-
Medical journals
- Career
The prevalence of decreased glomerular filtration rate in patients with monoclonal gammopathy of undetermined significance
Authors: T. Šálek 1; D. Moravčíková 2; P. Humpolíček 3; M. Tichý 4; V. Palička 4
Authors‘ workplace: Department of Clinical Biochemistry, Tomas Bata Regional Hospital in Zlín a. s., Havlíčkovo nábřeží 600, 76 75 Zlín 1; Faculty of Humanities, Institute of Health Care Studies, Degree Programme Midwifery, Tomas Bata University in Zlín, Mostní 51 9, 760 01 Zlín 2; Centre of Polymer Systems, Polymer Centre, Tomas Bata University in Zlín, T. G. Masaryka Sq. 5555, 760 01 Zlín 3; Institute of Clinical Biochemistry and diagnostics, Faculty of Medicine in Hradec Králové and University Hospital, 500 05 Hradec Králové 4
Published in: Klin. Biochem. Metab., 21 (42), 2013, No. 1, p. 25-29
Overview
Background:
Renal function is more frequently decreased in patients with monoclonal gammopathy. 10 % of patients with multiple myeloma present with acute renal failure and renal function is included in CRAB criteria for the diagnosis of multiple myeloma.Aim:
The aim of this study is to determine the prevalence of chronic renal failure (CRF), defined as a glomerular filtration rate (GFR) below 1.0 ml/s/1.73m2, in 101 patients with monoclonal gammopathy of undetermined significance (MGUS).Design:
retrospective studyMaterial and methods:
We compared GFR estimates from serum creatinine (MDRD GFR) and serum cystatin C (CysC GFR) and looked at the correlation between free light chains (FLC) concentration and renal markers. We performed serum protein electrophoresis (SPE) with quantification of monoclonal intact immunoglobulin (MIg) and measured serum creatinine, cystatin C, κ and λ FLC and β2microglobulin in all patients.Results:
We found CRF in 38.6% patients using estimation from serum cystatin C and 34.6 % using estimation from serum creatinine. B2microglobulin has the highest number of significant correlations; cystatin C (0.90), CysC GFR (-0.69), creatinine (0.70), MDRD GFR (-0.60), MIg concentration (0.24), κ FLC (0.22) λ FLC (0.34) and age (0.40). The best correlation results may be explained by the fact that β2microglobulin reflects both malignant cells burden and renal function.Conclusions:
We found CRF in 38.6 % MGUS patients using estimation from serum cystatin C and in 34.6 % using estimation from serum creatinine. The class of monoclonal immunoglobulin may influence results of glomerular filtration estimated from cystatin C but it does not influence GFR estimated from creatinine. Diabetic patients had not significantly lower GFR than patients without diabetes mellitus.Key words:
chronic renal failure, glomerular filtration rate, monoclonal gammopathy, free light chainsIntroduction
CRF is a very important medical, social and economic issue. One patient out of nine suffers from this condition. The current definition was established by National Kidney Foundation in 2002. The presence of CRF is defined as decreased level of GFR below 1.0 ml/s/1,73m2 with or without kidney damage for more than 3 months. Markers of kidney damage are pathological findings in urine (usually increased proteinuria or albuminuria), blood or pathological findings in imaging studies [1]. Five stages are defined according to the level of GFR [1]. Czech society for clinical biochemistry adopted the same staging system in its recommendation on determination of GFR. The most commonly used method to measure renal function is GFR [2]. The most frequently used substances to determine GFR are serum or plasma creatinine and cystatin C. MDRD equation and Grubb equation are recommended to estimate GFR [2]. Creatinine is not an ideal marker of renal function. It is influenced by factors such as age, gender, muscle mass and physical activity [3, 18], race, catabolic or anabolic state [3, 18] and is insensitive to small decreases in GFR [2]. Serum creatinine is an unreliable indicator in the elderly whose muscle mass has been reduced and therefore their results must be interpreted carefully [5]. Substances with low molecular weight such as cystatin C (13.3kDa) or β2microglobulin (11.8 kDa) appear to be better indicators of GFR [4, 18]. Cystatin C, a cysteine protease inhibitor [2, 18], is a relatively new marker of establishing GFR [6, 18]. It seems to be better than creatinine because its concentration in serum is independent of muscle mass. It is unlikely to be influenced by age or gender [7] and it is easily filtered by the glomerulus [6, 8]. However, cystatin C can be influenced by extra renal factors such as corticosteroid administration and thyroid dysfunction [7, 9, 18]. The current reference method for determination of GFR is a DTPA isotope method. This method is invasive, time consuming and available only in specialised centres [10].
Major risk factors for chronic renal failure include diabetes mellitus, hypertension, atherosclerosis and family history of renal disease [1, 11]. Monoclonal gammopathies may also cause renal damage [12, 16]. Renal insufficiency is defined as serum creatinine levels > 177 µmol/l as part of the CRAB criteria for diagnosis of multiple myeloma described by the International Myeloma Working group in 2003 [13].
[C] Calcium elevation in the blood S. Calcium >2.75 mmol/l
[R] Renal insufficiency S. Creatinine > 177 µmol/l
[A] Anemia Hemoglobin < 100 g/l or 20g/l < normal
[B] Lytic bone lesions or osteoporosis
MGUS is an asymptomatic pre-malignant disorder introduced in 1978 by Dr. Robert A. Kyle and characterized by clonal proliferation of plasma cells. The etiology of this disease is unknown, but it is related to genetic predisposition and the environmental factors [14, 15]. MGUS is frequently presented as an idiopathic rearrangement of immunoglobulin genes, which cau-ses production of an M-protein [15] and when accompanied by production of monoclonal FLC can cause kidney disease [16]. In the kidney nephron, FLC pass through fenestrated epithelia of the glomerular wall and when their quantity overwhelms the renal reabsorption capacity, they cause nephrotoxicity, such as the formation of waxy casts. Production of monoclonal FLC and abnormal κ/λ ratio predict the development of MGUS to further stages [17, 20].
Materials and methods
This study included 101 consecutive patients with MGUS from Thomas Bata Regional Hospital in Zlín from January 2009 to June 2011. This group consisted of 35 Caucasian men and 66 Caucasian women, with age ranged from 43 to 95 years. We have created 3 age groups: 40 – 59 years, 60 – 79 years and 80 – 99 years. 32 patients suffered from diabetes mellitus. All patients had an intact monoclonal immunoglobulin mole-cule; we did not include Light Chain only MGUS in our patient cohort. We retrospectively evaluated patients for the presence of CRF. The CRF is defined accor-ding to National Kidney Foundation as GFR below 1.0 ml/s/1,73m2 with or without kidney damage. It includes stage 3, stage 4 a stage 5 according to the level of GFR. It is not a particular clinical diagnose, it is the level of GFR. We originally planned to assess proteinuria, as a marker of kidney damage, but did not have urine samples from large proportion of the patients. Fasting blood samples were collected during routine medical check-up. We compared two methods of GFR estimation. The first one was estimation from serum cystatin C (CysC GFR). Cystatin C was measured by an immunoturbidimetric technique using DAKO calibration on an Abbott Architect analyser. CysC GFR was calculated by Grubb equation: CysC GFR= 1.4115 x cystatin C (mg/L)–1.680, (multiplied by 0.948 in females). The second one was an estimation from serum creatinine (MDRD GFR), using a standardized photometric enzymatic method traceable to NIST SRM 967 reference material on an Abbott Architect analyser [18]. Estimation of GFR from serum creatinine was calculated using the four parameter MDRD equation: MDRD GFR = 515.3832 x creatinine -1.154 x age -0.203, (multiplied by 0.742 in females) [18,19].
All patients had determined serum levels of β2microglobulin determined using an immunoassay on the Axsym analyser; the concentration of intact monoclonal immunoglobulin was performed using SPE densitometry and calculation from total protein; κ and λ FLC and their ratios were determined by particle enhanced immunoturbidimetric assays (Freelite; The Binding Site, Birmingham, UK) using an Olympus analyser. We compared GFR in patients with and without diabetes mellitus.
The study has been approved by The Ethical Committee of Tomas Bata Hospital. We were allowed to collect and anonymously report retrospective data of patients. Now we realized that we did not need this approval for this article. The Spearman correlation coefficient was used to assess correlation between the following parameters: cystatin C, creatinine, monoclonal immunoglobulin concentration, κ and λ FLC, FLC κ/λ ratio, β2microglobulin, gender and age. The influence of monoclonal immunoglogulin class on markers of GFR was evaluated using a mixed linear model (MLM).
Results and Discussion
The studied population had an average CysC GFR value of 1.23 ± 0.60 ml/s/1.73m2 and MDRD GFR of 1.16 ± 0.37 ml/s/1.73m2. Results show that GFR estimates from cystatin C were over decision limit (1.0 ml/s/1.73m2) in 62 patients and 39 patients had CRF (stage 3 had 29 patients, stage 4 had 8 patients and stage 5 had 2 patients). GFR estimates from creatinine were over decision limit in 66 patients and 35 patients had CRF (stage 3 had 34 patients, stage 4had 1 patient, nobody had stage 5). Discrepancies were founded in 14 cases. 9 cases had CysC GFR in the range of CRF and MDRD GFR was over decision point 1.0 ml/s/ 1.73m2. 5 patients had CysC GFR over decision limit 1.0 ml/s/1.73m2, while MDRD GFR was in the range of CRF. Overall, more than 1/3 of patients had CRF by cystatin C or creatinine.
The Spearman correlation analysis is shown in (Table 1). B2microglobulin has the highest number of significant correlations: cystatin C (0.90), CysC GFR (-0.69), creatinine (0.70), MDRD GFR ( - 0.60), MIg concentration (0.24), κ FLC (0.22) λ FLC (0.34) and age (0.40). The best correlation results may be explained by the fact that β2microglobulin reflects both malignant cells burden and renal function [18]. The higher correlation with cystatin C compared to creatinine can be explained by decreased muscle mass in elderly patients [3]. The Spearman coefficient of correlation between κ/λ FLC ratio and CysC GFR and MDRD GFR was approaching zero. We can explain it as the majority of our patients had physiological κ/λ FLC ratio.
1. Spearman correlation coefficients between observed parameters 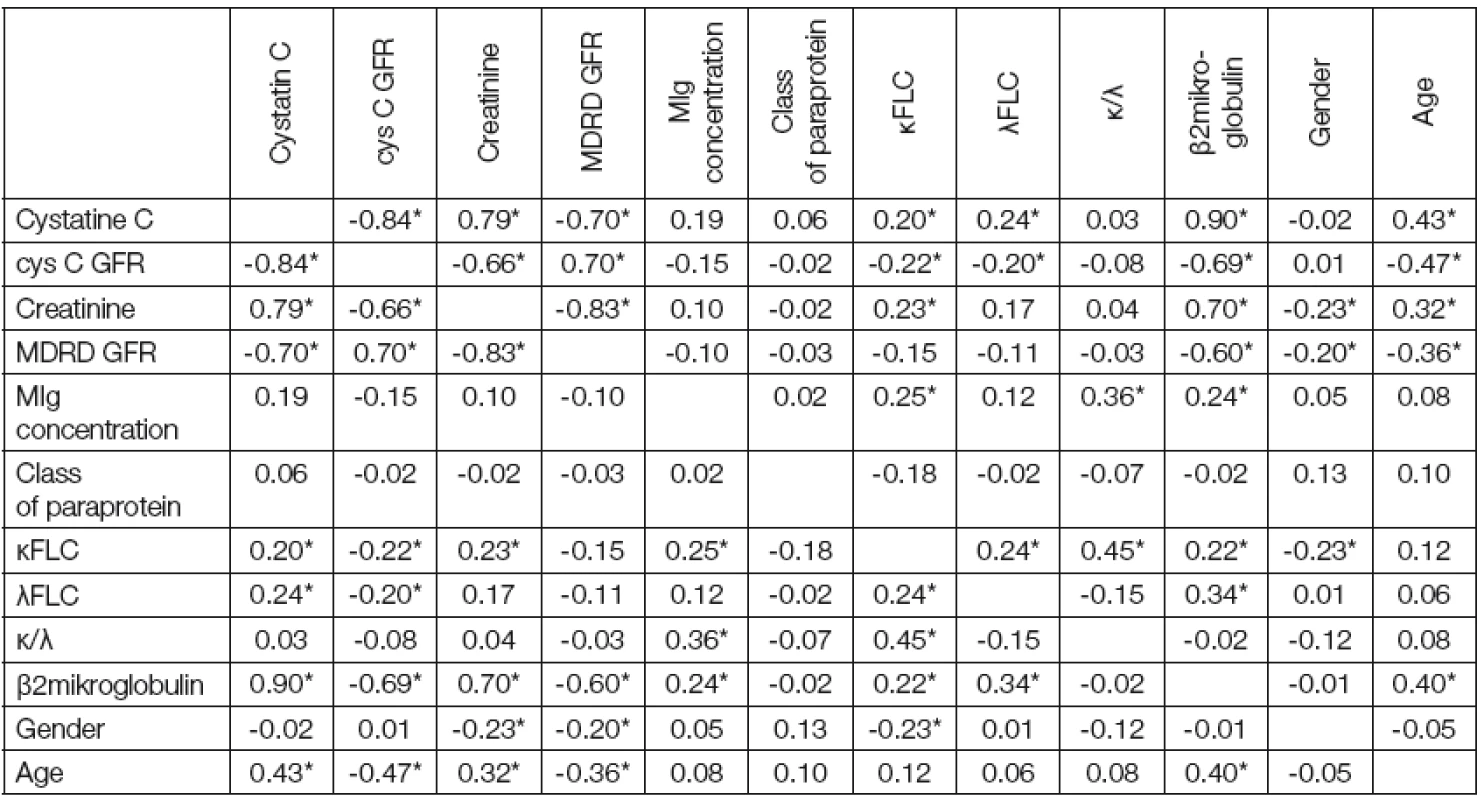
Note: Marked correlation are significant on level of probability P ≤ 0.05 (*) MIg concentration has only two significant correlations: κFLC and β2microglobulin. Increased serum β2microglobulin concentration probably reflects higher number of plasma cells in bone marrow [18]. Κ/λ FLC ratio was significant only in two cases: with MIg concentration (0.36) and with κ FLC (0.45).
The influence of monoclonal immunoglobulin class on markers of GFR is shown in (Table 2). We can see that CysC GFR shows statistically significant diffe-rences between biclonal paraproteins and IgA λ. There is an average value 0.92 with standard deviation 0.19 for biclonal paraproteins and 1.6 as an average value with standard deviation 0.24 for IgA λ. We can conclude that the class of monoclonal immunoglobulin may influence results of glomerular filtration estimated from cystatin C but it does not influence GFR estimated from creatinine. We do not have explanation for these results. Gender was also evaluated as a factor to influence results of GFR (Table 3). The age-dependent differences in estimated GFR were statistically significant in both types of estimation (CysC GFR or MDRD GFR) (Table 4). There must be relationship between age and estimated GFR because the age is included in these equations [19]. The age dependent GFR decrease is sharper in CysC GFR than in MDRD GFR. This may be explained by more rapid decrease in muscle mass in elderly patients due to chronic di-seases associated with aging [3]. Diabetic patients had not significantly lower GFR than patients without diabetes mellitus (Table 5).
2. Differences between classes of paraprotein by GFR (LSM ± SD) 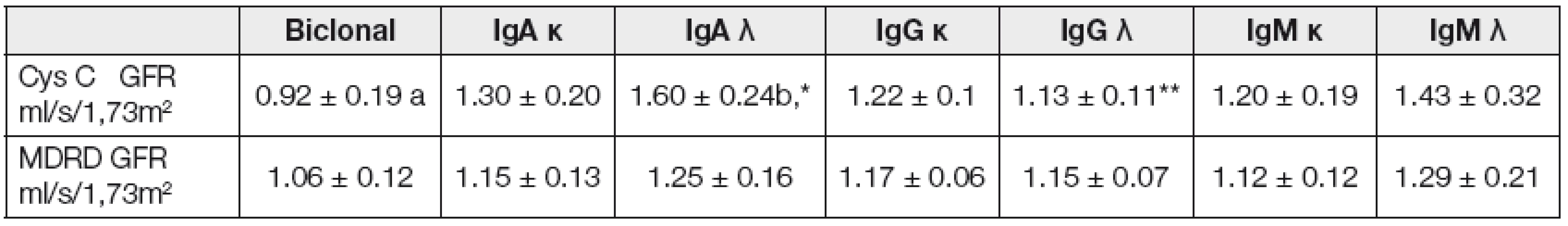
Note: Values with different superscripts show significance level within rows: P ≤ 0.05 (a/b); P ≤ 0.1 (*/**) 3. Differences in gender by GFR (LSM ± SD) 
Note: Values with different superscripts show significance level within row P ≤ 0.05 (a/b) Conclusions
We have found CRF in 38.6 % patients using GFR estimation from cystatin C and in 34.6% patients using GFR estimation from creatinine. B2microglobulin has the highest number of significant correlations: cystatin C (0.90), CysC GFR ( - 0.69), Creatinine (0.70), MDRD GFR (-0.60), MIg concentration (0.24), κ FLC (0.22) λ FLC (0.34) and age (0.40). The best correlation results may be explained by the fact that β2microglobulin reflects both malignant cells burden and renal function. The class of monoclonal immunoglobulin may influence results of GFR estimated from cystatin C but it does not influence GFR estimated from creatinine. We do not have explanation for these results. Diabetic patients had not significantly lower GFR than patients without diabetes mellitus. Further studies are needed to evaluate which parameter of GFR is most suitable and practical for patients with monoclonal gammopathies, and to evaluate treatment options for patients with elevated FLC.
Do redakce došlo 11. 5. 2012
Adresa pro korespondenci
MUDr. Tomáš Šálek
Záhumení 789
687 22 Ostrožská Nová Ves
e-mail: tsalek@seznam.cz
Sources
1. Levey, S., Coresh, J., Balk, E. et al. National Kidney Foundation practice guidelines for chronic renal failure: evaluation,classification and stratification. Ann Intern Med., 2003, vol. 139, s. 137-147.
2. Zima, T., Teplan, V., Tesař, V. et al. Doporučení České nefrologické společnosti a České společnosti klinické biochemie ČLS JEP k vyšetřování glomerulární filtrace. Klin. Biochem. Metab., 2009, č. 2, s. 109–117.
3. Larson, A., Flodin, M., Hanson, L. O., Carlsson, L. Patient selection has a strong impact on cystatin C and Modification of Diet in Renal Disease (MDRD) estimated glomerular filtration rate. Clinical Biochemistry, 2008, vol. 41, s. 1355 – 1361.
4. Donadio, C., Lucchesi, A., Ardini, M., Giordani, R. cystatin C, β2microglobulin, and retinol-binding protein as indicators of glomerular filtration rate: comparison with plasma creatinine. Journal of pharmaceutical and biomedical analysis, 2001, vol. 24, s. 835-42.
5. Hsu, C. Y., Chertow, G. M., Curhan, G. C. Metho-dological issues in studying the epidemiology of mild to moderate chronic renal insufficiency. Kidney Int., 2002, vol. 61, s. 1567 – 1576.
6. Narvaez-Sanchez, R., Gonzales, L., Salamanca, A., Silva, M., Rios, D. et al. cystatin C could be a replacement to serum creatinine for diagnosing and monitoring kidney function in children. Clinical Biochemistry, 2008, vol. 41(7-8), s. 498-503.
7. Daniel, K. M., Cason, C. L. Estimating Glomerular Filtration Rate in the Eldery. The Journal for Nurse Practicioners – JNP, 2007 (3), 4, s. 242-244.
8. Newman, D. J., Thakkar, H., Edwards, R. G., Wilkie, M., White, T. et al. Serum cystatin C measured by automated immunoassay: a more sensitive marker of ganges in GFR than serum creatinine. Kidney Int., 1995, vol. 47, s. 312 – 318.
9. Chen, H. H. Is cystatin C an Important Prognostic Mar-ker Independent of Renal Function. JACC, 2010, vol. 56, s. 1937 – 1938.
10. Vižďa, J., Lepej, J., Křížková, H., Urbanová, E. Atlas scintigrafie ledvin. Praha: Agentura Pankrác spol. s.r.o. 2002, s. 5-10.
11. Schoolwerth, A. C., Eengelgau, M. M., Hostetter, T. H. et al. Chronic kidney disease: a public health problem that needs a public health action plan. Prev Chronic Dis., 2006, vol. 3, s. A57.
12. Bradwell, A. R. Serum free light chain analysis fourth edition. U.S.A. Binding Site Inc: SanDieg, Ca 92121, s.173-182.
13. The international myeloma working group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J. Haematol., 2003, vol. 121, s. 749-757.
14. Madan, S., Greipp, P. R. The incidental monoclonal protein: Current approach to management of monoclonal gammopathy of undetermined significance (MGUS). Blood Rev., 2009, vol. 23, s. 257 – 265.
15. Munshi, N. C. Monoclonal gammopathy of undetermined significance. genetic vs. environmental etiologies. Mayo Clin Proc., 2007, vol. 82, s. 1457 – 1459.
16. Hutchison, C. A., Basnayake, K., Cockwell, P. Serum free light chain assessment in monoclonal gammopathy and kidney disease. Nat. Rev. Nephrol., 2009, vol. 5, s. 621 – 627.
17. Ščudla, V., Schneiderka, P., Pika, T. Clinical importance of evaluating serum levels of free light chains of immunoglobulins in monoclonal gammopathies. Klin. Biochem. Metab., 2008, č. 2, s. 76–83.
18. Delanghe, J. R. GFR – Where are we now? New trends in classification, diagnosis and management of renal diseases. Zagreb: Medicinska naklada 2008, s. 63 – 67.
19. Levey, S., Coresh, J., Greene, T., Marsh, J., Stevens, L. A. et al. Expressing the Modification of Diet in Renal Disease Study Equation for Estimating Glomerular Filtration Rate with Standardized Serum Creatinine Values. Clin. Chem., 2007, vol. 53, s. 766 – 772.
20. Rajkumar, S. V., Kyle, R. A., Therneau, T. M., Melton, L. J. RD, Bradwell A. R., et al. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood, 2005, vol. 106, s. 812 – 817.
Labels
Clinical biochemistry Nuclear medicine Nutritive therapist
Article was published inClinical Biochemistry and Metabolism

2013 Issue 1-
All articles in this issue
- Number of cells in the cerebrospinal fluid, energy relations in the cerebrospinal fluid compartment and intensity of inflammatory response in the central nervous system
- Significance and possibilities to examine brain metabolism in neurointensive care by microdialysis
- Changes in serum levels of markers in early detection of prostate cancer (pilot study)
- New regulation hormones of the breast milk
- The prevalence of decreased glomerular filtration rate in patients with monoclonal gammopathy of undetermined significance
- Clinical Biochemistry and Metabolism
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Significance and possibilities to examine brain metabolism in neurointensive care by microdialysis
- Number of cells in the cerebrospinal fluid, energy relations in the cerebrospinal fluid compartment and intensity of inflammatory response in the central nervous system
- Changes in serum levels of markers in early detection of prostate cancer (pilot study)
- New regulation hormones of the breast milk
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

