-
Medical journals
- Career
Single-incision endoscopic-assisted temporoparietal fascia harvest for single stage auricular reconstruction
Authors: Ha. Nguyen H.; Tran T. T. Huyen; Nguyen T. T. Hang
Authors‘ workplace: Department of Maxillofacial-Plastic-Aesthetic Surgery, Viet-Duc University Hospital, Hanoi, Vietnam
Published in: ACTA CHIRURGIAE PLASTICAE, 64, 2, 2022, pp. 62-68
doi: https://doi.org/10.48095/ccachp202262Introduction
Auricular reconstruction for acquired auricular defects or congenital microtia can be divided into two broad groups based on the use of autologous costal cartilage or porous polyethylene (PPE) frameworks. The autologous costal cartilage technique requires 2–4 surgical stages. Thus, microtia patients often have to wait up to 8–12 years old to have sufficient costal cartilage [1–6]. Surgeons are always looking for new methods with fewer surgery stages and, most importantly, this operation can be done earlier in 3–5-year-old children before they enter the school. Since the 1990s, Reinisch pioneered the early single-stage auricular reconstruction for 3–5-year-old children using a PPE framework. The key to increasing the success rate of single-stage reconstruction is to have a thin yet durable tissue layer to cover the framework well. A temporoparietal fascia flap (TPFF) is an optimal choice to minimalize framework exposure complications of this technique [3–5,7,8].
TPFF has been used for a long time to cover cartilage frameworks in one or two-stage ear reconstruction [1,2,9,10]. However, to harvest TPFFs, most of the surgeons often have to make an Y - or zigzag-shaped incision > 20 cm long on the patient's scalp, but all agreed that this surgical technique could have bad scarring and hair loss complication, especially for people with short hair. To avoid that, some authors used a loupe with headlights and/or other light sources to harvest the flap. However, this method presented huge technical difficulties as the dissection progresses toward the top of the head and it is more difficult to observe vascular branches due to the head contour. Even damages done to a very small vascular branch could increase the risk of necrosis, framework exposure, and infection which ultimately lead to complete failure of the operation [11–16]. Therefore, only a few surgeons achieved high success rates in auricular reconstruction with a PPE framework. Since the 2000s, some authors had used endoscopic techniques in TPFF harvesting to better visualize the surgical field, which in turn helps to decrease complication rates [10,17–19].
To sufficiently dissect the TPFF, all the above authors still have to make one auricular horizontal incision plus 1 or 2 additional incisions on the hair-bearing temporal scalp. The size of the endoscopic case series is also limited to 1–10 flaps [10,17–19]. In this report, we present a 60-patient series of single-stage auricular reconstruction using costal cartilage or PPE framework, covered by 61 TPFFs with the help of endoscopic techniques via only one cosmetic auricular skin incision.
Patients and methods
The study was conducted in patients with congenital microtia or acquired auricular defects who underwent single-stage auricular reconstruction using autologous costal cartilage or PPE framework from June 2018 to September 2021.
The costal cartilage framework was sculpted from the 6th–9th costal cartilages and adjusted to match the opposite ear’s size. The PPE framework was Omnipore (Matrix Surgical USA). Pre-op flap design/measurement, framework projection/fixation, flap/skin graft coverage, dressing, and post-op care were all conducted according to the technique described by Reinisch (Fig. 1). Flap dissection was innovated using endoscopic techniques via a single auricular incision.
Fig. 1. A) A 5-year-old girl with right sided microtia. White line, auricular incision; it can be extended horizontally across the upper sideburn if necessary to facilitate dissection. Blue dotted line, estimated outline of the temporoparietal fascia flap. Red dotted line: two branches of superficial temporal artery. Yellow dotted line: estimated path of the frontal branch of the facial nerve (Pitanguy’s line); B) delivered flap, no incisions in hair bearing scalp; C) porous polyethylene framework; D) after suction, the thin and vascular-rich flap, fully covered with the polyethylene framework, with nice ear shape; E) good projection, posterior side after 3 months; (F) result after 6 months. 
Typically, we harvest TPFFs of an approx. size of 13 × 10 cm (Fig. 1) via a horizontal 4–4.5 cm auricular incision along the hairline above the estimated helix rim, using the endoscopic technique with 30 degrees 10 mm optic and small electrocauteries probes with different lengths. A horizontal extension of the incision across the upper sideburn can be made to facilitate dissection of the TPFF and vascular pedicle protection (Fig. 1A). Dissecting the plane between the scalp and the flap, we pay attention not to damage the hair follicles or superficial temporal artery (STA) branches (Fig. 2A–C). Two branches of the STA are the parietal artery (PA, also called posterior branch) and the frontal artery (FA, also called anterior branch). The FA often divides into 3 branches near lateral border of the orbit: the posterio/centro/anteriofrontal artery. The posteriofrontal artery (PFA) travels posteriorly to connect with the parietal artery. The PFA is around 12 cm from the supposed ear canal, which is why we often harvest a 13 cm long flap. We always try to preserve both PA and PFA to ensure flap survivability. In some certain cases, with the help of endoscope magnification, we can discover 1–2 smaller artery(ies) which branch out earlier from the FA than from PFA (pre-posteriofrontal artery 1 and 2 or PPFA 1 and 2). These arteries can help to adjust flap design in case the frontal branch of the facial nerve is too close to the PFA (Fig. 2D–F).
Fig. 2. A) Endoscopic technique advantages; B) clear distinguish between the flap and subcutaneous fat layer with hair follicles; C) with high image quality, even small bubbles inside arteries are visible (black arrow); D) pre-operative Doppler found only two branches of the superficial temporal artery: the parietal and the frontal arteries. The posteriofrontal artery travels posteriorly around 12 cm from the supposed ear canal; E) thanks to endoscopic technique, we can see more branches (the pre-posteriofrontal artery 2 is undetectable by pre-operative Doppler); F) superficial temporal artery with parietal artery and posteriofrontal artery and two smaller arteries which branch out earlier from the frontal artery than from the posteriofrontal artery (pre-posteriofrontal arteries 1 and 2). These two small branches are undetectable by pre-operative Doppler (Fig. 2D). 
Patient demographic data such as age and sex were reviewed. Specific data regarding the patient’s ear laterality, the existence of a syndrome, framework material, and surgical complications were also included. All patients using open surgery or endoscopic technique but required extra incisions on the hair-bearing temporal scalp were excluded.
We evaluated the result based on the survivability of the flap/grafted skin, the shape of the auricle, as well as the framework. Complications are categorized as severe complications such as severe infection, framework exposure, bleeding, necrosis of the flap leading to the failure of the entire operation which required frameworks replacement or removal; mild complications such as superficial infection, small framework exposure, and small necrosis of the distal part of the flap which self-resolved or only required local intervention.
Results
A total of 60 patients with microtia and trauma-induced auricular defect, aged 3–50 years, underwent single-stage total auricular reconstruction; in 15 patients, autologous costal cartilages were used and in 45 patients, 46 PPE frameworks were used.
The age of the autologous costal cartilage group ranged from 9 to 24 years, while that of PPE ranged from 3 to 50 years; of these patients, up to 20 patients who were 6 years old or younger had early surgery before going to school. The male : female ratio was 1.7 : 1. Fifty-six patients had congenital microtia (53 unilateral and 3 bilateral) and 4 patients had a trauma-induced auricular defect (Tab. 1).
1. Patients’ demographic data (N = 60). 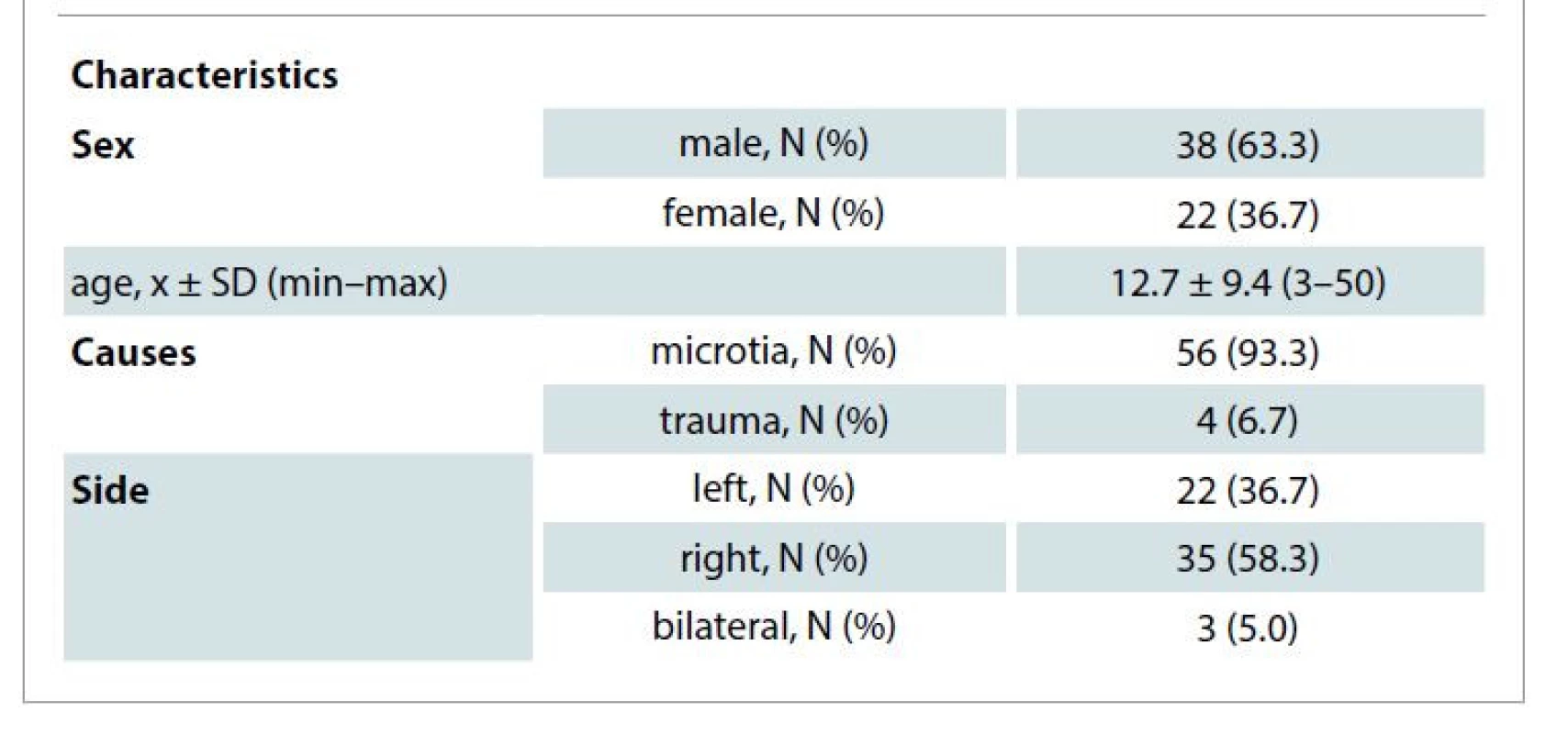
All the TPFFs were harvested by improved endoscopic technique with only one cosmetic incision across the hairline without any additional incisions on the hair-bearing temporal skin. The flaps were fully viable immediately after harvesting, both parietal and frontal arteries along with their connections were all sufficiently preserved, and flap edges perfusion was also good (Fig. 2F). There was no bleeding peri-/post-operatively. After 2 weeks of gauze compression and external splinter fixation, all flaps had fully survived. There were no severe complications which would require framework removal or replacement. Among autologous costal cartilage (15 flaps) there was 1/15 flap (6.7%) which had a 3 mm framework exposure due to superficial infection which resolved on its own thanks to granulation tissue formation. In the group of 46 PPE frameworks, 3/46 flaps (6.5%) of superficial infection caused framework exposure and 3/46 flaps (6.5%) had pressure-induced framework exposures, all of which were small (3–7 mm) in size and required only local flaps coverage without having to replace or remove the implant framework. One case had frontal nerve injury (Tab. 2).
2. Flaps demographic data (N = 61). 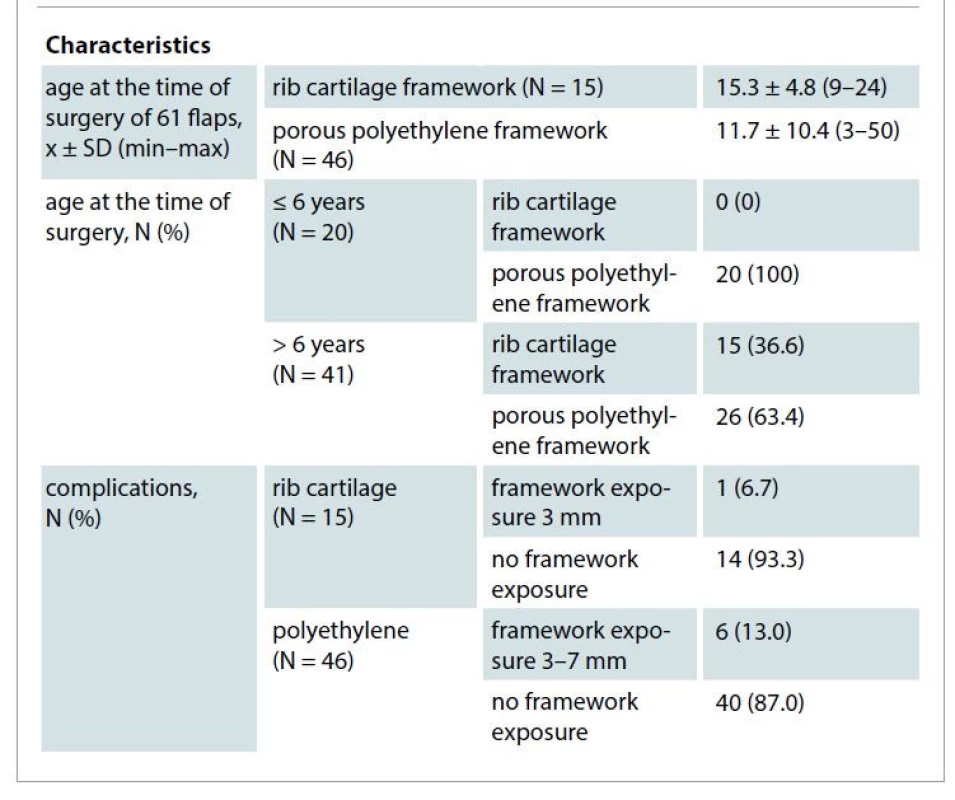
Post-operative follow-up was 6–36 months and its results were as follows: there was no framework exposure or break. Grafted skin fully survived and could move freely above the frameworks. Autologous costal cartilage frameworks had less projection than implanted ones. Aesthetically, ears shaped by implant frameworks have a more cleared and aesthetic outline than the autologous cartilaginous framework.
Discussion
Until now, auricular reconstruction methods are mostly consisting of multiple (2–4) stages of surgery. The reconstruction materials were mostly autologous costal cartilages. Thus, the authors advise that it is desirable to wait until the patients already have sufficiently large costal cartilages, usually at the age of 8–12 [1–6,9,20]. This long period of postponing causes psychological impacts on the patients and families, especially when the children reach school age.
Single-stage auricular reconstruction reduces both the number of operations and the treatment duration [3,5,14,21,22]. Plastic surgeons have continuously improved their techniques, collaborating with manufacturers to improve artificial materials to suit clinical needs. Since the 1990s, Reinisch have been a pioneer in the development of early single-stage auricular reconstruction using the PPE framework [7,8,12]. It can spare thoracic damage and related complications like hemo/pneumothorax and requires less surgery. However, it is still a difficult technique that requires finesse, meticulousness, and a long learning process, so only a few surgeons are able to master it. The most frequent and also most catastrophic complication of PPE is framework extrusion, which can lead to total surgical failure [4,14–16,22]. To minimize that risk, it is vital to have a soft, thin, yet sufficiently large TPFF with both parietal and frontal arterial branch and their connections to cover the framework. When encountered difficulty due to head contour or scalp limited elasticity, Reinisch suggested making a second horizontal temporal incision [8]. This helps the dissection but increases the risk of bad scars and alopecia (Fig. 3A).
Fig. 3. Examples of bad scar alopecia patient open or extra horizontal incision, which has been excluded from research. A) A 5-year-old boy with additional horizontal incision, result after 4 months; B) a 14-year-old boy with an open surgery scar after 1 year. 
The idea of ear reconstruction with TPFF has been around for a long time. In 1983, Brent has used the TPFFs to cover the autologous costal cartilage framework in post-traumatic auricular defects [1]. Nagata (1993) used TPFFs to cover the posterior surface of the reconstructed ears to perfect his innovative two-stage reconstruction technique [2]. In 1999, Park reported the effective use of 123 TPFFs in open surgery auricular reconstruction [9]. If the dissection plane is too close to the skin to protect vascular pedicles, hair follicles will be damaged and vice versa. Although open surgery with a magnifying loupe was performed, he still encountered 1 flap with an injury of the frontal branch of the facial nerve, and 21 flaps of vascular damages which required repair by microsurgery, of which 5 flaps were partially necrotic, and 2 flaps of an infected framework required removal [9]. In the literature, all authors agreed that open surgery can lead to bad scarring and hair loss complication which affect the cosmetic results (Fig. 3B). Furthermore, intraoperative blood loss and postoperative pain/discomfort are also concerned [10,17,19,23].
The application of endoscopy in surgery has brought about a new revolution by projecting the operating field on a large screen, allowing surgeons to observe detailed anatomical structures and preserving the supplying vessels for the TPFF during the harvesting process. In 2000, Takushima successfully harvested a TPFF using the endoscopic technique to cover the posterior side during Nagata-style second stage auricular projection. This yielded positive results but still required 3 incisions: auricular and 2 horizontal incisions, each approx. 2 cm long, on the upper border of the planned TPFF. Among 9 flaps, there were 2 incidents of perioperative uncontrollable bleeding which required incision extension to facilitate coagulation. One TPFF far end became nonviable and the exposed framework had to be covered with a local skin flap [10]. In 2002, Chung et al used TPFF in 5 patients with upper limb and hand defects. Through a 4 cm preauricular incision, they managed to harvest TPFF with maximal dimensions of 10 × 6 cm. Three flaps were fully viable, 1 with total necrosis and 1 with partial necrosis. One donor site bleeding incident required additional surgical drainage [23].
In 2008, Helling and Wang implemented an endoscopic technique to harvest large TPFF to cover Medpor auricular framework in 9 patients [17]. Still, they need a 2–3 cm V-shaped extra access incision between the upper third and middle third of the temporal region to counter head contour during dissection. These incisions could affect the cosmetic result.
In 2014 Hempel et al performed partial auricular reconstruction in 10 patients with PPE frameworks and TPFFs. Harvesting techniques were similar to that of Helling with two skin incisions and flaps were generally smaller. Eighty percent of patients were satisfied while the remaining 20 were not since there was 1 framework extrusion and 1 grafted skin loss, both required additional surgery [18].
In 2015 Nataliya Biskup reported successful single-stage auricular reconstruction with rib cartilage framework in 1 low hairline patient based on Helling and Wang technique. The small sample size is the limitation of this report [19].
Since 2017 we have applied Reinisch’s technique to perform single-stage auricular reconstruction with both open and endoscopic-assisted TPFF harvesting. At the beginning, for the endoscopic technique, we still need to use an extra incision - a superior access port incision described by Helling. After following up, we found out that both open and extra horizontal incision techniques could leave an unaesthetic scar and alopecia (Fig. 3A, B). This is the reason why we decided to improve the technique, abandon the horizontal incision and use only one single auricular incision to harvest TPFF with endoscopic assistance.
Due to the nature of our department where endoscopy is applied widely in craniomaxillofacial trauma, skull base tumors, and cosmetic surgeries, we are comfortable with the endoscopic-assisted TPFF harvesting technique. Along with innovative instruments and 30° 10 mm optic, we were able to harvest 13–14 cm or larger and fully viable TPFF with both arterial branches even when encountered head contour through only one single auricular incision (Fig. 4, 5). Endoscopic technique also allows good coordination and comfortable posture, requires no loupe or headlight. Large screen projection is also good for teaching purpose and thus makes the technique reproducible and reliable. Precise sharp dissection can be made which diminishes blood loss and post-operative pain comparing with an open technique.
Fig. 4. A) A 12-year-old male, auricular reconstruction with a rib cartilage framework; B) endoscopic-assisted flap harvesting yielded a 13 cm long temporoparietal fascia flap; C) a six-month follow-up result. Good framework coverage, no scar in the scalp. 
Fig. 5. A) A 50-year-old female’s auricular reconstruction with a polyethylene framework; B) a 13 cm long temporoparietal fascia flaps with all arterial and superficial venous branches; C) a 3-month follow-up result showed no hair loss. Superficial veins are preserved so there is no prolonged swelling like in other adult patients who underwent non-endoscopic flap harvesting technique. 
Our technique achieved both reconstructive requirements and cosmetic results. All flaps were fully viable, with no peri-/post-operative bleeding or framework extrusion that required framework replacement/removal. One patient out of 15 (6.7%) in the autologous rib cartilage group had self-resolved framework exposure. Six patients out of 46 (13.0%) in the PPE framework group had 3–7 mm framework exposure which was covered by a local skin flap.
Constantine (2014) performed 36 open surgery auricular reconstructions using 18 rib cartilages and 18 PPE frameworks. One rib framework exposure had self-resolved and 2 (11.1%) PPE exposures required replacement by rib cartilages [4].
In the early years (1993–1995), Reinisch et al had a complication rate of up to 44% of patients with framework exposure and 25% with framework breakage. Continuous technique improvement helped him and his colleagues to reduce the complication rate (1996–1997) to 7.3% of patients with framework exposure and 2.7% patients with framework breakage [7].
Kim et al (2017) report rib cartilage and Medpor open surgery auricular reconstruction in 149 patients. Fourteen patients out of 149 (9,4%) had framework exposure (3/48 (6.3%) in the rib cartilage group, 11/101 (10.9%) in the Medpor group) and all required deep temporal and mastoid fascia flap coverage [16].
Frontal branch of the facial nerve injury is another possible complication but is less commonly reported. We encountered one case of paralysis of the frontal branch of the facial nerve. Park (1999) and Koulaxouzidis (2011) each also reported similar cases [9,24]. In case the course of FA is too anterior, it may then occur too close to the frontal branch of the facial nerve. This may be the cause of its injury when the arterial branches are involved during harvesting of the flap. Reinisch suggested altering the flap design and including the medial and lower portion of the frontal artery only [7]. However, this raised a question since the upper portion along with the PFA is crucial to the flap viability. Fortunately, thanks to the advantages of endoscopic techniques, we were able to clearly identify smaller branches originating from the FA (PPFA 1, 2, etc.) which were previously undetectable by clinical examination / pre-operative Doppler / 3D CT angiography. TPFFs can be harvested based on these artery’s paths to avoid nerve injury while maintaining flap survivability. For example, with the presence of PPFA 1 and 2, flap anterior border can go between PFA and PPFA 2, or PPFA 2 and PPFA 1 (Fig. 2D–F).
There is currently a very limited number of open surgery PPE framework auricular reconstruction reports and even fewer endoscopy-assisted ones. We only found 4 authors (mentioned above) reporting the results of auricular reconstruction, with the application endoscope in TPFFs harvesting with a modest number of 1–10 flaps. Thus, we are confident that this is the largest case series of auricular reconstruction with endoscopy-assisted TPFFs harvest (61 flaps) yet and that our center is one of the very few to routinely perform the endoscopy-assisted temporoparietal flap harvesting technique via only one single cosmetic incision.
Conclusions
Single-stage auricular reconstruction with autologous costal cartilage or PPE framework covered with TPFFs is a difficult technique, requiring extensively trained and experienced surgeons. Especially, PPE framework enables reconstructive surgery on pre-school age children. Combining the knowledge of flap transfer and with endoscopic technique allows obtaining sufficiently large TPFFs with high survivability ensuring full coverage of autologous costal cartilage or PPE framework. This method is reliable, reproducible with advanced training. There is only a limited number of reports/cases about this technique in the literature. Our cases series are the largest endoscopic-assisted TPFF harvesting series and the first to implement single-incision endoscopic technique in auricular reconstruction.
Acknowledgments: We would like to thank Prof. John Reinisch and Dr. Ken Stewart for sharing their precious experience in microtia ear reconstructions.
Financial disclosure statement: None of the authors has financial interest in companies producing or distributing products use for this study.
Conflict of interest: The authors declare that they have no conflicts of interest.
Compliance with ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent form was signed for all the patients who underwent surgery.
Role of authors: All authors have been actively involved in the planning, preparation, analysis and interpretation of the findings, enactment and processing of the article with the same contribution.
Ha H. Nguyen
Department of Maxillofacial -Plastic -Aesthetic Surgery
Viet-Duc University Hospital
40 Trang Thii
Hanoi
Vietnam
e-mail: nhadr4@gmail.com
Submitted: 3. 5. 2021
Accepted: 20. 7. 2022
Sources
1. Brent B., Byrd HS. Secondary ear reconstruction with cartilage grafts covered by axial, random, and free flaps of temporoparietal fascia. Plast Reconstr Surg. 1983, 72(2): 141–152.
2. Nagata S. A new method of total reconstruction of the auricle for microtia. Plast Reconstr Surg. 1993, 92(2): 187–201.
3. Baluch N., Nagata S., Park C., et al. Auricular reconstruction for microtia: a review of available methods. Plast Surg. 2014, 22(1): 39–43.
4. Constantine KK., Gilmore J., Lee K., et al. Comparison of microtia reconstruction outcomes using rib cartilage vs porous polyethylene implant. JAMA Facial Plast Surg. 2014, 16(4): 240–244.
5. Ali K., Trost JG., Truong TA., et al. Total ear reconstruction using porous polyethylene. Semin Plast Surg. 2017, 31(3): 161–172.
6. Tahiri Y., Reinisch J. Porous polyethylene ear reconstruction. Clin Plast Surg. 2019, 46(2): 223–230.
7. Reinisch JF., Lewin S. Ear reconstruction using a porous polyethylene framework and temporoparietal fascia flap. Facial Plast Surg. 2009, 25(3): 181–189.
8. Reinisch J., Tahiri Y. Polyethylene ear reconstruction: a state-of-the-art surgical journey. Plast Reconstr Surg. 2018, 141(2): 461–470.
9. Park C., Lew DH., Yoo WM. An analysis of 123 temporoparietal fascial flaps: anatomic and clinical considerations in total auricular reconstruction. Plast Reconstr Surg. 1999, 104(5): 1295–1306.
10. Takushima A., Asato H., Harii K. Endoscopic-assisted harvest of the temporoparietal fascial flap. Ann Plast Surg. 2000, 45(4): 382–385.
11. Lewin S. Complications after total porous implant ear reconstruction and their management. Facial Plast Surg. 2015, 31(6): 617–625.
12. Reinisch J. Ear reconstruction in young children. Facial Plast Surg. 2015, 31(6): 600–603.
13. Stephan S., Reinisch J. Auricular reconstruction using porous polyethylene implant technique. Facial Plast Surg Clin North Am. 2018, 26(1): 69–85.
14. Oliver JD., Rodriguez D., Scott D., et al. Alloplastic auricular reconstruction: review of implant types, adverse events, and aesthetic outcomes. J Craniofac Surg. 2020, 31(6): 1593–1596.
15. Reinisch JF., van Hövell Tot Westerflier CVA., Gould DJ., et al. Secondary salvage of the unsatisfactory microtia reconstruction. Plast Reconstr Surg. 2020, 145(5): 1252–1261.
16. Kim YS., Yun IS., Chung S. Salvage of ear framework exposure in total auricular reconstruction. Ann Plast Surg. 2017, 78(2): 178–183.
17. Helling ER., Okoro S., Kim G., et al. Endoscope-assisted temporoparietal fascia harvest for auricular reconstruction. Plast Reconstr Surg. 2008, 121(5): 1598–1605.
18. Hempel JM., Braun T., Patscheider M., et al. Partial auricular reconstruction with porous polyethylene frameworks and superficial temporoparietal fascia flap. Eur Arch Otorhinolaryngol. 2014, 271(10): 2761–2766.
19. Nataliya B., Martin MC. A true single-stage reconstruction of a projected auricle for concha-type microtia incorporating endoscopically harvested temporoparietal fascia. J Craniofac Surg. 2015, 26(6): 1930–1932.
20. Zhang Q., Quan Y., Su Y., et al. Expanded retroauricular skin and fascial flap in congenital microtia reconstruction. Ann Plast Surg. 2010, 64(4): 428–434.
21. Romo T., Fozo MS., Sclafani AP. Microtia reconstruction using a porous polyethylene framework. Facial Plast Surg. 2000, 16(1): 15–22.
22. Berghaus A., Stelter K., Naumann A., et al. Ear reconstruction with porous polyethylene implants. Adv Otorhinolaryngol. 2010, 68 : 53–64.
23. Chung KC., Cederna PS. Endoscopic harvest of temporoparietal fascial free flaps for coverage of hand wounds. J Hand Surg Am. 2002, 27(3): 525–533.
24. Koulaxouzidis G., Torio-Padron N., Momeni A., et al. Soft tissue reconstruction with a temporoparietal fascial flap (TPFF). Oper Orthop Traumatol. 2012, 24(1): 32–42.
Labels
Plastic surgery Orthopaedics Burns medicine Traumatology
Article was published inActa chirurgiae plasticae

2022 Issue 2-
All articles in this issue
- Editorial
- Single-incision endoscopic-assisted temporoparietal fascia harvest for single stage auricular reconstruction
- Temporary skin closure in extremity soft tissue sarcoma – our indications
- Modified harvesting technique for pedicled pectoralis major muscle flap after extended manubrial resection in case of recurrent cervicothoracic junction tumors
- Bilateral latissimus dorsi for breast reconstruction in one stage
- Subcutaneous shoulder hibernoma presenting as an atypical lipomatous tumor – a case report
- Salvage of a large exposed cranial implant on irradiated necrosed scalp using free latissimus dorsi and forehead flaps – a case report
- Single-stage reconstruction of a large lower eyelid defect using a full-thickness bilamellar autograft
- Hook nail treatment – a bulky palmar flap as an alternative to the “antenna” procedure and to the thenar flap for fingertip coverage
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Single-incision endoscopic-assisted temporoparietal fascia harvest for single stage auricular reconstruction
- Salvage of a large exposed cranial implant on irradiated necrosed scalp using free latissimus dorsi and forehead flaps – a case report
- Bilateral latissimus dorsi for breast reconstruction in one stage
- Hook nail treatment – a bulky palmar flap as an alternative to the “antenna” procedure and to the thenar flap for fingertip coverage
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

