-
Medical journals
- Career
Combining Systemic Therapies with Radiation in Non ‑ small Cell Lung Cancer
Authors: K. Adamowicz; E. Goszczynska‑ matysiak
Authors‘ workplace: Department of Oncology, Regional Oncology Center of Gdansk, Poland
Published in: Klin Onkol 2015; 28(5): 321-331
Category: Reviews
doi: https://doi.org/10.14735/amko2015321Overview
Radiotherapy has been the mainstay of treatment of stage III non‑small cell lung cancer patients. In the early 90s, combined treatment with chemotherapy was introduced. In 1995, a meta‑analysis showed improved treatment outcome of the sequential use of cisplatin‑based chemotherapy and radiotherapy compared to radiotherapy alone. Subsequent randomized studies and two meta‑analyses demonstrated that concurrent radiochemotherapy is superior (local control and overall survival) to sequential usage of both method. However, several questions remain unanswered concerning the optimal chemotherapy regimen and radiotherapy doses and techniques in terms of treatment outcome and toxicity profile. Targeted therapies represent a new class of drugs, which interfere with specific molecular targets (typically proteins) playing critical roles in tumor growth and progression. Some combinations appear to be too toxic, such as the vascular epithelial growth factor antibody bevacizumab. The feasibility of adding the epidermal growth factor receptor inhibitor cetuximab has been recently reported for non‑small cell lung cancer patients. Strategies to incorporate safely novel antiangiogenic agents into combined ‑ modality therapy in lung cancer are needed. Hopefully, rapid development of molecular oncology will contribute to better patient selection to particular strategies and to treatment optimization. Increasing radiotherapy doses applied according to up ‑ to ‑ date techniques and combinations with new biologicals might lead to further treatment improvements.
Key words:
lung neoplasms – chemotherapy – radiotherapy – drug therapyIntroduction
Lung cancer is the most common cause of cancer death globally [1]. Most cases of lung cancer occur around the age of 60 – 70 years [2].
Treatment of non‑small cell lung cancer (NSCLC) is challenging in many ways. Until the 1990s, radiotherapy alone was the standard treatment for stages IIIA and IIIB of NSCLC. With the standard dose of 60 Gy in 30 doses, survival rates were extremely poor [3]. Indeed, technical developments allowing the administration of higher radiation doses resulted in strategies to improvetreatment results include increasing doses of radiotherapy and decreasing overall treatment time [4]. For NSCLC, a dose‑effect relationship exists: the higher the radiation dose, the greater the probability of tumor control improved local control and OS [5]. The theoretical solution of simply increasing radiation doses to high biologically effective doses, ideally above the threshold of 100 Gy, has been suggested by several groups [6 – 9]. However, radiation dose escalation does not address the issue of distant or out ‑ of ‑ field relapses. Therefore, a different option is to combine radiotherapy with chemotherapy. The first report on improved OS after adding chemotherapy to radiation was published more than 20 years ago [10]. Over the past decades, concomitant chemotherapy and radiotherapy have become the established treatment for patients with stage III NSCLC. In this review, we present current clinical knowledge on combining available systemic therapies with radiation.
Radiochemotherapy in locally advanced NSCLC
The strategy of exclusive radiotherapy for locally advanced inoperable NSCLC has been challenged after publication of meta‑analysis by the Non ‑ small Cell Lung Cancer Collaborative Group in 1995 [11]. Since than, a combination of chemotherapy and radiotherapy is the recommended treatment in this group of patients. Radiotherapy preceded by (usually) two courses of chemotherapy yielded an improvement of the 2‑year overall survival rate (OS) from 21% to 25%. The 5‑year survival increased from 6% to 8% provided that the chemotherapy regimen included cisplatin. The effect was explained by a reduction of distant metastases. Until now, this effect of a lower distant metastasis rate was observed in one study only [12]. In this study, Le Chevalier et al. compared radiotherapy alone to chemotherapy and radiotherapy. However, patients with adenocarcinoma were excluded. Since an important proportion of NSCLC patients were not included, results might not be representative. The 2‑year survival rate was 14% in radiotherapy alone group and 21% in combined treatment group. The 3‑year survival rate was 12% for the combination arm vs. 4% for the radiotherapy arm (p < 0.02) and local control was poor in both groups (17% and 15%, resp.). To our knowledge, these results have never been confirmed. Until recently, sequential cisplatin‑containing radiochemotherapy has been the standard treatment for inoperable stage IIIA and IIIB disease. Various chemotherapy schedules have been applied, but the treatment outcome did not differ significantly.
Despite this progress, both loco ‑ regional and distant failures are frequent. Over the last 20 years, concomitant use of radiotherapy and chemotherapy have been extensively studied in various malignancies, including NSCLC, rectal cancer, anal cancer and head and neck cancers, and has currently replaced radiotherapy alone in patients with good performance status. This strategy, through superadditive effect, not only improves local tumor control but also increases overall survival [13]. The benefits of concomitant radiochemotherapy include a potential synergism between both modalities and avoiding the delay of radiotherapy. Therefore, there is a rationale for considering concomitant chemo ‑ radiation also in patients with high‑risk lung cancer. Attempts to improve the loco ‑ regional control included increasing radiotherapy dose using altered fractionation regiments and combining chemotherapy with radiotherapy. After phase I and phase II studies, the EORTC started a 3 - arm phase III trial comparing split ‑ course radiotherapy of 55 Gy using the same radiotherapy scheme, concurrently combined with 30 mg/ m2 cisplatin once a week or 6 mg/ m2 daily [14]. No improvement was seen after treatment with radiotherapy and weekly cisplatin. The 6 mg/ m2 cisplatin daily added to radiotherapy improved OS due to improved control of local disease. The difference was also significant after adjustment for known prognostic factors in a multivariate analysis. There was no effect on the distant metastasis rate, and late toxicity was not increased. These data demonstrated that cisplatin improved the radiotherapy effect by radiosensitization. The most frequently reported acute side effects were nausea and vomiting. In 1992, Trovo et al. [15] also published their randomized phase III study. Three weeks of radiotherapy, to a dose of 45 Gy, were compared to the same radiotherapy dose with the addition of 6 mg/ m2 cisplatin daily. In this study, no significant advantage of the combined treatment over radiation therapy only was found. However, this result may be due to the lower dosage of radiation used in the study (Tab. 1).
1. Chemoradiotherapy in locally advanced NSCLC. 

IMRT – intensity-modulated radiotherapy, KPS – Karnofsky performance status, ECOG – Eastern Cooperative Oncology Group performance status, WHO – World Health Organization performance status, ENI – elective nodal irradiation, OS – overall survival, PFS – progression-free survival, PR – partial remission, CR – complete remission, TTP – time to progresion, NR – not reported All phase III trials were included in a meta‑analysis including 12 trials and 1921 patients by Aupérin et al. [16] indicated a 4% survival gain at two years and 2% at five years for concurrent chemoradiation vs. radiotherapy alone, a comparable improvement to that observed with the sequential combination. Even though this meta‑analysis was based on individual patient data, it did not allow to accurately define the size of such a potential treatment benefit and the optimal schedule of chemotherapy. The efficacy of concurrent chemoradiotherapy vs. radiotherapy also was compared in a metaanalysis including 14 randomized studies (and 2,393 patients) in 2010 [17]. A Cochrane meta‑analysis confirmed these conclusions: concurrent chemoradiotherapy was associated with 14% reduction in the risk of death at two years compared to sequential chemoradiotherapy, and a 7% reduction compared to radiotherapy alone.
If sequential and concurrent radiochemotherapy improved overall survival, so there is another question: which is better? In several trials, improved 1 - and 2‑year overall survival rates in favor of the concurrent arm were reported [18 – 23]. Most of these trials were included in a new meta‑analysis based on individual patient data by Aupérin et al. [24] who concluded that concurrent radiochemotherapy yielded superior results compared to sequential combinations. There was a significant benefit of concomitant radiochemotherapy on overall survival (p = 0.004), with an absolute benefit of 5.7% (from 18.1% to 23.8%) at three years and 4.5% at five years. There was no significant difference regarding acute pulmonary toxicity. Concomitant treatment decreased locoregional progression, but concomitant radiochemotherapy increased acute esophageal toxicity (grade 3 – 4) from 4% to 18%. This improved OS was accomplished because of an improved locoregional control. There were no significant differences between the regimens: single or double high‑dose chemotherapy or daily low‑dose cisplatin. No differences in distant metastasis rate were observed between the two approaches.
Within a few months, a meta‑analysis was published by O‘Rourke et al. reporting a 10% absolute survival benefit at two years [25]. Six trials (1,024 patients) of concurrent vs. sequential chemoradiation were included. A significant benefit of concurrent treatment was shown in overall survival (hazard ratio – HR 0.74, 95% CI 0.62 – 0.89). More treatment‑related deaths (4% vs. 2%) were reported in the concurrent arm without statistical significance (relative risk – RR 2.02, 95% CI 0.90–4.52). There was increased severe esophagitis with concurrent treatment (RR 4.96, 95% CI 2.17 – 11.37). The most important acute but manageable side effect was esophagitis grade 3 to 4 in 18% of the patients treated with concurrent radiochemotherapy vs. 4% in patients treated with sequential arm.
The role of timing and sequencing of the treatment may also depend on the tumor type, the degree of oxygenation of tumor cells and other biochemical processes occurring during radiation. In clinical practice, a compromise option is the alternation of radiotherapy and chemotherapy, for example by insertion of radiotherapy after 2 – 3 cycles of chemotherapy. The clinical efficacy of this strategy has, however, not been verified in prospective clinical studies. Concurrent chemoradiation is at present the treatment of choice for patients with locally advanced NSCLC. However, due to its higher toxicity, this combination is mostly restricted to patients in a good general condition, minimal comorbidity and who are relatively young [26 – 29].There is a question what proportion of patients would be suitable for concurrent chemoradiation. We found only one report on a population‑based study that prospectively evaluated comorbidities in all patients diagnosed with lung cancer, stage III for NSCLC [30]. In this prospective, population‑based study, more than half of the patients with stage III NSCLC were not eligible for concurrent chemoradiation on the basis of criteria of age and important comorbidities that were present at diagnosis. Less toxic alternatives are needed for these patients. So, there are arguments for sequential treatment, such as “safe” delivery of full dose of radiotherapy and chemotherapy, but there are also problems like delayed radiotherapy delivery especially in patients’ slow recovery from chemotherapy. El Sharouni [31] shows that in the time interval between the end of induction chemotherapy and the start of radiotherapy rapid tumor progression occurs as a result of accelerated tumor cell proliferation: mean tumor doubling times are much shorter than those in not treated tumors. As a consequence, the gain obtained with induction chemotherapy with regard to volume reduction was lost in the waiting time for radiotherapy (chemo‑resistant stem cells that persist and can give rise to tumor regrowth). A correlation was observed between the amount of delay and degree of regrowth for percent volume and percent tumor diameter change. A delay between induction chemotherapy and radiotherapy greater than 21 days produced greater increases in percent volume change and percent diameter than lesser delays [32]. Also, a retrospective analysis of a total of 474 patients demonstrates a correlation between prolonged overall radiotherapy treatment time and survival in patients with locally advanced NSCLC, even when concurrent chemotherapy is used [33]. It is recommended to diminishthe time interval between chemo ‑ and radiotherapy to as short as possible.
Targeted therapies and radiotherapy in locally advanced NSCLC
Targeted therapies represent a new class of drugs, which interfere with specific molecular targets (typically proteins) playing critical roles in tumor growth and progression. The approved targeted therapies in lung cancer include erlotinib, gefitinib (a small‑molecule tyrosine kinase inhibitors) and bevacizumab (a monoclonal anti‑VEGF antibody). The accepted dogma is that antiangiogenic therapy destroys or blocks the function of tumor‑associated vessels to deprive the tumor of oxygen and nutrients, thereby inhibiting tumor growth. Numerous preclinical studies indicated synergistic activity of various antiangiogenic or antivascular therapies with single‑dose or fractionated radiotherapy in human and murine tumors [34,35]. However, since multiple variables contribute to the sensitivity of tumors to radiation or antiangiogenic treatment, the most effective way of their combining is virtually unknown [36,37]. Blocking survival signaling in endothelial cells after irradiation seems to increase the radiation response considerably [38]. Moreover, sensitization of endothelial cells just before exposure to radiation may be the most effective way to improve response of tumor cells to radiation [39,40]. On the other hand, induction of hypoxia via blood vessel damage may potentially induce radioprotection of the tumor. A logical and clearly proven premise for optimal multimodality therapy is therefore necessary for efficient translation of promising preclinical strategies into clinical applications. Many new biologicals have entered the therapeutic domain; several were combined with concurrent RCT regimens. Some combinations appear to be too toxic, such as the vascular epithelial growth factor antibody bevacizumab [41]. Also, the use of bevacizumab and erlotinib is not recommended given the lack of an efficacy signal and the substantial risk of esophageal toxicity (Tab. 2) [42].
2. Targeted therapies and radiotherapy in locally advanced NSCLC. 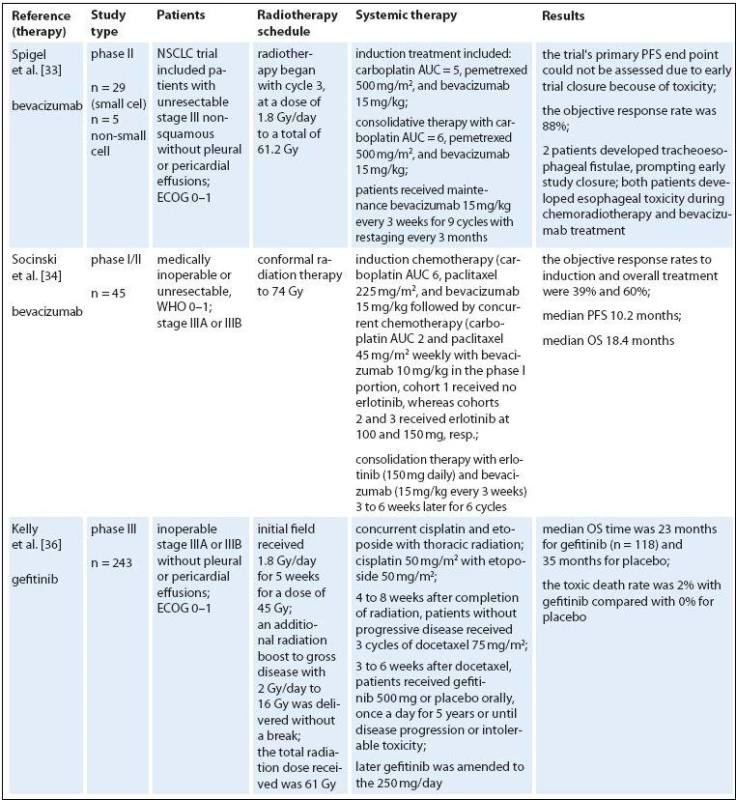

IMRT – intensity-modulated radiotherapy, KPS – Karnofsky performance status, ECOG – Eastern Cooperative Oncology Group performance status, WHO – World Health Organization performance status, ENI – elective nodal irradiation, OS – overall survival, PFS – progression-free survival, PR – partial remission, CR – complete remission, TTP – time to progresion, NR – not reported Erlotinib and gefitinib are small molecule inhibitors that reversibly target tyrosine kinase activity of the epidermal growth factor receptor (EGFR). EGFR is overexpressed and/ or mutated in many cancer types, and its activation triggers pathways involved in cell growth and proliferation. Early clinical studies with gefitinib showed promising efficacy and mild toxicity in patients with advanced NSCLC. In clonogenic in vitro survival experiments, gefitinib had significant radiosensitizing effects on NSCLC cell lines [43]. Gefitinib enhances radioresponse of NSCLC cells by suppressing cellular DNA repair capacity. But in unselected population, gefitinib did not improve OS [44]. The trial from the National Cancer Institute of Canada Clinical Trials Group showed that erlotinib monotherapy prolonged OS in patients with advanced NSCLC who had progressed after standard chemotherapy [45], and erlotinib is approved in this setting. Interestingly, EGFR expression does not seem to correlate with response to EGFR inhibitors but a recent analysis of data from this trial indicated that EGFR mutations and high copy number are predictive of response to erlotinib [46]. In addition, EGFR fluorescence in situ hybridization score was a significant predictive marker of differential survival benefit of erlotinib. Erlotinib‑induced apoptosis was augmented by radiation in cells with very high expression of HER1/ EGFRonly. In conclusion, high HER1/ EGFR expression may result in a high degree of radiosensitization with erlotinib combined with radiation [47]. A strong rationale may exist for combining erlotinib with radiotherapy. Erlotinib helps disrupt cell growth pathways and enhances the sensitivity of cells to the effects of radiotherapy [48–50]. It is also possible that radiotherapy enhances the effectiveness of erlotinib by cytoreducing the tumor and creating a hypoxic environment [51]. Several studies in NSCLC were conducted to evaluate erlotinib in combination with radiotherapy. A prospective phase II study found that radiotherapy and concurrent erlotinib used in the treatment of patients with unresectable NSCLC shows promising results without an increase in toxicity [52]. Adverse events related to radiotherapy included esophagitis, radiation dermatitis, and pneumonitis. The addition of erlotinib to radiotherapy did not appear to increase radiotherapy associated toxicities. Erlotinib‑related adverse events included mild to moderate skin rash (61.5%) and diarrhea (23%). The RR was 55.5% in the radiotherapy ‑ alone arm compared with 83.3% in the erlotinib arm. The Cancer and Leukemia Group Bis conducting a phase II trial, CALGB 30605, of paclitaxel followed by radiotherapy and erlotinib in patients with unresectable stage III NSCLC. The study is evaluating induction chemotherapy consisting of paclitaxel and carboplatin. Patients with no disease progression outside the planned radiation field will continue to receive concurrent erlotinib and radiotherapy. Results from current studies are eagerly awaited.
Several drugs interfering with the EGFR signaling pathway have been developed e. g. cetuximab (a human ‑ murine chimeric IgG1 monoclonal antibody that binds to the extracellular region of the EGFR). Under experimental laboratory conditions in animal models, cetuximab increases tumor radiocurability (fractionated and single dose irradiation) [53,54]. The feasibility of adding the epidermal growth factor receptor inhibitor cetuximab has been recently reported for NSCLC patients [55]. We found a few phase II clinical trials of cetuximab combined with radiotherapy for NSCLC. Two of them have combined radiotherapy and cetuximab without any chemotherapy in patients who are not candidates for chemoradiation [56,57]. Combined radioimmunotherapy with cetuximab was safe and feasible, especially in elderly patients with multiple comorbidities. Another study included patients with inoperable stage III disease and good performance status after induction chemotherapy [58,59]. Induction chemotherapy followed by concurrent cetuximab and radiotherapy to 68 Gy was clearly feasible with promising OS. Toxicity, like pneumonitis and esophagitis was low compared to most schedules with concurrent chemotherapy. The last study published by Radiation Therapy Oncology Group (RTOG) was a phase II study of chemoradiotherapy with carboplatin and paclitaxel plus cetuximab in patients with stage III NSCLC [60]. The combination of cetuximab with CRT is feasible and shows promising activity. The overall survival achieved with this regimen was longer than any previously reported by the Radiation Therapy Oncology Group with median survival 22.7 months, and 24 - month overall survival – 49.3%. The second trial in this category with several important differences (mandatory PET, higher radiation dose of 70 Gy, only seven weeks of cetuximab concomitant to radiotherapy, chemotherapy with carboplatin and pemetrexed) was done [61]. Median OS was 25.2 months and failure‑free survival 12.3 months.
Until now, no definite data can be reported. Further basic research and appropriately designed clinical studies are clearly needed to optimize scheduling of combined radiation and molecular targeted therapies. The results of the published clinical trials (one of them was a phase III study) suggest that larger randomized trials are warranted. It is very important to include the right patient population, especially patients with the right genetics/ mutations for these clinical trials.
Conclusions
Patients with stage III disease differ with regard to primary tumor volume and proximity/ infiltration of surrounding structures, extent of lymphatic spread, cancer biology, and host factors such as age, cardiopulmonary function and other comorbidity. Treatment recommendations have to take into account these differences and stratify patients according to technical resectability, ability to tolerate high‑dose radiotherapy and chemotherapy, and many more. Many patients with inoperable stage III disease are candidates for combined modality chemo ‑ and radiotherapy. In conclusion, after two decades of mainly sequentially combined treatment, concurrent radiochemotherapy is nowadays the standard treatment of locally advanced NSCLC. However, there are some doubts.
Firstly, it should be realized that the trial data were collected in a period before routine staging with FDG ‑ PET and MRI of the brain. The routine use of these tests definitely changed the population of patients enrolled in radiochemotherapy.
Secondly, the other topics for future research are RCT with more sophisticated radiotherapy techniques allowing possibly higher tumor doses and/ or lower toxicities in surrounding healthy tissues. For patients with larger tumor volumes, possibilities to increase the radiation dose were limited by normal tissue constraints (esophagus and spinal cord). Conventionally fractionated radiotherapy for stage I NSCLC has shown inferior outcomes than surgery, and these results are linked to insufficient radiation doses. After the impact of radiotherapy dose for lung cancer was established, a number of trials were structured in the quest for better local control and overall survival by either dose escalation or shortening the total treatment time through conventional/ altered fractionation, even in combination with chemotherapy. The delivery of 60 Gy resulted in a 5‑year survival rate of 38% for patients with primary tumors less than 2 cm in size, 22% for tumors 2 – 3 cm in size, 5% for tumors 3 – 4 cm in size, and 0% for larger tumors [62]. Based on biological and statistical modelling of tumor responses to various radiation dose levels, it has been shown that doses as high as 80 to 90 Gy ensure a progression‑free survival rate of 50% [63]. The majority of studies concluded that patients receiving higher radiation doses have better treatment outcomes [64,65].
New technical advances in the application of radiotherapy enhanced the ability of targeted treatment and sparing of normal tissues, making high BED studies possible. Intensity ‑ modulated radiotherapy (IMRT) has the potential benefit to further increase the dose that can be safely prescribed in lung cancer patients due to a better conformity index [66 – 68]. In Stereotactic Radiation Therapy (SABR), high doses per limited number of fractions are used, although the actual biologically equivalent dose (BED) for the eradication is not yet completely understood [69]. When a sufficient dose (BED ≥ 100 Gy) is used, it has been noted in most clinical studies that the success rate of local control is over 90%. In particular, the surprising results of the RTOG 0617 trial [70] drove attention to the importance of adverse effects, once again emphasizing that future research should focus on quality of life.
Thirdly, in most of these studies, authors did not include factors such as histological type, age, comorbid conditions into their analyses. Since the incidence of NSCLC is high among elderly patients and many of them have history of smoking, the majority of these patients have severe comorbidities. Therefore, aggressive combined modality treatment might be contraindicated or poorly tolerated. However, age is not an independent prognostic factor in stage III and IV NSCLC, and epidemiological studies show that, with increasing age, the percentage of people treated with chemotherapy decreases [71 – 73]. Elderly patients with marginal renal function (creatinine clearance < 70 mL/min) or marginal cardiac function are eligible for administration of daily low‑dose cisplatin, while administration of full‑dose chemotherapy is often contraindicated. Combination of concurrent daily cisplatin with radiation appears to be a good alternative, especially in these elderly, frail patients [74,75]. Also, preclinical studies on RCT support the use of daily administration for optimal radiosensitizing effects [76]. This approach, delivered in a short overall treatment time, is suitable for both the elderly and for patients with comorbidities. It also offers the opportunity to combine concomitant radiochemotherapy with new agents. Existing data concerning targeted therapies in conjunction with radiotherapy are inconsistent and do not allow for firm conclusions. The optimal timing of the administration of radiotherapy and EGFR kinase inhibitors has yet to be determined. Strategies to safely incorporate novel antiangiogenic agents into combined ‑ modality therapy in lung cancer are needed. Studies using targeted therapies, in particular addressing their optimal integration with radiotherapy, are still in their infancy. Rapid development of molecular oncology will hopefully contribute to better patient selection to particular strategies and to treatment optimization.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE recommendation for biomedical papers.
Submitted: 27. 7. 2015
Accepted: 14. 9. 2015
Krzysztof Adamowicz, MD, PhD
Department of Oncology
Regional Oncology Center of Gdansk
Aleja Zwycięstwa 31/32
80-219 Gdansk
Poland
e-mail: krzys.adamowicz@gmail.com
Sources
1. Ferlay J, Shin HR, Bray F et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127(12): 2893 – 2917. doi: 10.1002/ ijc.25516.
2. Jemal A, Siegel R, Ward E et al. Cancer Statistics, 2007. CA Cancer J Clin 2007; 57(1): 43 – 66.
3. Perez CA, Stanley K, Grundy G et al. Impact of irradiation technique and tumor extent in tumor control and survival of patients with unresectable non‑oat cell carcinoma of the lung: report by the Radiation Therapy Oncology Group. Cancer 1982; 50(6): 1091 – 1099.
4. Saunders MI, Dische S. Continuous, hyperfractionated, accelerated radiotherapy (CHART) in non‑small cell carcinoma of the bronchus. Int J Radiat Oncol Biol Phys 1990; 19(5): 1211 – 1215.
5. Kong FM, Ten Haken RK, Schipper MJ et al. High‑dose radiation improved local tumor control and overall survival in patients with inoperable/ unresectable non‑small-‑cell lung cancer: long‑term results of a radiation dose escalation study. Int J Radiat Oncol Biol Phys 2005; 63(2): 324 – 333.
6. Wurstbauer K, Weise H, Deutschmann H et al. Non ‑ small cell lung cancer in stages I – IIIB: Long‑term results of definitive radiotherapy with doses ≥ 80 Gy in standard fractionation. Strahlenther Onkol 2010; 186(10): 551 – 557. doi: 10.1007/ s00066 ‑ 010 ‑ 2108 ‑ 3.
7. Machtay M, Bae K, Movsas B et al. Higher biologically effective dose of radiotherapy is associated with improved outcomes for locally advanced non‑small cell lung carcinoma treated with chemoradiation: an analysis of the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 2012; 82(1): 425 – 434. doi: 10.1016/ j.ijrobp.2010.09.004.
8. Guckenberger M, Wilbert J, Richter A et al. Potential of adaptive radiotherapy to escalate the radiation dose in combined radiochemotherapy for locally advanced non‑small cell lung cancer. Int J Radiat Oncol Biol Phys 2011; 79(3): 901 – 908. doi: 10.1016/ j.ijrobp.2010.04.050.
9. De Ruysscher D, Faivre‑Finn C, Nestle U et al. European Organization for Research and Treatment of Cancer recommendations for planning and delivery of high‑dose, high‑precision radiotherapy for lung cancer. J Clin Oncol 2010; 28(36): 5301 – 5310. doi: 10.1200/ JCO.2010.30.3271.
10. Dillman RO, Seagren SL, Propert KJ et al. A randomized trial of induction chemotherapy plus high‑dose radiation versus radiation alone in stage III non‑small‑cell lung cancer. N Engl J Med 1990; 323(14): 940 – 945.
11. Collaborative Group. Chemotherapy in non‑smallcell lung cancer: a meta‑analysis using updated data on individual patients from 52 randomized clinical trials. BMJ 1995; 311(7010): 899 – 909.
12. Le Chevalier T, Arriagada R, Quoix E et al. Radiotherapy alone versus combined chemotherapy and radiotherapy in nonresectable non‑small‑cell lung cancer: first analysis of a randomized trial in 353 patients. J Natl Cancer Inst 1991; 83(6): 417 – 423.
13. Jassem J, Begg AC, Stewart F et al. Combined chemotherapy and radiotherapy In: Peckham M, Pinedo HM, Veronesi U (eds). Oxford textbook of oncology. Oxford: Oxford University Press 1995 : 811 – 823.
14. Schaake ‑ Koning C, Van den Bogaert W, Dalesio O et al. Effects of concomitant cisplatin and radiotherapy on inoperable non‑small‑cell lung cancer. N Engl J Med 1992; 326(8): 524 – 530.
15. Trovo MG, Minatel E, Franchin G et al. Radiotherapy versus radiotherapy enhanced by cisplatin in stage III non‑small cell lung cancer. Int J Radiat Oncol Biol Phys 1992; 24(7): 11 – 15.
16. Aupérin A, Le Péchoux C, Pignon JP et al. Concomitant radio ‑ chemotherapy based on platin compounds in patients with locally advanced non‑small cell lung cancer (NSCLC): a meta‑analysis of individual data from 1,764 patients. Ann Oncol 2006; 17(3): 473 – 483.
17. Rowell NP, O’rourke NP. Concurrent chemoradiotherapy in non‑small cell lung cancer. Cochrane Database Syst Rev 2004; (4): CD002140.
18. Clamon G, Herndon J, Eaton W et al. A feasibility study of extended chemotherapy for locally advanced non‑small cell lung cancer: a phase II trial of cancer and leukemia group B. Cancer Invest 1994; 12(3): 273 – 282.
19. Fournel P, Robinet G, Thomas P et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non‑small‑cell lung cancer: groupe Lyon ‑ Saint ‑ Etienne d’Oncologie Thoracique ‑ Groupe Français de Pneumo ‑ Cancérologie NPC 95 – 01 Study. J Clin Oncol 2005; 23(25): 5910 – 5917.
20. Furuse K, Fukuoka M, Kawahara M et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non‑small‑cell lung cancer. J Clin Oncol 1999; 17(9): 2692 – 2699.
21. Ulutin HC, Güden M, Oysul K et al. Split ‑ course radiotherapy with or without concurrent or sequential chemotherapy in non‑small cell lung cancer. Radiat Med 2000; 18(2): 93 – 96.
22. Zatloukal P, Petruzelka L, Zemanova M et al. Concurrent versus sequential chemoradiotherapy with cisplatin and vinorelbine in locally advanced non‑small cell lung cancer: a randomized study. Lung Cancer 2004; 46(1): 87 – 98.
23. Belderbos J, Uitterhoeve L, van Zandwijk N et al. Randomized trial of sequential versus concurrent chemo ‑ radiotherapy in patients with inoperable non‑small cell lung cancer (EORTC 08972 – 22973). Eur J Cancer 2007; 43(1): 114 – 121.
24. Aupérin A, Le Péchoux C, Rolland E et al. Meta‑analysis of concomitant versus sequential radiochemotherapy in locally advanced non‑small‑cell lung cancer. J Clin Oncol 2010; 28(13): 2181 – 2190. doi: 10.1200/ JCO.2009.26.2543.
25. O‘Rourke N, Roqué I Figuls M, Farré Bernadó N et al. Concurrent chemoradiotherapy in non‑small cell lung cancer. Cochrane Database Syst Rev 2010; 6: CD002140. doi: 10.1002/ 14651858.CD002140.pub3.
26. Auperin A, Rolland E, Curran W Jr et al. Concomitant radio ‑ chemotherapy (RT ‑ CT) versus sequential RT ‑ CT in locally advanced non‑small cell lung cancer (NSCLC): a meta‑analysis using individual patient data (IPD) from randomized clinical trials (RCTs). J Thorac Oncol 2007; 2 (Suppl 4): S310.
27. Robinson LA, Ruckdeschel JC, Wagner H Jr et al. Treatment of non‑small cell lung cancer‑stage IIIA: ACCP evidence‑based clinical practice guidelines (2nd ed.). Chest 2007; 132 (Suppl 3): 243S – 265S.
28. Jett JR, Schild SE, Keith RL et al. Treatment of non‑smallcell lung cancer, stage IIIB: ACCP evidence‑based clinical practice guidelines (2nd ed). Chest 2007; 132 (Suppl 3): 266S – 276S.
29. Mayor S. NICE issues guidance for diagnosis and treatment of lung cancer. BMJ 2005; 330(7489): 439.
30. De Ruysscher D, Botterweck A, Dirx M et al. Eligibility for concurrent chemotherapy and radiotherapy of locally advanced lung cancer patients: a prospective, population‑based study. Ann Oncol 2009; 20(1): 98 – 102. doi: 10.1093/ annonc/ mdn559.
31. El Sharouni SY, Kal HB, Battermann JJ. Accelerated regrowth of non‑small‑cell lung tumours after induction chemotherapy. Br J Cancer 2003; 89(12): 2184 – 2189.
32. Chen CP, Weinberg VK, Jahan TM et al. Implications of delayed initiation of radiotherapy: accelerated repopulation after induction chemotherapy for stage III non‑smallcell lung cancer. J Thorac Oncol 2011; 6(11): 1857 – 1864. doi: 10.1097/ JTO.0b013e318229a41e.
33. Machtay M, Hsu C, Komaki R et al. Effect of overalltreatment time on outcomes after concurrent chemoradiation for locally advanced non‑small‑cell lung carcinoma: analysis of the Radiation Therapy Oncology Group (RTOG) experience. Int J Radiat Oncol Biol Phys 2005; 63(3): 667 – 671.
34. Hanna NN, Seetharam S, Mauceri HJ et al. Antitumor interaction of short ‑ course endostatin and ionizing radiation. Cancer J 2000; 6(5): 287 – 293.
35. Dings RP, Williams BW, Song CW et al. Anginex synergizes with radiation therapy to inhibit tumor growth by radiosensitizing endothelial cells. Int J Cancer 2005; 115(2): 312 – 319.
36. Citrin D, Menard C, Camphausen K. Combining radiotherapy and angiogenesis inhibitors: clinical trial design. Int J Radiat Oncol Biol Phys 2006; 64(1): 15 – 25.
37. Fogarty M. Learning from angiogenesis trial failures. The Scientist 2002; 16 : 33 – 35.
38. Gorski DH, Beckett MA, Jaskowiak NT et al. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res 1999; 59(14): 3374 – 3378.
39. Abdollahi A, Lipson KE, Sckell A et al. Combined therapy with direct and indirect angiogenesis inhibition results in enhanced antiangiogenic and antitumor effects. Cancer Res 2003; 63(24): 8890 – 8898.
40. Winkler F, Kozin SV, Tong RT et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin‑1, and matrix metalloproteinases. Cancer Cell 2004; 6(6): 553 – 563.
41. Spigel DR, Hainsworth JD, Yardley DA et al. Tracheoesophageal fistula formation in patients with lung cancer treated with chemoradiation and bevacizumab. J Clin Oncol 2010; 28(1): 43 – 48. doi: 10.1200/ JCO.2009.24.7353.
42. Socinski MA, Stinchcombe TE, Moore DT et al. Incorporating bevacizumab and erlotinib in the combined ‑ modality treatment of stage III non‑small‑cell lung cancer: results of a phase I/ II trial. J Clin Oncol 2012; 30(32): 3953 – 3959. doi: 10.1200/ JCO.2012.41.9820.
43. Tanaka T, Munshi A, Brooks C et al. Gefitinib radiosensitizes non‑small cell lung cancer cells by suppressing cellular DNA repair capacity. Clin Cancer Res 2008; 14(4): 1266 – 1273. doi: 10.1158/ 1078 ‑ 0432.CCR ‑ 07 ‑ 1606.
44. Kelly K, Chansky K, Gaspar LE et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non‑small‑cell lung cancer: SWOG S0023. J Clin Oncol 2008; 26(15): 2450 – 2456. doi: 10.1200/ JCO.2007.14.4824.
45. Shepherd FA, Rodrigues Pereira J, Ciuleanu T et al. Erlotinib in previously treated non‑small‑cell lung cancer. N Engl J Med 2005; 353(2): 123 – 132.
46. Zhu CQ, da Cunha Santos G, Ding K et al. Role of KRAS and EGFR as biomarkers of response to erlotinib in National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol 2008; 26(26): 4268 – 4275. doi: 10.1200/ JCO.2007.14.8924.
47. Kim JC, Ali MA, Nandi A et al. Correlation of HER1/ EGFR expression and degree of radiosensitizing effect of the HER1/ EGFR ‑ tyrosine kinase inhibitor erlotinib. Indian J Biochem Biophys 2005; 42(6): 358 – 365.
48. Chinnaiyan P, Huang S, Vallabhaneni G et al. Mechanisms of enhanced radiation response following epidermal growth factor receptor signaling inhibition by erlotinib (Tarceva). Cancer Res 2005; 65(8): 3328 – 3335.
49. Nyati MK, Morgan MA, Feng FY et al. Integration of EGFR inhibitors with radiochemotherapy. Nat Rev Cancer 2006; 6(11): 876 – 885.
50. Baumann M, Krause M, Dikomey E et al. EGFR ‑ targeted anti‑cancer drugs in radiotherapy: preclinical evaluation of mechanisms. Radiother Oncol 2007; 83(3): 238 – 248.
51. Tortora G, Gelardi T, Ciardiello F et al. The rationale for the combination of selective EGFR inhibitors with cytotoxic drugs and radiotherapy. Int J Biol Markers 2007; 22 (Suppl 4): S47 – S52.
52. Martinez E, Martinez M, Viñolas N et al. Feasibility and tolerability of the addition of erlotinib to 3D thoracic radiotherapy (RT) in patients (p) with unresectable NSCLC: a prospective randomized phase II study. J Clin Oncol 2008; 26: abstr. 7563.
53. Milas L, Fan Z, Andratschke NH et al. Epidermal growth factor receptor and tumor response to radiation: in vivo preclinical studies. Int J Radiat Oncol Biol Phys 2004; 58(3): 966 – 971.
54. Nasu S, Ang KK, Fan Z et al. C225 antiepidermal growth factor receptor antibody enhances tumor radiocurability. Int J Radiat Oncol Biol Phys 2001; 51(2): 474 – 477.
55. Govindan R, Bogart J, Stinchcombe T et al. Randomized phase II study of pemetrexed, carboplatin, and thoracic radiation with or without cetuximab in patients with locally advanced unresectable non‑small‑cell lung cancer: Cancer and Leukemia Group B trial 30407. J Clin Oncol 2011; 29(23): 3120 – 3125. doi: 10.1200/ JCO.2010.33.4979.
56. Jatoi A, Schild SE, Foster N et al. A phase II study of cetuximab and radiation in elderly and/ or poor performance status patients with locally advanced non‑small-‑cell lung cancer (N0422) Ann Oncol 2010; 21(10): 2040 – 2044. doi: 10.1093/ annonc/ mdq075.
57. Jensen AD, Münter MW, Bischoff HG et al. Combined treatment of nonsmall cell lung cancer stage III with intensity ‑ modulated radiotherapy and cetuximab: the NEAR trial. Cancer 2011; 117(13): 2986 – 2994. doi: 10.1002/ cncr.25888.
58. Hallqvist A, Wagenius G, Rylander H et al. Concurrent cetuximab and radiotherapy after docetaxel‑cisplatin induction chemotherapy in stage III NSCLC: satellite – a phase II study from the Swedish Lung Cancer Study Group. Lung Cancer 2011; 71(2): 166 – 172. doi: 10.1016/ j.lungcan.2010.05.011.
59. Hughes S, Liong J, Miah A et al. A brief report on the safety study of induction chemotherapy followed by synchronous radiotherapy and cetuximab in stage III non‑small cell lung cancer (NSCLC): SCRATCH study. J Thorac Oncol 2008; 3(6): 648 – 651. doi: 10.1097/ JTO.0b013e3181757a60.
60. Blumenschein GR Jr, Paulus R, Curran WJ et al. Phase IIstudy of cetuximab in combination with chemoradiation in patients with stage IIIA/ B non‑small‑cell lung cancer: RTOG 0324. J Clin Oncol 2011; 29(17): 2312 – 2318. doi: 10.1200/ JCO.2010.31.7875.
61. Govindan R, Bogart J, Stinchcombe T et al. Randomized phase II study of pemetrexed, carboplatin, and thoracic radiation with or without cetuximab in patients with locally advanced unresectable non‑small‑cell lung cancer: Cancer and Leukemia Group B Trial 30407. J Clin Oncol 2011; 29(23): 3120 – 3125. doi: 10.1200/ JCO.2010.33.4979.
62. Noordijk EM, Poest Clement E et al. Radiotherapy as an alternative to surgery in elderly patients with resectable lung cancer. Radiother Oncol 1988; 13(2): 83 – 89.
63. Onishi H, Shirato H, Nagata Y et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage Inon‑small cell lung cancer: updated results of 257 patients in a Japanese multi‑institutional study. J Thorac Oncol 2007; 2 (Suppl 3): 94 – 100.
64. Sibley GS. Radiotherapy for patients with medically inoperablestage I nonsmall cell lung carcinoma: smaller volumes and higherdoses – a review. Cancer 1998; 82(3): 433 – 438.
65. Timmerman R, Paulus R, Galvin J et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010; 303(11): 1070 – 1076. doi: 10.1001/ jama.2010.261.
66. Grills IS, Yan D, Martinez AA et al. Potential for reduced toxicity and dose escalation in the treatment of inoper-able non‑small‑cell lung cancer: a comparison of intensity ‑ modulated radiation therapy (IMRT), 3D conformal radiation, and elective nodal irradiation. Int J Radiat Oncol Biol Phys 2003; 57(3): 875 – 890.
67. Murshed H, Liu HH, Liao Z et al. Dose and volume reduction for normal lung using intensity ‑ modulated radiotherapy for advanced‑stage non‑small‑cell lung cancer. Int J Radiat Oncol Biol Phys 2004; 58(4): 1258 – 1267.
68. Schwarz M, Alber M, Lebesque JV et al. Dose heterogeneity in the target volume and intensity ‑ modulated radiotherapy to escalate the dose in the treatment of non‑small‑cell lung cancer. Int J Radiat Oncol Biol Phys 2005; 62(2): 561 – 570.
69. Park C, Papiez L, Zhang S et al. Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys 2008; 70(3): 847 – 852. doi: 10.1016/ j.ijrobp.2007.10.059.
70. Bradley JD, Paulus R, Komaki R et al. A randomized phase III comparison of standard‑dose (60 Gy) versus high‑dose (74 Gy) conformal chemoradiotherapy with or without cetuximab for stage IIIa/ IIIb non‑small cell lung cancer: preliminary findings on radiation dose in RTOG 0617. 53rd Annual Meeting of the American Society of Radiation Oncology; Miami, FL, USA. 2 – 6 October 2011.
71. Janssen ‑ Heijnen ML, Smulders S, Lemmens VE et al. Effect of comorbidity on the treatment and prognosis of elderly patients with non‑small cell lung cancer. Thorax 2004; 59(7): 602 – 607.
72. Pignon T, Gregor A, Schaake Koning C et al. Age has no impact on acute and late toxicity of curative thoracic radiotherapy. Radiother Oncol 1998; 46(3): 239 – 248.
73. Socinski MA. Clinical issues in the management of non‑small‑cell lung cancer and the role of platinum‑based therapy. Clin Lung Cancer 2004; 5(5): 274 – 289.
74. Takata I, Ueoka H, Kiura K et al. Daily low‑dose cisplatin and concurrent thoracic irradiation for poor ‑ risk patients with unresectable non‑small‑cell lung cancer. Acta Med Okayama 2002; 56(5): 261 – 266.
75. Uitterhoeve AL, Koolen MG, van Os RM et al. Accelerated high‑dose radiotherapy alone or combined with either concomitant or sequential chemotherapy; treatments of choice in patients with non‑small cell lung cancer. Radiat Oncol 2007; 2(1): 27.
76. Bartelink H, Kallman RF, Rapacchietta D et al. Thera-peutic enhancement in mice by clinically relevant dose and fractionation schedules of cis‑diamminedichloropla-tinum (II) and irradiation. Radiother Oncol 1986; 6(1): 61 – 74.
Labels
Paediatric clinical oncology Surgery Clinical oncology
Article was published inClinical Oncology

2015 Issue 5-
All articles in this issue
- Assessment of Heavy/ Light Chain Pairs of Immunoglobulin (Hevylite™ assay) – Benefit for Stratification of Multiple Myeloma?
- Methods of Assesing Quality of Life in Women with Breast Cancer – Overview and Basic Characteristics
- The Relevance of MicroRNAs in Glioblastoma Stem Cells
- Surgical Treatment of Lung Metastases of Colorectal Carcinoma – Survival and Prognostic Factors
- Combining Systemic Therapies with Radiation in Non‑ small Cell Lung Cancer
- Utilization of Prognostic Indexes for Patients with Brain Metastases in Daily Radiotherapy Routine – is the Complexity and Intricacy Still an Issue?
- Forbidden to Drive – a New Chemotherapy Side Effect
- Clinical Oncology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Methods of Assesing Quality of Life in Women with Breast Cancer – Overview and Basic Characteristics
- Surgical Treatment of Lung Metastases of Colorectal Carcinoma – Survival and Prognostic Factors
- Forbidden to Drive – a New Chemotherapy Side Effect
- Combining Systemic Therapies with Radiation in Non‑ small Cell Lung Cancer
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career


