-
Medical journals
- Career
HPV related deregulation of selected microRNAs in sinonasal squamous cell carcinoma
Authors: H. Kovaříková 1; I. Baranová 1; J. Laco 2; K. Rozkošová 2; H. Vošmiková 2; M. Vošmik 3; P. Dundr 4; K. Němejcová 4; J. Michálek 5; V. Palička 1; M. Chmelařová 1
Authors‘ workplace: Institute of Clinical Biochemistry and Diagnostics, Charles University, Faculty of Medicine in Hradec Králové and University Hospital Hradec Králové 1; The Fingerland Department of Pathology, Charles University, Faculty of Medicine in Hradec Králové and University Hospital Hradec Králové 2; Department of Oncology and Radiotherapy, Charles University, Faculty of Medicine in Hradec Králové and University Hospital Hradec Králové 3; Department of Pathology, Charles University, First Faculty of Medicine and General University Hospital in Prague 4; Department of Clinical and Molecular Pathology, Palacky University Olomouc, Faculty of Medicine and Dentistry and University Hospital Olomouc 5
Published in: Klin. Biochem. Metab., 27, 2019, No. 3, p. 100-106
Overview
Objective: In the present study, we used quantitative real-time PCR to investigate relative expression values of miR-145-5p, miR-484 and miR-99a-5p in HPV positive and HPV negative samples of sinonasal squamous cell carcinoma.
Design: Original Article.
Settings: Institute of Clinical Biochemistry and Diagnostics, Charles University, Faculty of Medicine in Hradec Králové and University Hospital Hradec Králové, Sokolská 581, 500 05 Hradec Králové
Materials and Methods: Quantitative real-time PCR with TaqManTM Advanced miRNA Assays was used to investigate relative expression values of selected microRNAs (miR-145-5p, miR-484 and miR-99a-5p) in a unique set of formalin-fixed paraffin-embedded tissue samples obtained from 46 patients with sinonasal squamous cell carcinoma. Statistical analysis (Student’s t-test, one-way analysis of variance and regression analysis) was performed to compare relative expression miRNA values and recorded clinicopathological data such as HPV status.
Results: Our results show statistically significant downregulation of miR-145-5p (P < 0.001 and Fold change = -2.78) in sinonasal squamous cell carcinoma samples. The presence of HPV infection correlated with the expression levels of all three studied miRNAs. The expression of miR-145-5p was lower in HPV negative samples (P = 0.019), miR-99a-5p was upregulated in HPV positive samples (P = 0.058) and miR-484 was downregulated in HPV positive samples (P = 0.016). Patients with recorded history of smoking or currents smokers showed downregulation of miR-99a-5p (P = 0.060). Perineural invasion was linked to significant downregulation of miR-99a-5p (P = 0.0055). Distinctive downregulation of miR-145-5p was linked to vascular invasion (P = 0.037) and regional recurrence (P = 0.076).
Conclusion: We have found correlation between studied miRNA expression and presence of HPV infection in the patient samples. Our results suggest that miR-145, miR-484 and miR-99a are involved in HPV pathogenesis. However, the actual pathway of HPV related miRNA involvement in sinonasal tumor development is not yet known.
Keywords:
sinonasal carcinoma – microRNA – human papilloma virus.
Introduction
Sinonasal carcinomas (SNC) are group of malignancies developing in nasal and paranasal sinuses. They represent up to 3 % – 5 % of all head and neck cancers and 1 % of all kinds of tumors [1]. The most common subtype is sinonasal squamous cell carcinoma (SSCC) (65 – 75%). However, squamous cell tumors are less prevalent than in other types of head and neck cancers. SSCCs are further classified as non-keratinizing or keratinizing, when non-keratinizing tumors are more likely to be linked to high-risk HPV (HR-HPV) infection [2].
In 2016, incidence of SNCs in the Czech Republic was 0.5/100 000 and mortality was 0.23/100 000 [3]. The tumors are more common in men than women and appear mostly in advanced age [4]. Early symptoms of the disease can be rhinorrhea, epistaxis, epiphora, and nasal obstruction. Advanced lesion symptoms might include blurred vision, diplopia or proptosis [5]. Treatment is usually based on radical surgical resection with postoperative radiotherapy [6].
Risk factors for the disease development involve smoking and professional exposure to specific cancerogenous substances such as wood-dust, leather dust and formaldehyde [7, 8].
Influence of HPV infection on SNC carcinogenesis has been researched recently. Approximately 20 – 30 % of SNC tumors harbor transcriptionally active HPV infec-tion, which is smaller amount in comparison to other head and neck tumors [9]. High-risk HPV genotype 16 is the most aggressive type, which has been found in about 90 % of HPV positive cases of SSCC. Patients with HPV positive tumors have much better prognosis than patients with HPV negative tumors [10-12].
microRNAs are short (~23 nucleotides) non-coding RNA molecules taking part in gene expression regula-tion. The first evidence of their existence was published by Lee et al. in 1993 [13] as an outcome of experiments with Caenorhabditis elegans. All information about annotated mature miRNAs and their hairpin sequences are summarized in searchable online reference database miRbase („microRNA registry“). The newest version (Release 22.1, October 2018) of miRbase contains 38,589 entries in total, from which 1,917 are human miRNA precursors and 2,654 human mature miRNA entries [14, 15]. Primary function of miRNAs is negative translation regulation as part of RISC (RNA induced silencing complex) by translational repression and/or mRNA degradation [16].
In the present study, we used quantitative real-time PCR to investigate relative expression values of miR-145-5p, miR-484 and miR-99a-5p in a unique set of formalin-fixed, paraffin-embedded (FFPE) tissue samples obtained from patients with SSCC. As in some of the samples HPV infection was detected, correlation between HPV positivity and relative miRNA expression was investigated.
Material and Methods
A total of 63 FFPE tissue samples from SSCC patients and normal sinonasal tissue were analyzed: 46 were cancer cases and 17 were control samples. Only tumors primarily originating from the nasal cavity, maxillary sinuses, and ethmoid complex were included, while no tumors were found in the frontal or sphenoid sinuses. Due to retrospectivity of the study, the samples used as controls were 8 mucosal specimens from the nasal cavity and 9 from the maxillary sinus which were obtained from patients treated for a non-malignant diagnosis such as chronic polypoid sinusitis and rhinitis (10 women and 7 men with median age of 54).
Paraffin samples for this study were obtained from Departments of Pathology of three University Hospitals in the Czech Republic (Hradec Kralove, Prague, Olomouc) and all malignancies were diagnosed between August 1995 and August 2014. Carcinoma cases were classified according to the current World Health Organization (WHO) classification (2017) and all the samples were reviewed by experienced head and neck pathologist (JL). The study was approved by the Ethics Committee of University Hospital Hradec Kralove (201511 S27P).
The tumor types included exclusively squamous cell carcinoma (conventional, verrucous, papillary, basaloid, spindle cell, acantholytic and adenosquamous). Vascular invasion, perineural spread, status of resection margins (in the case of radical surgery), and microscopic findings in the surrounding mucosa were described.
Data such as gender, age at the time of diagnosis, smoking history (non-smoker vs. ex-smoker vs. current smoker), occupation (high-risk such as rubber industry worker, joiner, locksmith, woodworker and miller vs. low-risk), tumor localization (nasal cavity, maxillary sinus and ethmoid complex), laterality and clinical/pathological TNM (7th edition classification) were recorded for every patient (Table 1). During the follow-up period (until February 2016) local recurrence, regional recurrence, distant recurrence, death, and tumor related death staging were documented. The patients were treated with radical surgery (35 patients), 17 of them were treated in combination with radiotherapy and 10 in combination with radiotherapy and chemotherapy. 5 of the patients were treated by radiotherapy and 4 patients were treated by combination of radiotherapy and chemotherapy.
1. Deregulace vybraných mikroRNA u karcinomu sinonasálních dlaždicových buněk související s HPV 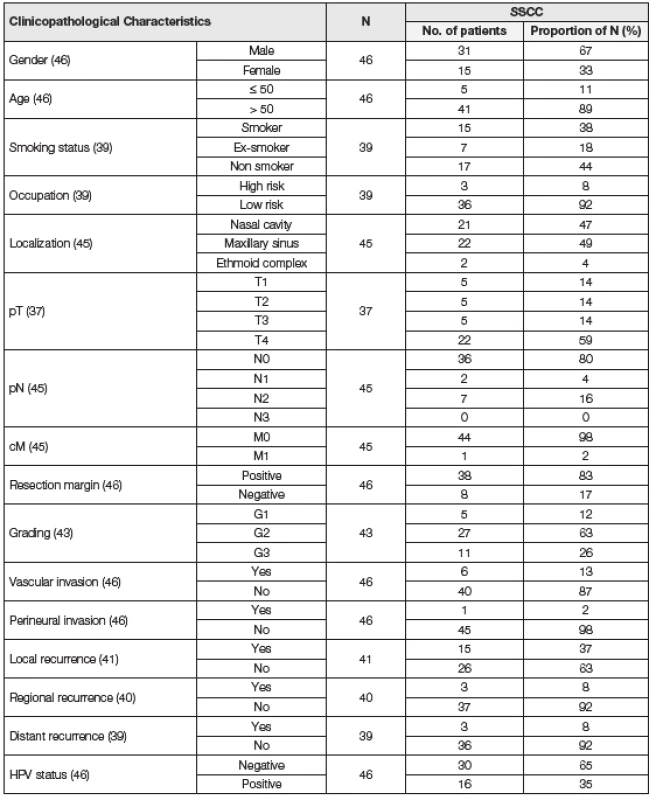
HPV status was analyzed using HPV DNA in situ hybridization (ISH), HPV E6/E7 mRNA ISH, HPV DNA polymerase chain reaction (PCR) and typing, and HPV E6/E7 mRNA reverse transcription and polymerase chain reaction (RT PCR). For the purpose of statistical analysis, a case was considered HPV positive if it was positive for HPV DNA ISH/PCR and/or HPV E6/E7 mRNA ISH/PCR. For detailed information about HPV analysis see manuscript by Laco et al. (2015) [12].
RNA isolation
Two to four 5-µm-thick sections were cut from FFPE tissue samples and deparaffinized using xylene and ethanol. Total RNA including miRNAs was isolated from FFPE tissue samples using RNeasy FFPE Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. The extracted RNA was ultimately eluted in 30 µl of RNase free water. Concentration and purity of the isolated RNA was determined by NanoDrop ND 1000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA) by measuring the optical density at 260 nm and 280 nm (A260/280 ratio). After isolation, the samples were immediately processed or stored at -80 °C.
Quantitative real-time PCR of microRNAs
The synthesis of cDNA was done using TaqManTM Advanced miRNA cDNA Synthesis Kit with universal reverse transcription primers (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s protocol with 8 – 10 ng of total RNA in the reaction. Real-time PCR was done with TaqManTM Fast Advanced Master Mix (Applied Biosystems) and specific TaqManTM Advanced miRNA Assays (Applied Biosystems) on Rotor Gene Q (Qiagen). Assays for hsa-miR-145-5p, hsa-miR-484 and hsa-miR-99a-5p and hsa-miR-361-5p (endogenous control) were used. All steps were performed following the manufacturer’s protocol. Studied miRNAs were selected based on review of literature dealing with deregulation of miRNA in various types of head and neck cancer.
All reactions were performed in triplicates and the reaction volume was 10 µl with 2.5 µl of the sample. The reaction conditions were set according to the manufacturer’s protocol and it involved enzyme activation at 95 °C for 20 s followed by 40 cycles of denaturation at 95 °C for 3 s and annealing/extension at 60 °C for 30 s. Fluorescence data were analyzed in the Rotor Gene Q Series Software. Relative expression of each miRNA was determined using the 2-ΔΔCt method [17] with expression levels of miR-361 for data normalization. This workflow was chosen based on literature review and manufacturer’s recommendation for endogenous controls listed in the user guide for TaqManTM Advanced miRNA Assays.
Statistical Analysis
All statistical analyses were performed using STATISTICA (data analysis software system) version 13 (TIBCO Software Inc., Tulsa, OK, USA). MiRNA expression values were log transformed to a normal distribution of data for parametric tests and extreme values were excluded from the analysis. Student’s t-test was used to compare miRNA expression levels in tumor and non tumor samples. The null hypothesis was based on theory that there was no difference between expression levels of studied microRNAs between tumor samples and control samples.
One way analysis of variance (ANOVA) and regression analysis were used to analyze the correlation between expression level of miRNA and various clinicopathological features. All tests were two tailed and P < 0.05 was considered statistically significant.
Results
In this study we analyzed expression levels of miR-145-5p, miR-484 and miR-99a-5p using TaqManTM Advanced miRNA Assays and real-time PCR method in 46 SSCC and 17 control samples. One of the three studied miRNAs was downregulated – miR-145-5p (P < 0.001 and Fold change = -2.78). The other two miRNAs were deregulated, however, the results were not statistically significant, because fold change value was more than -2 and P value was more than 0.05.
Correlation with clinicopathological characteristics
Age range of the patients at the time of diagnosis was between 27 and 82 years and median age was 65 years. During the follow-up period, 26 patients died, of whom 11 due to the tumor. Control samples (n = 17) were acquired from 7 male and 10 female patients. The youngest patient was 24 years old and the oldest one was 74 years old while median age was 54 years. Using statistical analysis, we compared relative expression levels of examined miRNA with clinicopathological characteristics described earlier (Table 2). Only statistically significant results and results approaching statistical significance will be described in the following text.
2. Correlations of miRNA expression with clinicopathological data sinonasal squamous cell carcinoma samples. 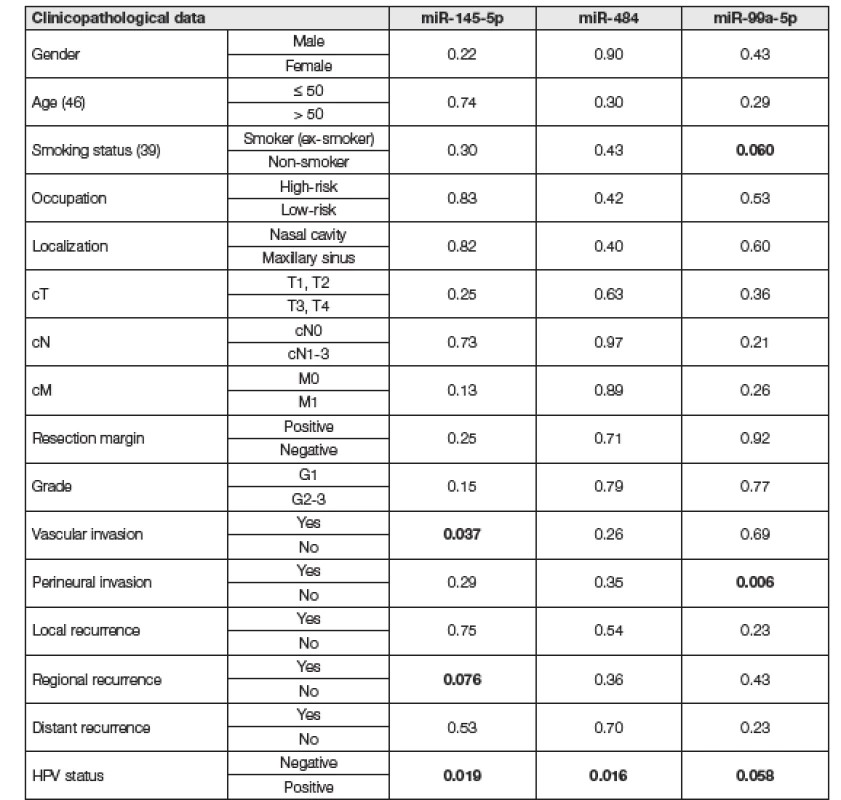
Statistically significant results and results approaching statistical significance and are marked in bold. Patients with recorder history of smoking or current smokers showed downregulation of miR 99a-5p, whereas nonsmokers had normal levels of this miRNA (P = 0.06). Moreover, perineural invasion was linked to significant downregulation of miR-99a-5p (P = 0.0055). Distinctive downregulation of miR-145-5p was linked to vascular invasion (P = 0.037) and regional recurrence (P = 0.076).
HPV status was positive in 16 patients and negative in the other 30 patients. The presence of HPV infection correlated with the expression levels of all three studied miRNAs. The expression of miR-145-5p was lower in HPV negative samples (P = 0.019). Moreover, miR-99a-5p was downregulated in HPV negative samples and upregulated in HPV positive samples approaching statistical significance (P = 0.058). On the other hand, miR-484 was downregulated in HPV positive samples and no deregulation was found in HPV negative samples (P = 0.016) (Fig. 1).
1. Box plot of relative expression of miR-99a-5p, miR-145-5p and miR-484 grouped by HPV status. Boxes represent mean ± standard error of the mean and whiskers represent mean ± confidence interval. Outliers are symbolized as dots and extremes are symbolized as asterisks. 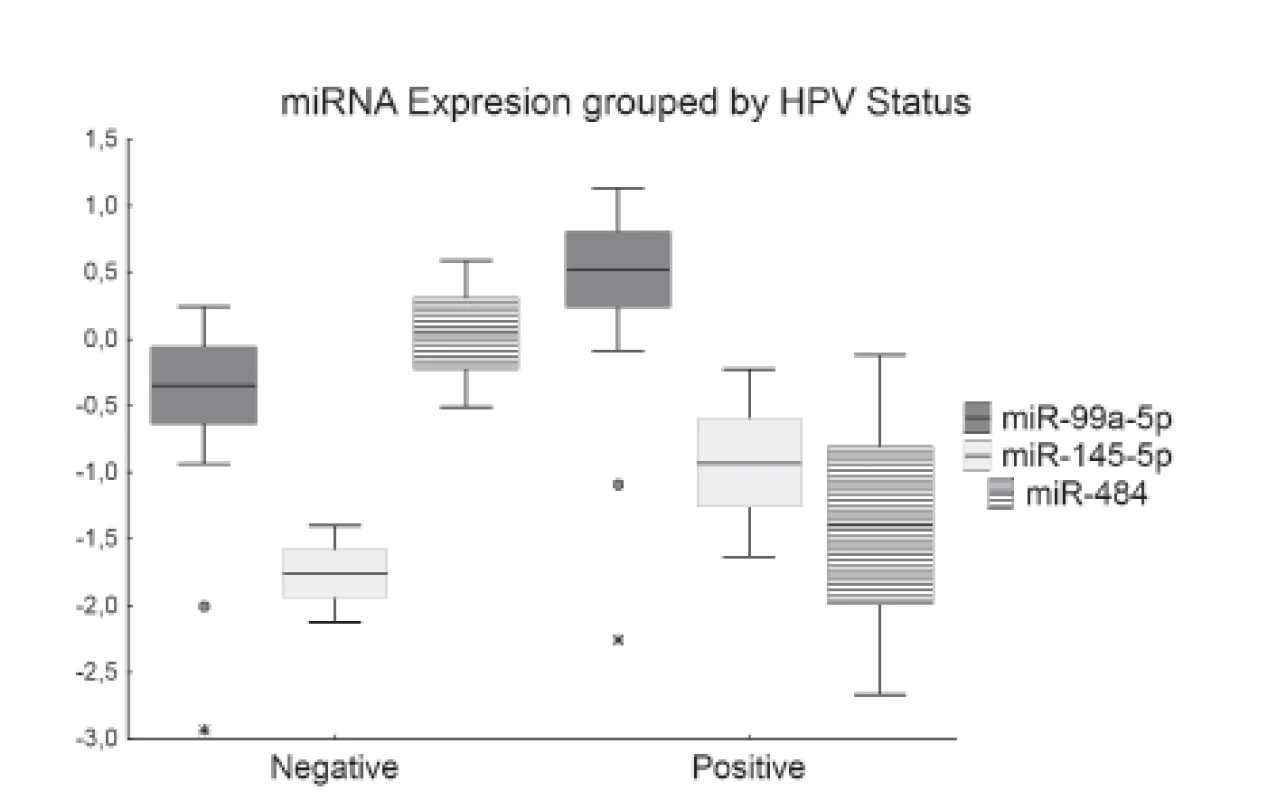
Discussion
microRNAs have been recognized as key molecules in cancer development and progression in various types of tumors. They have been established as promising prognostic cancer biomarkers due to their resistance to degradation in many tissue types (including FFPE tissues) and various body fluids (such as plasma, serum, urine, or saliva) [18, 19].
Number of studies focused on miRNA expression in sinonasal cancer has been limited. Ogawa et al. [20] studied correlation between downregulation of miR-34a and resistance to cis-platinum treatment. The downregulation of miR-34a in SSCC was further confirmed by Zhao and Wang [21] and further associated it with poor prognosis of the patients. HPV positivity is currently the hot topic especially in oropharyngeal cancer research. Deregulation of various miRNAs (such as miR-21 miR-31, miR-24, miR-146a, etc.) has been previously described [22, 23].
Expression levels of miR-145-5p were significantly lower in our set of sinonasal squamous cell samples compared to control samples. According to literature miR-145-5p is deregulated in various types of cancers such as melanoma [24] and even in head and neck cancers such as laryngeal squamous cell carcinoma [25]. miR-145 has been established as an important tumor suppressor and its deregulation has an impact on the cell proliferation, apoptosis, migration and invasion of the tumor cells [26]. In concordance, we found correlation between miR-145-5p downregulation and vascular invasion and regional recurrence of the tumors. These findings confirm involvement of the miRNA deregulation in aggressiveness of the tumor.
Reports of deregulation of miR-99a in head and neck cancer have been published, which we could not confirm in SNC. However, we found downregulation of miR-99a in patients with recorded history of smoking, which might be connected to possible suggested regulation of miR-99a DNA damage response [27].
Finally, all three miRNAs correlated with the presence of HPV infection in the patients’ samples. It has been reported that approximately 20 – 30 % sinonasal tumors are HPV positive but the role of HPV status in SNC has not been well-established yet (opposed to other types of head and neck cancer such as oropharyngeal cancer). HPV positivity is more often detected in non-keratinizing, basaloid and papillary tumors rather than in keratinizing tumors. HPV infection might act in SSCC pathology by p53 tumor suppressor gene mutation. On the other hand, HPV positivity was identified in up to 100 % of SSCC tumors developed from inverted papillomas. As a result, it is believed that transition from inverted papilloma to carcinoma is the other probable mechanism of HPV involvement in SNC pathology [9]. High risk HPV type 16 is the most aggressive type human papillomavirus, which has been found in about 80 % of HPV+ cases of SSCC. Patients with HPV+ tumors have much better prognosis than patients with HPV - tumors [28, 12].
Downregulation of miR-145 has been described in HPV related tumors such as oropharyngeal cancer (which is nowadays predominantly caused by HPV infection) and cervical cancer which is mostly HPV related [29]. Lu et al. 2015 [30] suggested a synergic effect of miR-145-5p and long non-coding RNA MALAT1 in HPV+ positive cervical cancer causing reduction of cancer colony formation, cell cycle regulation and induction of apoptosis. Correspondently to HPV pathology, it has been reported that miR-145 regulates cell proliferation and apoptosis induced by p53 activator by targeting NRAS gene.
Vojtechova et al. [31] have reported miR-99a to be specifically downregulated in HPV negative oropharyngeal squamous cell carcinomas, which correspond with the trend of low expression miR-99a in our sinonasal squamous cell carcinoma samples. It has been shown that miR-99a inhibits cell proliferation, migration and invasion by targeting FGFR3 [32]. miR-484 was downregulated in HPV positive samples of SNC in comparison to HPV negative samples. Interestingly, downregulation of this miRNA has been reported in HPV linked cervical tumors. Moreover, miR-484 has been reported to inhibit cell proliferation and EMT processes by targeting ZEB1 and SMAD2 genes in HPV+ related cervical cancer [33].
Conclusion
We have found correlation between studied miRNA expression and presence of HPV infection in the samples. Our results suggest that miR-145, miR-484 and miR-99a are involved in HPV pathogenesis. However, the actual pathway of HPV related miRNA involvement in sinonasal tumor development is not yet known.
Research funding: The study was supported by the program MH CZ - DRO (UHHK, 00179906), SVV grant 260 398/2017, by the program PROGRES Q40/11, by European Regional Development Fund-Project BBMRI-CZ.: Biobank network – a versatile platform for the research of the etiopathogenesis of diseases No. EF16 013/0001674.
Korespondující autor
Ing. Helena Kovaříková
ÚKBD FN Hradec Králové
Sokolská 581
500 05 Hradec Králové
Tel: + 420 495 833 864
e mail: Helena.Kovarikova@fnhk.cz
Sources
. Kawaguchi, M., Kato, H., Tomita, H., et al. Imaging Characteristics of Malignant Sinonasal Tumors. J Clin Med, Dec 6 2017, 6(12).
2. Stelow, E. B. and Bishop, J. A. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Tumors of the Nasal Cavity, Paranasal Sinuses and Skull Base. Head Neck Pathol, Mar 2017, 11(1), 3-15.
3. Dušek, L., Mužík, J., Kubásek, M., et al. Epidemiologie zhoubných nádorů v České republice. 2005 Masarykova Univerzita, Version 7.0 [2007], ISSN 1802 – 8861 [cit. 2018/05/30]. Available from: http://www.svod.cz].
4. Dutta, R., Dubal, P. M., Svider, P. F., et al. Sinonasal malignancies: A population-based analysis of site-specific incidence and survival. Laryngoscope, Nov 2015, 125(11), 2491-2497.
5. Barnes, L., Eveson, J. W., Reichart, P. and Sidransky, D. Tumours of the Nasal Cavity and Paranasal Sinuses. In World Health Organization Classification of Tumours. Pathology and Genetics of Head and Neck Tumours. Lyon: IARC Press, 2005, p. 9 - 80.
6. Bossi, P., Saba, N. F., Vermorken, J. B., et al. The role of systemic therapy in the management of sinonasal cancer: A critical review. Cancer Treat Rev, Dec 2015, 41(10), p. 836-843.
7. T Mannetje, A., Kogevinas, M., Luce, D., et al. Sinonasal cancer, occupation, and tobacco smoking in European women and men. Am J Ind Med, Jul 1999, 36(1), p. 101-107.
8. Mensi, C., Consonni, D., Sieno, C., et al. Sinonasal cancer and occupational exposure in a population-based registry. Int J Otolaryngol, 2013, 2013, 7 pages.
9. Kilic, S., Kilic, S. S., Kim, E. S., et al. Significance of human papillomavirus positivity in sinonasal squamous cell carcinoma. Int Forum Allergy Rhinol, Oct 2017, 7(10), p. 980-989.
10. Alos, L., Moyano, S., Nadal, A., et al. Human papillomaviruses are identified in a subgroup of sinonasal squamous cell carcinomas with favorable outcome. Cancer, Jun 15 2009, 115(12), p. 2701-2709.
11. Syrjanen, K. and Syrjanen, S. Detection of human papillomavirus in sinonasal carcinoma: systematic review and meta-analysis. Hum Pathol, Jun 2013, 44(6), p. 983-991.
12. Laco, J., Sieglova, K., Vosmikova, H., et al. The presence of high-risk human papillomavirus (HPV) E6/E7 mRNA transcripts in a subset of sinonasal carcinomas is evidence of involvement of HPV in its etiopathogenesis. Virchows Arch, Oct 2015, 467(4), p. 405-415.
13. Lee, R. C., Feinbaum, R. L. and Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell, Dec 3 1993, 75(5), p. 843-854.
14. All Homo sapiens miRNAs. 2019, [cit. 12/11/2019]. Available from: http://www.mirbase.org/summary.shtml?org=hsa.
15. Kozomara, A. and Griffiths-Jones, S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res, Jan 2014, 42(Database issue), D68-73.
16. Iwakawa, H. O. and Tomari, Y. The Functions of MicroRNAs: mRNA Decay and Translational Repression. Trends Cell Biol, Nov 2015, 25(11), p. 651-665.
17. Schmittgen, T. D. and Livak, K. J. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc, 2008, 3(6), p. 1101-1108.
18. Ferracin, M. and Negrini, M. Micromarkers 2.0: an update on the role of microRNAs in cancer diagnosis and prognosis. Expert Rev Mol Diagn, 2015, 15(10), p. 1369-1381.
19. Moody, L., He, H., Pan, Y.-X. and Chen, H. Methods and novel technology for microRNA quantification in colorectal cancer screening. Clin Epigenetics, 2017-10-24 2017, 9(1), 119.
20. Ogawa, T., Saiki, Y., Shiga, K., et al. miR-34a is downregulated in cis-diamminedichloroplatinum treated sinonasal squamous cell carcinoma patients with poor prognosis. Cancer Science, 2012, 103(9), p. 1737-1743.
21. Zhao, Y. and Wang, X. miR-34a targets BCL-2 to suppress the migration and invasion of sinonasal squamous cell carcinoma. Oncol Lett, Nov 2018, 16(5), p. 6566-6572.
22. Gao, G., Gay, H. A., Chernock, R. D., et al. A microRNA expression signature for the prognosis of oropharyngeal squamous cell carcinoma. Cancer, Jan 1 2013, 119(1), p. 72-80.
23. Kovarikova, H., Bubancova, I., Laco, J., et al. Dere-gulation of selected microRNAs in sinonasal carcinoma: Value of miR-21 as prognostic biomarker in sinonasal squamous cell carcinoma. Head & Neck, sep 2017, 39(12), p. 2528-2536.
24. Liu, S., Gao, G., Yan, D., et al. Effects of miR-145-5p through NRAS on the cell proliferation, apoptosis, migration, and invasion in melanoma by inhibiting MAPK and PI3K/AKT pathways. Cancer Med, Apr 2017, 6(4), p. 819-833.
25. Karatas, O. F., Yuceturk, B., Suer, I., et al. Role of miR-145 in human laryngeal squamous cell carcinoma. Head Neck, Feb 2016, 38(2), p. 260-266.
26. Pashaei, E., Guzel, E., Ozgurses, M. E., et al. A Meta-Analysis: Identification of Common Mir-145 Target Genes that have Similar Behavior in Different GEO Datasets. PLoS One, 2016, 11(9), e0161491.
27. Chen, D., Cabay, R. J., Jin, Y., et al. MicroRNA Dere-gulations in Head and Neck Squamous Cell Carcinomas. J Oral Maxillofac Res, 2013, 4(1), e2.
28. Chen, C. C., and Yang, S. F. Human Papillomavirus-Related Carcinoma With Adenoid Cystic-like Features of the Sinonasal Tract (Also Known as Human Papillomavirus-Related Multiphenotypic Sinonasal Carcinoma). Arch Pathol Lab Me, 2019.
29. Lajer, C. B., Garnaes, E., Friis-Hansen, L., et al. The role of miRNAs in human papilloma virus (HPV)-associated cancers: bridging between HPV-related head and neck cancer and cervical cancer. Br J Cancer, Apr 24 2012, 106(9), p. 1526-1534.
30. Lu, H., He, Y., Lin, L., et al. Long non-coding RNA MALAT1 modulates radiosensitivity of HR-HPV+ cervical cancer via sponging miR-145. Tumour Biol, 2016, 37(2), p. 1683-1691.
31. Vojtechova, Z., Sabol, I., Salakova, M., et al. Comparison of the miRNA profiles in HPV-positive and HPV-negative tonsillar tumors and a model system of human keratinocyte clones. BMC Cancer, Jul 4 2016, 16, 382.
32. Wu, D., Zhou, Y., Pan, H., et al. microRNA-99a inhibi-ting cell proliferation, migration and invasion by targeting fibroblast growth factor receptor 3 in bladder cancer. Oncol Lett, 2014 Vol. 7, p. 1219-1224.
33. Hu, Y., Xie, H., Liu, Y., et al. miR-484 suppresses proliferation and epithelial–mesenchymal transition by targeting ZEB1 and SMAD2 in cervical cancer cells. Cancer Cell Int. 2017, vol. 17. ISSN 1475-2867 (Electronic).
Conflict of interest: The authors declare no conflict of interest.
Labels
Clinical biochemistry Nuclear medicine Nutritive therapist
Article was published inClinical Biochemistry and Metabolism

2019 Issue 3-
All articles in this issue
- XIV. celostátní sjezd České společnosti klinické biochemie
- HPV related deregulation of selected microRNAs in sinonasal squamous cell carcinoma
- Assessment of methylated derivatives of glycine in patients with metabolic syndrome and prediabetes
- Laboratory diagnostics of patients with MGUS and results of their monitoring in a regional biochemical laboratory within 10 years
- Harmonization in clinical laboratories. Concepts and problems. Metrological continuity, standardization, harmonization
-
Klinické laboratoře na rozcestí
(23. IFCC EFLM Evropský kongres klinické chemie a laboratorní medicíny) - Sborník a Abstrakta přednášek a posterů
- Clinical Biochemistry and Metabolism
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Laboratory diagnostics of patients with MGUS and results of their monitoring in a regional biochemical laboratory within 10 years
- XIV. celostátní sjezd České společnosti klinické biochemie
- Harmonization in clinical laboratories. Concepts and problems. Metrological continuity, standardization, harmonization
- HPV related deregulation of selected microRNAs in sinonasal squamous cell carcinoma
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

