology Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. 6; Department of Advanced Medical Sciences and Technologies, School of Paramedicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran 7
Published in: Klin Onkol 2020; 33(3): 201-207
Category: Original Articles
doi: https://doi.org/10.14735/amko2020201Overview
Background: Several factors have been evaluated for their competency as applied biomarkers regarding diagnosis and therapy of ovarian cancer as one of the most cause of death due to the gynecologic malignancies. However, some Fox-factors have been shown to modulate cancer progression primarily by their impacts on the proliferation of the cells, the expression and potential function of FOXR2 (Forkhead Box R2), newly identified as a probable oncogene in a few human cancers, remains undecided in ovarian cancer. The aim of this study was to evaluate the FOXR2 and some epithelial-mesenchymal transition (EMT) -related gene expression profiles in epithelial ovarian cancer (EOC) tissues and their healthy samples as well as an ovarian cancer cell line (SKOV-3).
Methods: In this observational study, 20 epithelial ovarian adenocarcinoma and their marginal samples, obtained from 20 women with EOC, as well as SKOV-3, were investigated for the relative gene expression levels of FOXR2, CDH1 (encoding E-cadherin) and FN1 (encoding fibronectin-1) in 2 groups using qualitative real-time polymerase chain reaction technique (qRT-PCR).
Results: The findings demonstrated a significant up-regulation of FOXR2 and FN1 despite the CDH1 down-regulation in case samples compared to controls (P < 0.05). There was a significant correlation between FOXR2 gene expression profile and EMT-related markers in high-grade tumors. Furthermore, the biomarker index of 0.772 was obtained for FOXR2 gene expression levels.
Conclusions: The findings indicated that the expression levels of FOXR2 have a significant association with ovarian cancer as far as it can be used as a diagnostic and therapeutic molecular biomarker in ovarian cancer.
Keywords:
ovarian cancer – FOXR2 – Gene expression – epithelial-mesenchymal transition
Introduction
Epithelial ovarian cancer (EOC) is 5th and 7th major cause of deaths among females in the United States and worldwide, respectively [1,2]. There are many clinical complications about this cancer since almost all of the patients are diag-nosed late, usually at stages III and IV. As a result, EOC is the most lethal cancer among all gynecological malignancies and the five-year survival rate is < 5% in these patients [3,4]. Therefore, despite vigorous attempts to upgrade the diag-nosis and treatment, most women affected by EOC eventually pass away from their cancer. The disease is more prevalent with increasing of women‘s age and most frequently diagnosed women are 55–65 years old [5,6]. For each woman, the risk of ovarian cancer in her lifetime is estimated 1 in 78 and the probability of death from this disease is about 1 in 108 [6]. The EOC symptoms comprise frequent urination, difficulty in eating, abdominal mass, tiredness and pelvic pain [7]. The most usual treatments for this disease are surgery, chemotherapy and radiotherapy. The most functional chemotherapy agents as the standard treatment for patients with EOC are the platinum-based drugs, namely cisplatin and carboplatin. These drugs are commonly applied in combination with another cytotoxic agent in order to improve the disease [8]. The relapse and development of resistance to first-line chemotherapy routinely occurs among the majority of patients with EOC [9]. Hence, new strategies are required to identify novel prognostic and therapeutic markers for improving patients‘ long-term survival rate.
The epithelial to mesenchymal transition (EMT) is a key approach in embryogenesis that polarized epithelial cells lose their intercellular adhesion, then transformed to mesenchymal phenotype and gain migratory and invasive characteristics [10]. This process is identified by changes in cell markers like reducing in epithelial markers and increasing in mesenchymal markers [11]. EMT is crucial for numerous developmental processes, and accumulating findings suggest that EMT participates in the initiation of cancer metastasis [12].
The Forkhead box (Fox) proteins organize an extensive family of transcriptional modulators which are characterized by an evolutionary conserved „fork-head“ DNA-binding domain [13,14]. The Fox superfamily consists of multifunctional transcription factors, responsible for regulation of a broad range of transcriptional events, which are involved in various cellular events such as cell cycle progression, proliferation, differentiation, metabolism, aging, vitality and apoptosis. In addition to serving as transcription activators, Fox also act as leading factors with the capability of unwinding the compacted chromatin and make it accessible to other factors to bind [15]. Given the great importance of Fox proteins, it is noticeable that the remodelings of their transcriptional regulation and function will result in pathological conditions including cancer. One of the key members of these subfamilies, FOXR2, has been discussed here with an emphasis on its carcinogenesis role.
FOXR2 as one of the Fox family members, also known as FOXN6, was located on the human chromosome Xp11.21 within genome sequence RP11-167P23 [13]. FOXR protein contains the forkhead binding domain at its C-terminal that followed by a common domain with FOXN5 in N-terminal that is named FN56 [16]. FOXR2 has been found to be upregulated in breast cancer and is associated with its poor prognosis [17]. Consequently, FOXR2 has been reported to be overexpressed in prostate and colorectal cell lines and tissues; it was shown that its knockdown by siRNA could suppress cell migration and invasion [18,19]. However, the role of FOXR2 gene remains undecided in EOC until now. The main goal of the current prospective study was to characterize the expression profile of FOXR2 in EOC tissue samples as well as SKOV-3 and to investigate if FOXR2 expression levels were correlated with EMT indicators.
Materials and methods
Patients and tissue specimens
Human EOC tissues and their matching adjacent noncancerous tissues as the control samples were collected from 20 ovarian cancer patients at Shahid Sadoughi Hospital, University of Medical Sciences, Yazd. Not all patients have received any treatment or chemotherapy before tissue sample biopsies for this study. At first, each sample was evaluated by a pathologist to ensure that it contained more than 70 % tumor cells.
The main features of the tumor from patients who participated in the present study including tumor histology, stage and the grades of the tumor as well as the presence of metastasis were evaluated according to the International Federation of Gynecology and Obstetrics global standard [19]. Furthermore, some normal ovarian samples were provided from 3 women who did not have any ovarian disease or endometriosis and were referred to Shahid Sadoughi Hospital for ovarian cyst surgery. The use of all samples was approved by the legal standards in the Ethics Committee of Shahid Sadoughi Hospital. All participants signed the informed consent for this research.
Cell culture
The SKOV-3 cell line (NCBI code: C209, ATCC code: HTB-77) was obtained from the National Cell Bank of Iran (NCBI), Pasteur Institute, and was cultured in RPMI-1640 (Gibco, Invitrogen, USA) supplemented with 10% fetal bovine serum (FBS: Gibco, Invitrogen, USA) and 100 IU/ml penicillin-streptomycin (Invitrogen). The cell line was kept at 37 °C in a humidified incubator with 5% CO2. The culture medium was replaced every 3 days after initial cell culturing and based on cell density. Since reaching 90–95% confluence, the cells were split as a routine passage at the ratio of 1/3. All data represented a duplicate from 2 experiments.
Quantitative real-time PCR
The relative expression of FOXR2 gene was assessed by real-time polymerase chain reaction technique (RT-PCR). Total RNAs were extracted from the tissue samples and the cell line by using TRIzol reagent (Invitrogen, USA) considering the manufacturer’s instructions. After achieving RNA concentration and quality, the measurements of its purity and integrity with a nanodrop spectrophotometer were assessed using agarose gel electrophoresis. The RNAs extracted from 3 normal ovarian samples were pooled together and assessed for the purity and integrity as a unit. Then, the complementary DNA (cDNA) was synthesized in a reverse transcriptase reaction with the Revert Aid cDNA Synthesis kit (Thermo Scientific, USA) according to the manufacturer’s recommendations. We used 1µl of total RNA (5 µg) to generate first-stand cDNA as the initial step of RT-PCR protocol. Using specified primers (Tab. 1), relative expressions of target genes (FOXR2, CDH1 and FN1) were evaluated by quantitative real-time polymerase chain technique (qRT-PCR). The gene for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was applied as a reference gene. Consequently, the qRT-PCR run was carried out by using the QuantiTect SYRB Green dye (TaKaRa, Japan) and Applied Biosystems™ StepOne™ Real-Time PCR System (ABI, Applied Biosystems, USA). All samples were run with the PCR conditions of an initial denaturation step at 95 °C for 2 min, and then 40 cycles at 95 °C for 5 s and 58 °C for 35 s. All reactions were run in duplicates. The relative mRNA expression levels were measured using the comparative CT method 2 - (DDCT).
1. Oligonucleotid primer sequences. 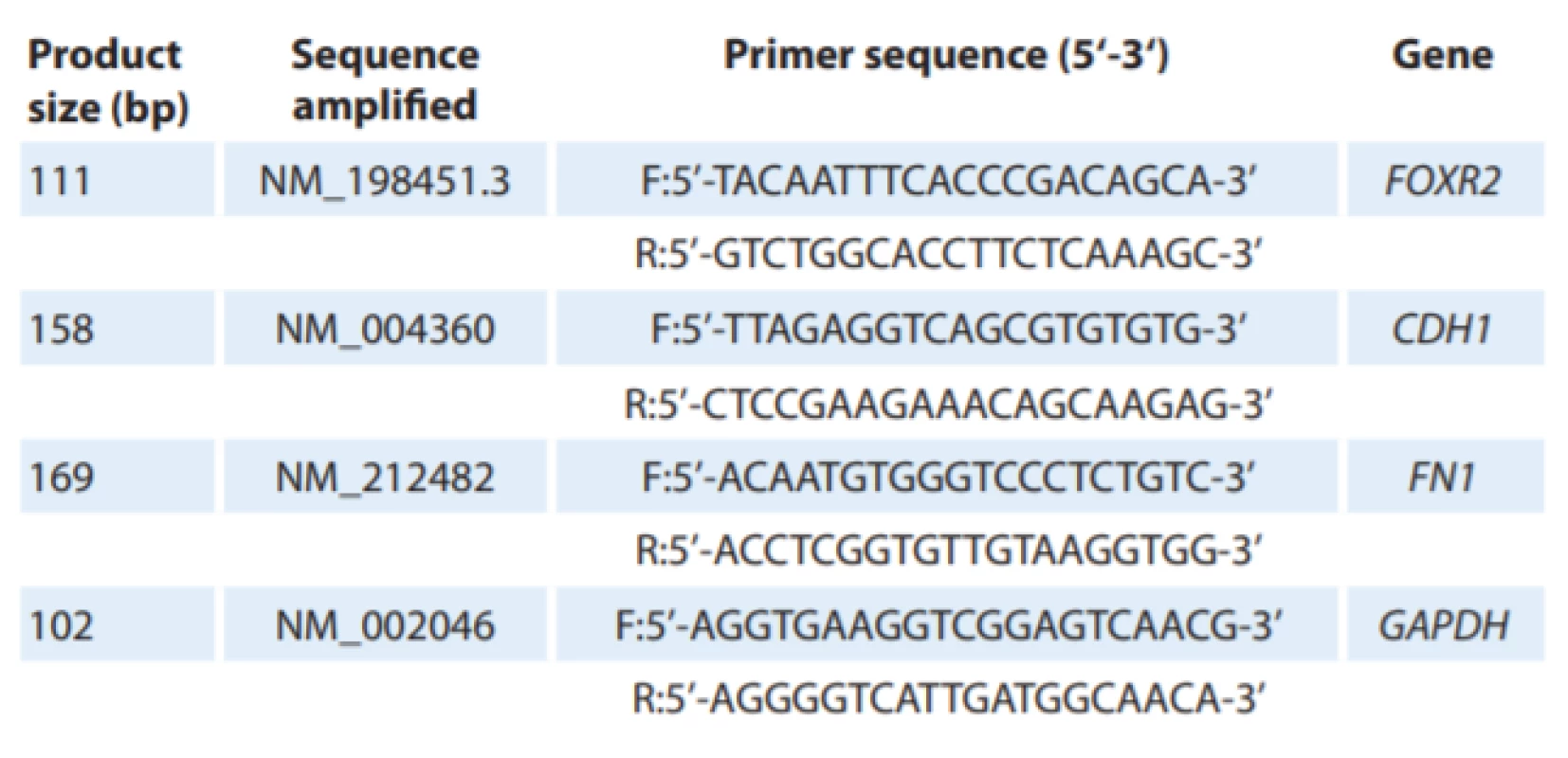
2. Oligonucleotid primer sequences. 
Statistical analysis
All data were analyzed using the SPSS standard software (Version 23.0, IBM, Armonk, NY, USA) and the results were reported as charts and graphical representation by Excel 2016 software. Based on the one-sample Kolmogorov-Smirnov test, our data did not have a normal distribution. Therefore, the comparisons between the two groups were performed by non-parametric tests. The independent sample t-test, Kruskal-Wallis test and Mann-Whitney test were used to examine the relationship between the quantitative variables and other clinical and pathological parameters. The quantitative data were expressed as the means ± standard deviation (SD). Moreover, a receiver-operating characteristic (ROC) curve was performed as a fundamental tool for evaluating a diagnostic performance and accuracy of the test to discriminate between the cases and controls. Indeed, the biomarker index of FOXR2 expression was assessed by the ROC curve in MedCalc Statistical Software. The P-values < 0.05 were considered as statistically significant for all data.
Results
Clinical and histopathological features
In this study, 20 patients with ovarian cancer with a mean age of 45.25 ± 2.56, ranged 19–64 years, were included. The general characteristics of the ovarian adenocarcinoma patients were presented in Tab. 2.
3. Characteristics of pataints with epithelial ovarian cancer. 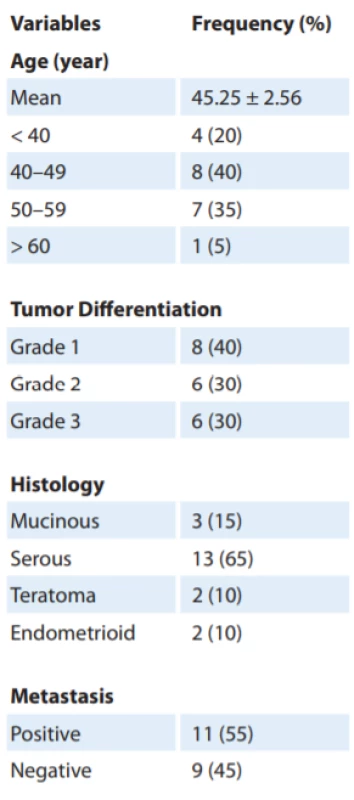
Assessment of RNAs quality
Fig. 1 showed both 18S and 28S rRNA appeared as typical bands after electrophoresis of total RNA, represented to be intact. The 28S rRNA band was of more intensity than the 18S rRNA. The 28S rRNA band was approximately twice as intense as the 18S rRNA. Also, the results from a nanodrop spectrophotometer showed that OD260/280 was 1.8–2 for all samples and the average of tissues RNA density was about 1,000 ng/μl (ranged 655–1,400 ng/μl) and it was 1,178 ng/μl for RNA extracted from the cell line.
1. Denaturing gel electrophoresis stained with ethidium bromide showing total RNA extracted from cell line (SKOV3) and ovarian tissue samples of patients with EOC. 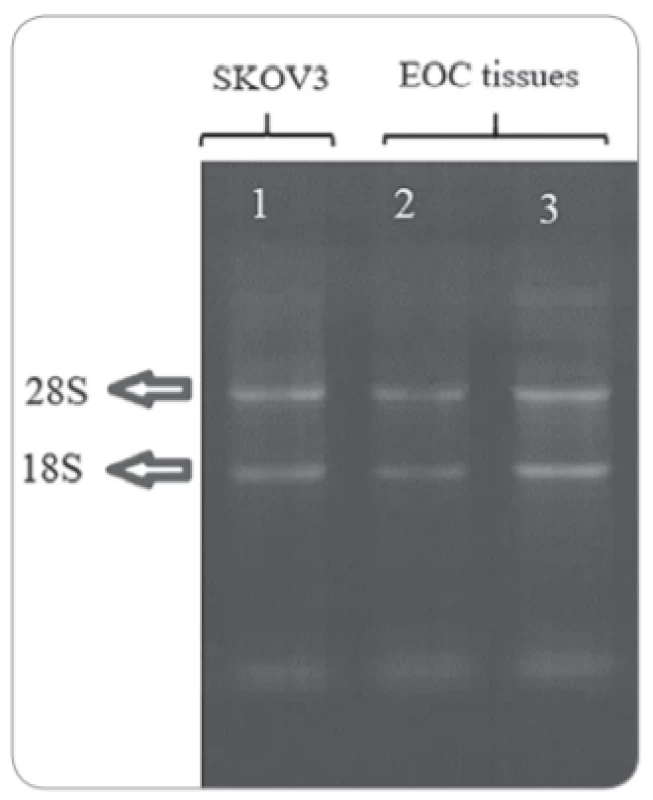
Total RNA (2 μl) was separated on agarose (1% w/v) gel. Expression of FOXR2 and EMT markers in EOC tissues and cell line
As we conducted RT-PCR to evaluate the relative expression levels of FOXR2 in case and control groups, there was a significant up-regulation (about 2-fold) in FOXR2 expression in EOC cases compared to non-cancerous controls (P = 0.015) (Fig. 2A). It was also found that the remarkable overexpression levels of FOXR2 in SKOV-3 cell line and 3 normal ovarian tissue samples pool (P = 0.035), but it was not significant statistically for FN1 (P = 0.09) and CDH1 (P = 0.3) genes (Fig. 2B). To investigate the expression level of CDH1 and FN1 genes as EMT-related markers (E-cadherin and fibronectin), no difference was found between case and control groups regarding CDH1 mRNA levels (P = 0.025). In contrast, the significantly higher expression levels were detected in the FN1 gene (2.8-fold) (P = 0.003) (Fig. 2A) as well as in SKOV-3 cell line (Fig. 2B).
2. A. Comparison of expression of target genes to GAPDH in epithelial ovarian tissues (EOC) obtained from EOC patients and SKOV-3 cell line by Mann-Whitney test. The ratio of genes expression of FOXR2, FN1, CDH1 and GAPDH. 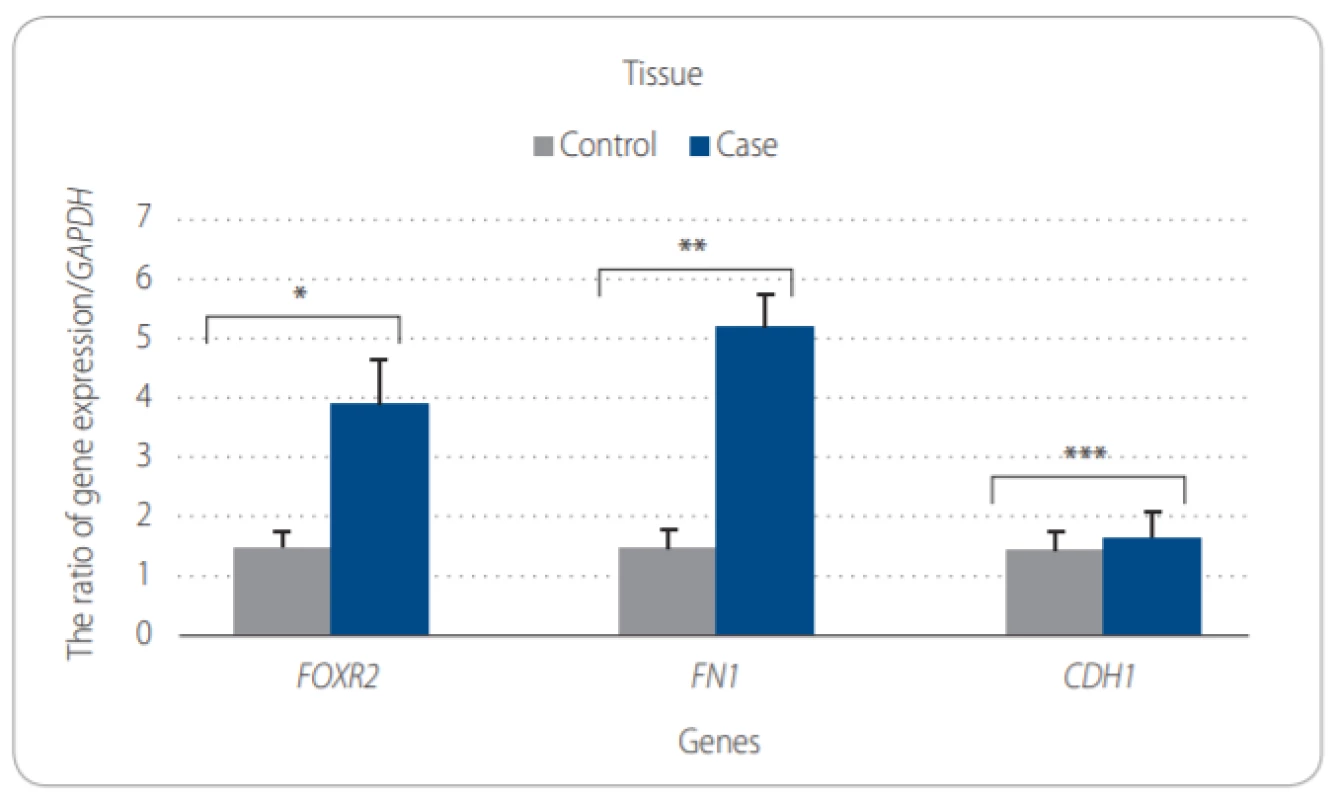
There were signifi cant diff erences between tumor (case) and control groups regarding mRNA levels of FOXR2 (*P = 0.015), FN1 (**P = 0.003) and CDH1 (***P = 0.025). Fig. 2B. Comparison of expression of target genes to GAPDH in epithelial ovarian tissues (EOC) obtained from EOC patients and SKOV-3 cell line by Mann-Whitney test. The ratio of genes expression of FOXR2, FN1, CDH1 and GAPDH. 
The mRNA expression levels of FOXR2 was remarkably upregulated in SKOV-3 cell lines (P = 0.035) when compared to the normal ovarian pool tissues. However, there were no signifi cant higher FN1 and CDH1 expression levels between SKOV-3 cell lines and normal controls (P = 0.09 and P = 0.3). P < 0.05 was considered as a signifi cant value. Association of gene expression and clinical aggressiveness of ovarian cancer
There was a positive relationship between FOXR2 mRNA levels and tumor grades. Despite insignificancy, the higher FOXR2 expression levels were associated with a high grade (grade III) of EOC compared to low and moderate grades – I and II) (P = 0.5) (Fig. 3A). We did not observe any significant change in FOXR2 expression related to the histological type and the metastatic features of tumor samples according to the Kruskal-Wallis test (P = 0.949 and P = 0.656, respectively) (Tab. 3). It was also demonstrated that the mRNA levels of EMT-related markers correlated with clinicopathological parameters so that FN1 expression increased in high-grade tumors (P = 0.8) despite almost no expression levels of CDH1 in high-grade tumors, compared to those with a low-grade status (P = 0.05) (Fig. 3B and 3C).
3. A. Gene expression levels of FOXR2 in patients with epithelial ovarian cancer based on tumor grades. 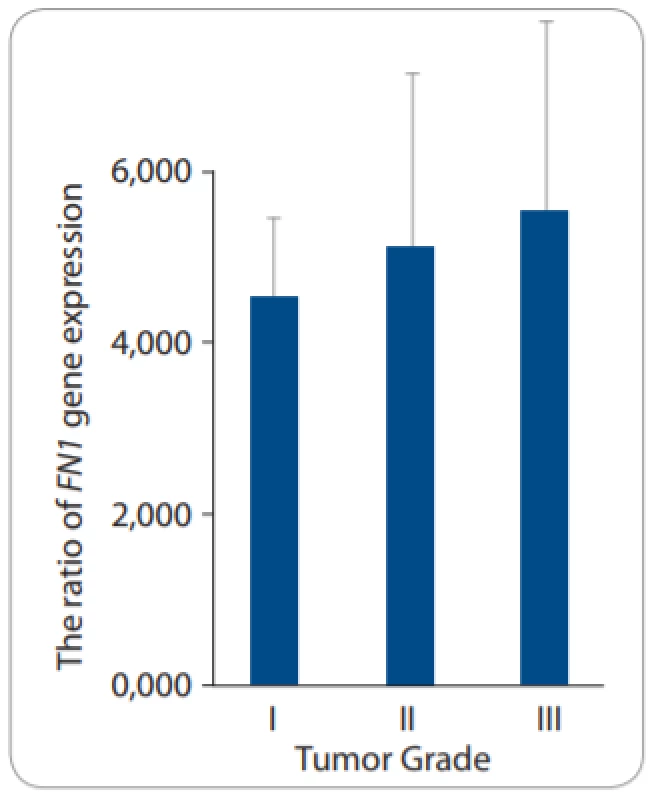
The rate of FN1 expression was no signifi - cantly increased among all tumors grades (P = 0.8). Fig. 3B. Gene expression levels of FOXR2 in patients with epithelial ovarian cancer based on tumor grades. 
There was almost no gene expression regarding CDH1 in high grade (III) compared to low (I) and moderate grade (II) tumors (P = 0.05). Fig. 3C. Gene expression levels of FOXR2 in patients with epithelial ovarian cancer based on tumor grades. 
There was a positive relationship between FOXR2 mRNA levels and tumor grades. A progressive increase was seen in FOXR2 mRNA levels and high tumor grade (III) based on Kruskal-Wallis test despite the insignifi cancy between the three tumor grades (P = 0.5). P < 0.05 was considered as a signifi cant value. 4. Correlations between FOXR2 expression levels and clinicopathological parameters of epithelial ovarian cancer. 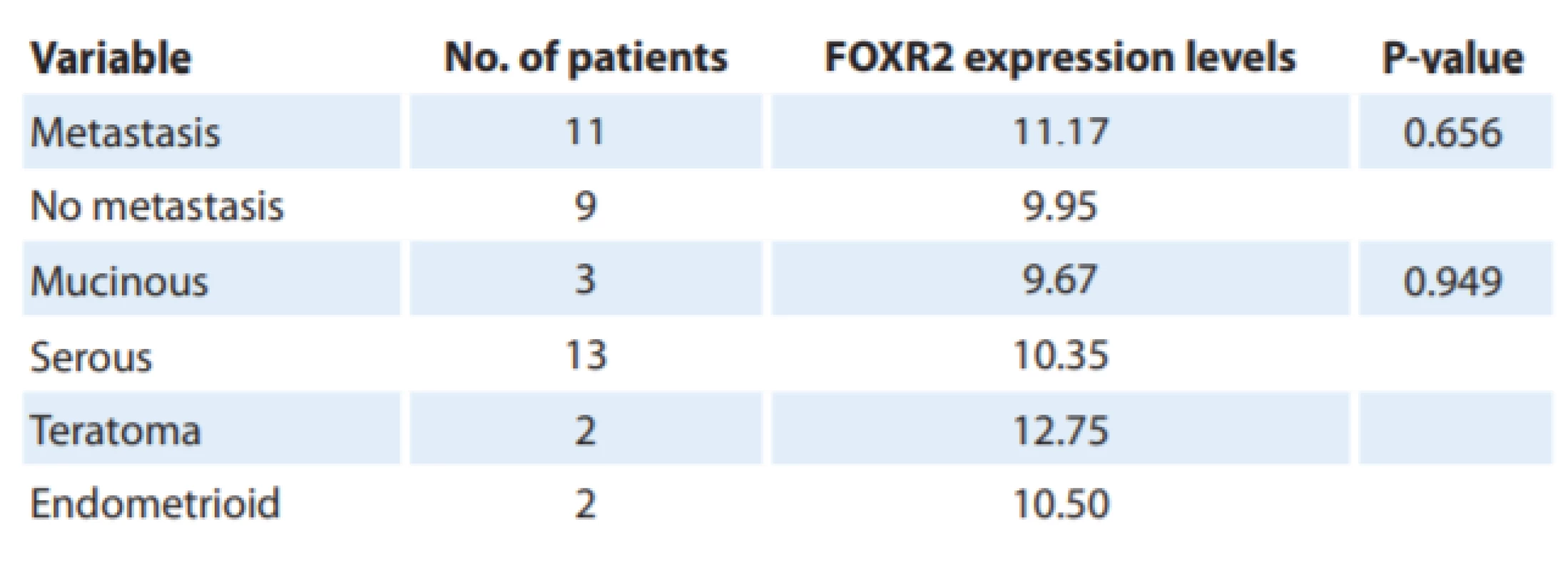
Correlation of FOXR2 expression and epithelial to mesenchymal transition
In addition, the data demonstrated a significant positive correlation between the rate of FOXR2 expressions and EMT-related markers (CDH1 and FN1) in EOC (P= 0.038, P = 0.007, respectively). Furthermore, the area under the curve (AUC) ROC demonstrated that the biomarker index for FOXR2 was about 0.722 with specificity and sensitivity of 80/00 and 55/00, respectively (P = 0.006) (Fig. 4 – available at www.linkos.cz).
Discussion
Ovarian carcinoma is a highly lethal cancer with an average of almost 30% of patients achieving the five-year survival [5]. Most of the women with ovarian cancer appear with late stage with expanding resistance to first-line chemotherapy caused by late diagnosis due to the obscure symptoms of ovarian cancer [20]. Therefore, identifying the novel prognostic and therapeutic biomarkers is crucial for improving the long-term survival rate of patients. Recently, the researchers suggested the biomolecular agents with important potentials for diagnosing and treating ovarian cancer.
The FOX gene family has frequently been studied and it has been reported to play a vital regulatory role as transcriptional factors in a broad spectrum of biological processes, especially during the development and tumorigenesis [14]. Furthermore, many of these family members can act as oncogenes in EOC. For example, overexpression of FOXM1 was reported by Zhao et al. in EOC [21]. Li et al. found that FOXC2 was significantly upregulated in cisplatin-resistant ovarian cancer tissues or ovarian cancer cell lines [22]. Also, in a new study, Li et al. identified that the upregulation of FOXR2 gene in ovarian cancer cell lines play a significant role in angiogenesis and malignancy in EOC by Sonic Hedgehog pathway [23].
In this study, the expression profile of FOXR2 gene in EOC samples was examined and it was compared with normal ovarian tissues for the first time. It was demonstrated that FOXR2 was overexpressed in EOC tissues/SKOV-3 cell line compared to controls. In addition, the elevated expression of FOXR2 was found to be significantly associated with a high grade (III) of EOC samples. FOXR2 is a scarcely studied member of the Fox protein superfamily [24]. Recently, the FOXR2 overexpression has been declared in various human malignancies including breast, prostate, colorectal and medulloblastoma tumors [17–19,25].
One of the most important processes in metastasis is EMT wherever polarized epithelial cells lose their intercellular adhesion, then transformed to mesenchymal phenotype and gain migratory and invasive characteristics [10]. Fibronectin was overexpressed, too; but E-cadherin had no significant difference between case and control samples. Therefore, it is likely that FOXR2 overexpression and its interaction with the other important transcription factors (TFs) MYC and MAX contribute to tumorigenesis of the ovary and maybe these stable TFs complexes affect the CDH1 promoter and inhibit in this gene expression. In previous studies, it was demonstrated that Fox proteins acted as the final effectors in various signaling pathways like Wnt/B-catenin, Shh, TGF-B1 and MAPK, leading to the cross-talks between parallel signaling pathways, so they caused more suitable cell responses to extracellular signals. Overall, the post-translational modifications on Fox proteins determined their target genes [14]. It was revealed that TFs are strongly regulated by the association with other proteins and these protein-protein interaction networks are crucial for their transcriptional activities via on and off chromatin [26]. A recent study showed that FOXR2 has a potential interaction with MYC and MAX in the cell nucleus. Indeed, FOXR2 promotes the effect of MYC transcriptional activity in tumor growth [27]. Despite the high expression levels of MYC, a recent study by Song et al. showed that E-cadherin expression was down-regulated in ovarian cancer [28,29]. It was established that the inhibition of FOXR2 reduced the migratory and the invasions abilities of cancer cells as well as their EMT [18,19].
Also, the samples of EOC patients with high-grade tumors showed no expression of CDH1 despite the FN1 with significantly up-regulations in all grades, specifically in grade III. It means that FOXR2 expression was significantly associated with high grades of ovarian cancer. These results indicated that FOXR2 inhibited the CDH1 expression rates and stimulated the cells to migration. Therefore, supplementary studies should be done to elucidate the exact mechanism of action of this gene in tumorigenesis of ovarian cancer.
Conclusions
The data declared that FOXR2 as an oncogene in EOC has a crucial impact on the EMT pathway, ovarian carcinogenesis and progression. The findings suggest that FOXR2 can serve as an independent prognostic factor as a new biomarker in early diagnosis of ovarian cancer or may be used as a novel drug agent for gene therapy in EOC patients, and therefore earlier treatments that lead to a reduction in mortality due to EOC.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
This study was supported by a grant of Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Studie byla fi nancována z grantu Univerzity lékařských věd Shahid Sadoughi, Yazd, Írán.
The authors would like to thank the staff of Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Autoři děkují personálu Výzkumného centra rekurentních potratů při Výzkumném a klinickém centru pro neplodnost, Univerzita lékařských věd Shahid Sadoughi, Yazd, Írán.
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
The authors declare they have no potential confl icts of interest concerning drugs, products, or services used in the study.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do bi omedicínských časopisů.
The Editorial Board declares that the manuscript met the ICMJE recommendation for biomedical papers.
Mahta Mazaheri Naeeini, MD, PhD
Department of Medical Genetics School of Medicine Shahid Sadoughi University of Medical Sciences Yazd Iran
e-mail: hn_1364@yahoo.com
Submitted/Obdrženo: 18. 9. 2019
Accepted/Přijato: 11. 11. 2019
Sources
1. Reid BM, Permuth JB, Sellers TA et al. Epidemiology of ovarian cancer: a review. Cancer Biol Med 2017; 14 (1): 9–32. doi: 10.20892/j.issn.2095-3941.2016.0084.
2. Kamali M, Hamadani S, Neamatzadeh H et al. Association of XRCC2 rs3218536 polymorphism with susceptibility of breast and ovarian cancer: a systematic review and meta-analysis. APJCP 2017; 18 (7): 1743–1749. doi: 10.22034/APJCP.2017.18.7.1743.
3. Rauh-Hain JA, Krivak TC, Del Carmen MG et al. Ovarian cancer screening and early detection in the general population. Rev Obstet Gynecol 2011; 4 (1): 14–21.
4. Karimi-Zarchi M, Forat-Yazdi M, Vafaeenasab MR et al. Evaluation of the effect of GnRH agonist on menstrual reverse in breast cancer cases treated with cyclophosphamide. Eur J Gynaecol Oncol 2014; 35 (1): 59–61.
5. Hunn J, Rodriguez GC. Ovarian Cancer. Clinic Obstet Gynecol 2012; 55 (1): 3–23. doi: 10.1097/GRF.0b0 13e31824b4611.
6. Torre LA, Trabert B, DeSantis CE et al. Ovarian cancer statistics, 2018. CA Cancer J Clin 2018; 68 (4): 284–296. doi: 10.3322/caac.21456.
7. Ng JS, Low JJH, Ilancheran A. Epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol 2012; 26 (3): 337–345. doi: 10.1016/j.bpobgyn.2011.12.005.
8. Ovarian epithelial, fallopian tube, and primary peritoneal cancer treatment (PDQ®): Patient version online. Available from: http: //www.ncbi.nlm.nih.gov/pubmed/26389163.
9. Ferrandina G, Corrado G. Treatment of platinum refractory or resistant ovarian cancer. Lancet Oncol 2018; 19 (9): 1147–1149. doi: 10.1016/S1470-2045 (18) 30428-5.
10. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 2009; 119 (6): 1420–1428. doi: 10.1172/JCI39104.
11. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 2014; 15 (3): 178–196. doi: 10.1038/nrm3758.
12. Acloque H, Adams MS, Fishwick K et al. Epithelial--mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest 2009; 119 (6): 1438–1449. doi: 10.1172/JCI38019.
13. Katoh M, Katoh M. Human FOX gene family (Review). Int J Oncol 2004; 25 (5): 1495–1500. doi: 10.3892/ijo.25.5.1495.
14. Benayoun BA, Caburet S, Veitia RA. Forkhead transcription factors: key players in health and disease. Trends Genet 2011; 27 (6): 224–232. doi: 10.1016/j.tig.2011.03.003.
15. Golson ML, Kaestner KH. Fox transcription factors: from development to disease. Development 2016; 143 (24): 4558–4570. doi: 10.1242/dev.112672.
16. Katoh M, Katoh M. Identification and characterization of human FOXN6, mouse Foxn6, and rat Foxn6 genes in silico. Int J Oncol 2004; 25 (1): 219–223.
17. Song H, He W, Huang X et al. High expression of FOXR2 in breast cancer correlates with poor prognosis. Tumour Biol 2016; 37 (5): 5991–5997. doi: 10.1007/s13277-015-4437-4.
18. Xu W, Chang J, Liu G et al. Knockdown of FOXR2 suppresses the tumorigenesis, growth and metastasis of prostate cancer. Biomed Pharmacother 2017; 87 : 471–475. doi: 10.1016/j.biopha.2016.12.120.
19. Lu SQ, Qiu Y, Dai WJ et al. FOXR2 promotes the proliferation, invasion and epithelial-mesenchymal transition in human colorectal cancer cells. Oncol Res 2017; 25 (5): 681–689. doi: 10.3727/096504016X14771034190471.
20. Kamal R, Hamed S, Mansour S et al. Ovarian cancer screening-ultrasound; impact on ovarian cancer mortality. British J Radiol 2018; 91 (1090): 20170571. doi: 10.1259/bjr.20170571.
21. Zhao F, Siu MK, Jiang L et al. Overexpression of forkhead box protein M1 (FOXM1) in ovarian cancer correlates with poor patient survival and contributes to paclitaxel resistance. PLoS ONE 2014; 9 (11): e113478. doi: 10.1371/journal.pone.0113478.
22. Li C, Ding H, Tian J et al. Forkhead box protein C2 (FOXC2) promotes the resistance of human ovarian cancer cells to cisplatin in vitro and in vivo. Cell Physiol Biochem 2016; 39 (1): 242–252. doi: 10.1159/000445620.
23. Li B, Huang W, Cao N et al. Forkhead-box R2 promotes metastasis and growth by stimulating angiogenesis and activating hedgehog signaling pathway in ovarian cancer. J Cell Biochem 2018; 119 (9): 7780–7789. doi: 10.1002/jcb.27148.
24. van Rijn S, Vouri M, Pfister SM et al. Embr-04. What the FOX say? A molecular analysis of FOXR2 in pediatric brain tumors. Neuro-Oncology 2018; 20 (Suppl 2): 69. doi: 10.1093/neuonc/noy059.189.
25. Koso H, Tsuhako A, Lyons E et al. Identification of FOXR2 as an oncogene in medulloblastoma. Cancer Res 2014; 74 (8): 2351–2361. doi: 10.1158/0008-5472.CAN-13-1523.
26. Li X, Wang W, Wang J et al. Proteomic analyses reveal distinct chromatin-associated and soluble transcription factor complexes. Mol System Biol 2015; 11 (1): 775. doi: 10.15252/msb.20145504.
27. Li X, Wang W, Xi Y et al. FOXR2 interacts with MYC to promote its transcriptional activities and tumorigenesis. Cell Rep 2016; 16 (2): 487–497. doi: 10.1016/j.celrep.2016.06.004.
28. Reyes-González JM, Armaiz-Peña GN, Mangala LS et al. Targeting c-MYC in platinum-resistant ovarian cancer. Mol Cancer Ther 2015; 14 (10): 2260–2269. doi: 10.1158/1535-7163.MCT-14-0801.
29. Song IH, Kim K-R, Lim S et al. Expression and prognostic significance of epithelial-mesenchymal transition-related markers and phenotype in serous ovarian cancer. Pathol Res Pract 2018; 214 (10): 1564–1571. doi: 10.1016/j.prp.2018.07.016.
Labels
Paediatric clinical oncology Surgery Clinical oncology
Article was published inClinical Oncology

2020 Issue 3-
All articles in this issue
- Importance of Aberrantly Activated Hedgehog/Gli Pathway in Tumour Progression
- Assessment of Quality of Life in Patients with Head and Neck Cancer
- Current Possibilities of Early Detection of Cardiotoxicity of Cytostatic Treatment
- Prognostic Survival Factors of Hepatocellular Carcinoma Treated with Transarterial Chemoembolization
- Complete Response to Chemotherapy in Metastatic Pancreatic Carcinoma Associated with Double Heterozygous Germline Mutation in BRCA2 and CHEK2 Genes – a Case Report
- Long-Term Effect of Erlotinib Therapy in the Third Line of Anticancer Treatment in a Patient with Non-Small-Cell Lung Cancer – a Case Report
- Therapy with Trifluridine/Tipiracil and Regorafenib in Patientswith Pre-treated Metastatic Colorectal Cancer – Experience from the Czech Republic
- Editorial
- Aktuality z odborného tisku
- Predstavujeme nové knihy
- Oncology in pictures
- Levels of NT-proBNP and Troponin T in Cancer Patients – Mini-Review
- Association of XPG rs17655G>C and XPF rs1799801T>C Polymorphisms with Susceptibility to Cutaneous Malignant Melanoma: Evidence from a Case-Control Study, Systematic Review and Meta-Analysis
- The Relationship of FOXR2 Gene Expression Profile with Epithelial-Mesenchymal Transition Related Markers in Epithelial Ovarian Cancer
- Clinical Oncology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Levels of NT-proBNP and Troponin T in Cancer Patients – Mini-Review
- Complete Response to Chemotherapy in Metastatic Pancreatic Carcinoma Associated with Double Heterozygous Germline Mutation in BRCA2 and CHEK2 Genes – a Case Report
- Assessment of Quality of Life in Patients with Head and Neck Cancer
- Importance of Aberrantly Activated Hedgehog/Gli Pathway in Tumour Progression
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

