-
Medical journals
- Career
Temporary skin closure in extremity soft tissue sarcoma – our indications
Authors: Matějovská J. 1; Christodoulou P. 1; Šorelová V. 1; Fridrichová M. 2; Matějovský Z. 3
Authors‘ workplace: Department of Plastic Surgery, University Hospital Bulovka, Institute for Postgraduate Studies, 1st Medical Faculty, Charles University, Prague 1; Department of Clinical Oncology, University Hospital Bulovka, Institute for Postgraduate Studies, 1st Medical Faculty, Charles University, Prague 2; Department of Orthopedics, University Hospital Bulovka, Institute for Postgraduate Studies, 1st Medical Faculty, Charles University, Prague 3
Published in: ACTA CHIRURGIAE PLASTICAE, 64, 2, 2022, pp. 69-75
doi: https://doi.org/10.48095/ccachp202269Introduction
The indication for temporary skin closure (TSC) after resection of soft tissue sarcomas (STS) of the extremities has been recently discussed on several meetings concerning the treatment of STS. Some have neglected its indication at all and others have claimed a wide indication. The aim of the study is to present our own experience with this method starting in 2004 and to discuss further possible theoretical indications.
Historically, packing was used for TSC in general surgery and porcine xenografts were used to cover defects after burns [1,2]. Later, different artificial TSC were being developed to replace porcine xenografts and to decrease the risk of infection, as well as to promote the growth of granulation tissue in order to prepare the surgical field for a final single stage or gradual wound closure [2,3]. These found their indications also in abdominal surgeries and neurosurgery [1,4].
The use of artificial TSC in oncological surgery is, with the exception of skin cancer, rare. The surgical principles for cancer and sarcomas differ grossly [5]. The basic principles of a wide resection in STS are well known [5]. In contrary to carcinomas or melanomas, a simple reresection of margins after an intralesional (R2) or marginal (R1) primary resection is not possible due to the contamination of the entire surgical field [5]. The basic principle of using the easiest effective method for closure of defects after STS resections is still valid [6]. With the evolution of new safer techniques, more sophisticated methods for covering defects after resections of STS are being used, prolonging the surgical time and increasing blood loss. This can lead to adverse effects in the immune system and general status of the patient, especially when receiving or planning systemic treatment [7,8]. For such cases the use of artificial TSC, enabling a safe two-stage surgery can be beneficial. An impending infection, like in ulcerating tumors, can also be the indication for artificial TSC, especially, when soaked with antibacterial solutions [9].
For these reasons we present our experience and indications for the use of TSC after resections of extremity STS. The purpose of our paper is not to evaluate the whole cohort of our patients with STS in detail, but to show indications when the use of TSC can be useful to solve certain situations or problems.
Materials and methods
In 2004, we first used Aquagel for a TSC and changed it for Parasorb in 2009 and for COM 30 (COM – a combined dressing fabric consisting of polyester mesh, polyurethane foam and polyamide knitted fabric) in 2015. The indication for the use of TSC was random, depending on the actual individual situation of every patient and the results were sometimes surprising. We present these indications and try to select those that we find correct or still appropriate. Out of 594 patients with STS treated surgically at the Department of Orthopedics, University Hospital Bulovka, Prague, between January 1st 2014 and January 31st 2022, 97 patients had a flap, TSC or both. One patient with a TSC had an initial diagnosis of malignant fibrous histiocytoma, but the final diagnosis was a melanoma and one patient with suspected melanoma had a final diagnosis of a clear cell sarcoma after detailed genetic sampling. Both were included into the cohort of patients with TSC evaluated in detail for location, diagnosis, secondary closure and clinical and local outcome. Two other patients had a TSC on the foot, but the diagnosis was a skin carcinoma so they were not included in the cohort.
As only one patient had a primary tumor located of the shin, where we do not indicate neoadjuvant radiation, and all of the other patients had recurrent tumors, where neoadjuvant radiation is not indicated, no patient had radiation as part of a planned neoadjuvant treatment prior to the use of TSC. No patient received postoperative radiation therapy, because in cases with local recurrence, the extent of the local spreading of the tumor from the time of primary surgery is unpredictable and radiotherapy only increases the risk of future surgeries. At the same time, it does not prevent local recurrence extending „outside“ of the scar area. The patients with systemic spread of the disease had systemic chemotherapy containing doxorubicin; some of them in combination with iphosphamide. Even if the margins of the resected local recurrence are clear (wide or radical resection), the procedure must be still considered intralesional due to the prior tumor spread in the operating field. Therefore, even if all but two patients, had a clear resection margin, we did not consider the histologic margin of the resected local recurrence as a relevant variable for further treatment. For such cases, we prefer to perform ultrasound or MRI local controls every 3 months in the first 2 years, instead of primary postoperative radiotherapy. Following these principals, small, possible consequent local recurrences can be detected early and easily excised [10].
TSC was replaced either by the plastic surgeon during wound checks and dressing changes, or during control smears to detect possible infections, or it was left in place until definitive surgery.
All patients had a regular follow up for local and systemic disease control [10].
As the indication is rare and therefore the cohort of patient was small, we did not perform any statistical evaluation.
Results
TSC was used in 11 patients (6 males and 5 females). All but one case on the shin were recurrent tumors at the time of surgery. Seven were high-grade sarcomas, 2 were synovial sarcomas, one was low-grade myxofibrosarcoma and one was low-grade malignant peripheral nerve sheath tumor.
In the first two patients with recurrent STS, we used Aquagel on the foot and they had a microsurgical free flap covering the tendons and bone in the second procedure (Fig. 1). One local recurrence was identified in each of the two patients by ultrasound controls and after its removal, no evidence of disease was found 18 and 17 years after the initial surgery.
Fig. 1. A 32-year-old female patient with a recurrent low-grade malignant schwannoma after primary resection at the age of 17 and resection of a local recurrence at the age of 26 years. A) Before surgery – local recurrence and extent of planed excision are marked; B) after planned excision; C) the defect covered with Aquagel; D) after covering the defect with a free vascular gracilis flap outward in 2005. 
In the next four cases, Parasorb was used to cover the defect on the foot and once in the popliteal region. All patients had a recurrent tumor. Primarily, one free flap and one local flap (after a negative smear of an originally ulcerating tumor) were used. In two cases, a split-thickness skin graft was used as granulating tissue evolved rapidly in the first case, where it covered the tendons in the popliteal region. In the other case, the planed free flap was contraindicated as the patient was scheduled for hyperthermic isolated limb perfusion (HILP) due to the change of the primary original diagnosis. After HILP, the failed split-thickness skin graft had to be changed for a local fascio-cutaneous sural perforator flap. All four patients had a local and systemic progression of the disease and died of it 5, 24, 27 and 29 months after our initial surgery.
In the last five cases, COM 30 was used on the foot (once), shin (once) and femur (three times). Except for the case over the tibia bone, all were recurrent tumors. On the dorsal part of the foot, the granulation tissue progressed rapidly over the tendons and COM was exchanged after 23 days for Mepilex AG and a split-thickness skin graft was successfully used 32 days later. The patient has no evidence of the synovial sarcoma 6 years after the surgery. The shin defect was covered, after a marginal excision, with a rotation flap. Seven days later, a small local recurrence was removed at the edge of the flap and the patient has no evidence of the disease 20 months after the initial surgery. On the femur, COM was indicated after failures of other local flaps in two cases. In the third case, it was indicated for a failed split-thickness skin graft in a locally infected area on the ventral proximal part of the femur. In this case, the application of COM over the femoral artery for Pseudomonas aeruginosa local infection led to an uncontrollable bleeding from a non-reconstructible defect in the femoral artery within 5 days after application of COM, necessitating exarticulation in the hip. The patient received chemotherapy and had no evidence of disease 33 months following the surgery. In patient No. 9, the local hatchet flap successfully covered the bone of the greater trochanter 7 days after application of COM. The patient died after local and systemic progression of the disease 21 months later. The patient with disseminated leiomyosarcoma from the femur died 5 months after surgery on local and systemic progression.
Six patients had systemic disease progression and died of the disease in average 18,5 (5–29) months after surgery. Five of them had a further local recurrence. Five patients with no evidence of the disease are surviving in average 109 (20–216) months. Three of them had a further single local recurrence that was detected and removed.
For further details, see Tab. 1.
1. Patients characteristics and outcome. 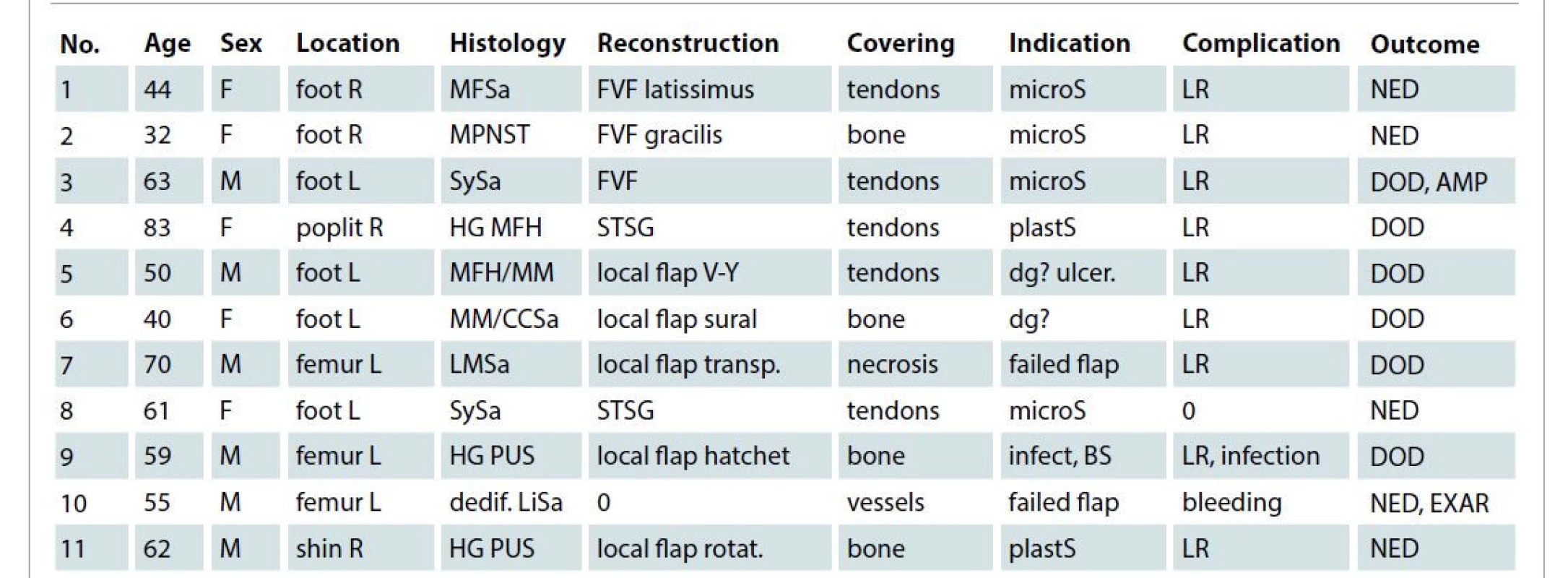
AMP – amputation, BS – bad patient status, CCSa – b-raf negative clear cell sarcoma, covering – area covered by TSC, dedif. LiSA – dedifferentiated MDM2 positive liposarcoma, dg? – diagnosis unclear, DOD – died of disease, EXAR – exarticulation, FVF – free vascular flap, HG MFH – high-grade malignant fibrous histiocytoma, HG PUS – high grade pleomorphic undifferentiated sarcoma, infect – infection, LMSa – leiomyosarcoma, LR – local recurrence, MFSa- myxofibrosarcoma, microS – microsurgeon not available, MM – malignant melanoma, MPNST – malignant peripheral nerve sheath tumor, NED – no evidence of disease at final control, No.– patient number, plastS – plastic surgeon consulted for unexpected local finding and indicated a delayed reconstruction, R/L – right left, rotat. – rotation, STSG – split-thickness skin graft, SySa – synovial sarcoma, transp. – transposition, ulcer. – ulcerating tumor Our indications for the use of artificial TSC were as follows:
1. delayed distant free flap when the microsurgeon was not available – four cases;
2. bad patient’s status or complications of anesthesia not allowing further prolongation of surgery or considering it at risk – one case;
3. high risk of infection, e.g., ulcerating tumors or failed closure as an alternative to vacuum assisted closure (VAC) system or when this is not possible – four cases;
4. planed split-thickness skin graft not feasible due to unsuitable tissue in the wound e.g., bone, tendons, vessels, nerves, when the surgeon is inexperienced in the use of local flaps or the plastic surgeon consulted by phone prefers a two-stage surgery – two cases;
5. suspicious melanoma or unclear histology in cases of planed extensive local flaps – two cases.
Further indications may include:
6. suspicious contamination of the wound in cases of planed extensive local flaps;
7. unexpected unclear dignity of the tumor e.g., in whoops lesions;
8. granulation tissue could improve the local situation and VAC system is not indicated – unexpectedly and unplanned in two cases;
9. technical problems to finish the planed surgery or to perform the planed reconstruction flap.
Discussion
All but one patient had recurrent tumors at the time of our surgery using TSC. Therefore, it is nearly impossible to evaluate the whole contaminated areas. This can explain why the local recurrences in low-grade tumors occurred at the edges of the flaps. Furthermore, most of them were high grade sarcomas, where the risk of skip lesions is high and it is difficult to differentiate them from local recurrences. In the high-grade tumors, the local recurrences occurred in most cases together with skip lesions, lymph node infiltrations or distant metastases. The histology of nearly all excisions was considered wide. Nevertheless, this evaluation also depends on the number of slices performed. Even if the rate of local recurrences was high, it was important that in all but two cases the patient had a functional limb until death. The amputation was indicated for local tumor progression and the exarticulation for infectious complications leading to uncontrollable bleeding. The rate of death is around 60% in high-grade or recurrent sarcomas [1]. This is similar to the survival rate in the small cohort of our patients, where 6 out of 11 patients dies of the disease.
In cases when a plastic surgeon is not available, or the conditions for microsurgery are not met, the covering of the defect by an artificial TSC and transferring the patient for planed definitive, mainly microsurgical, procedure to the plastic surgeon is, to our opinion, a good option, especially in the foot.
Except for cases after radiotherapy, closure on the femur or humerus is not a problem due to the removal of a large mass of tumor tissue. The problem arises on the lower extremity around the knee joint, on the front part of the shin over the tibia and on the dorsal part of the foot, especially over the tendons. Similarly, the difficult part on the upper extremity is the elbow forearm and hand. In specific cases, these areas require the use of large local or distant flaps. Easier reconstructions are performed in one session but more complex reconstructions can be done from several indications in a two-stage surgery. An artificial TSC is a good solution in these cases.
In contrary to the producer who does not recommend the use of COM for covering of tendons, nerves, vessels, bones and dry necrosis, our indication was mainly to temporarily cover tendons and bones in cases where a dermo-epidermal skin graft cannot be used and the final covering requires sufficient soft tissue coverage like a fascio-cutaneous or muscle flap. In two cases, we surprisingly observed, mainly around tendons, a rapid formation of granulation tissue that enabled a less extensive covering than initially planned. Others suggest that this could be faster using the VAC system [11]. The question arises as to the effect of COM on the uncontrolled bleeding in patient No 10. We believe that this was mainly due to the infection of Pseudomonas bacteria, but in the future, we will not indicate artificial TSC over large vessels.
Several artificial TSC as well as artificial permanent skin closures do exist [2,3]. Since 2004, we gradually used 3 types of TSM; at present, COM is our preferred type for these indications. In general, we set our indications for any readily available artificial TSC. The other two indications for the use of TSC were carcinoma on the foot with chronic osteomyelitis and carcinoma over the Achilles tendon. Both healed using a local flap over the foot and a split-thickness skin graft over the Achilles tendon.
With the improvement of cooperation between plastic and orthopedic surgeons and improved surgical techniques as well as improved anesthesia, we nowadays prefer a one stage surgery with a definitive closure in all situations where it is feasible concerning the patient status, planed systemic treatment and course of anesthesia. Still, in cases of complications during surgery, it is good to have a safe backup solution to quickly terminate the surgery and have the secondary skin and soft tissue closure performed when the patient returns to a good general condition. The healing and survival of free vascularized muscle flaps with micro-anastomosis or larger facio-cutaneous flaps is definitively better with sufficient blood perfusion, but the specific criteria are still to be set [12,13].
In general, the adverse effect of massive blood loss on the brittle balance of the patient’s immune system [14] and tumor progression is a well-known situation [8,15]. The effect of one prolonged anesthesia on the survival of oncologic patients compared to two shorter anesthesia is still to be evaluated, especially with the use of incomparable types and different courses of anesthesia [16]. Nevertheless, the increased risk of disseminated intravascular coagulation and other mainly vascular or hematological complications as well as increased hepatotoxic or nephrotoxic effects of prolonged anesthesia are a well-known complication of prolonged anesthesia in general [17,18]. These can later necessitate a preliminary termination of systemic treatment or lowering of the dose intensity, decreasing the chance of the patient for long-term survival. Whether these limits are 3, 5 or 10 hours of anesthesia are still to be set as well as the critical blood loss of 1 000, 3 000 or over 5 000 mL [19]. From our own experience with more than 500 surgically treated patients with extremity STS, we believe that a surgical time under 3 hours with a blood loss below 1 000 mL is safe and does not have any adverse effects on the course of the disease of the oncologic patient unless other severe comorbidities are present.
In general, immediate closure using local flaps is contraindicated in cases with localized disease where malignancy is not completely cleared from the wound bed. Also, distant or free flaps can hide early local recurrences increasing the risk of systemic spread of the disease. Furthermore, relative contraindications need to be taken into consideration as well. Smoking increases the risk of flap necrosis and/or infection. Most of the tumor resections on the foot are performed with the use of tourniquet for better visibility. This, of course, can impair blood perfusion, setting the flap at risk. Poor vascularity of the donor site increases the risk of these complications as well. The risk of hemorrhage is also a relative contraindication to flap surgery, as peri - or post-operative bleeding can severely impair flap viability. The presence of active infection at the recipient site represents another relative contraindication. While closure by a cutaneous or fasciocutaneous flap is contraindicated in such cases, it is possible to consider closing the wound with a muscle or musculocutaneous flap, as the muscle provides quality tissue coverage and fresh vascularity and should be especially considered in cases of chronic osteomyelitis and may even be considered in cases of exposed infected implants. The possible use of HILP is also considered a contraindication for complicated flaps.
In cases of suspicious bacterial contamination like in ulcerating tumors it can be beneficial to await the results of negative bacterial smears before performing large reconstructive plastic surgeries using different types of flaps [20]. For this a temporary treatment with artificial TSC, especially when it can be soaked with antiseptic solutions like povidone iodide or in antibiotics, can be beneficial. A VAC system, which has similar or even slightly better effects but the cost is higher and its use over vessels is also a risk, is an alternative for this [11].
The indication for delayed surgery in cases of suspicious tumor contamination is relative as a primarily wide resection should be always our goal, especially when the extent of the resection of skin and soft tissue is feasible due to extensive reconstruction possibilities of plastic and vascular surgery [1]. Nevertheless, there can be indications for clinically marginal resections around nerves and can also be a problem in other cases like vascular reconstructions after irradiation. In such cases, when a contamination needs to be histologically excluded, a temporary closure can be indicated. This also applies for some special histological types of tumors where the margins are difficult to be set, like in angiosarcoma or myxofibrosarcoma [1]. In these cases, it is important to have an exact histological diagnosis before planning an extensive plastic reconstruction. The precise histological diagnosis can be difficult in some cases. In one case, a melanoma was clinically suspected even if a revised histology stated a malignant fibrous histiocytoma. Another case diagnosed as a melanoma was finally diagnosed as a clear cell sarcoma or synovial sarcoma. The genetic differentiation then took further 4 weeks. In melanomas, the definite treatment considering re-excision of the margins differs from the recommendations for sarcomas; it is therefore beneficial to wait for final histology before definitive closure. If large local flaps are performed and the resection turns out to be contaminated, the whole surgical field must be irradiated. In case of circular irradiation of the extremity, severe vascular problems may arise [21]. In such cases, a more challenging free vascular distant flap may be a better solution and this can be assessed only after a precise histologic evaluation of the whole resected specimen. In case of a local recurrence, a circular or more extensive contamination is an indication for amputation in a sufficient height above the contaminated area in most cases of STS [1].
In cases of a so called “whoops procedure”, an unplanned excision of a “whoops lesion” i.e., a superficial subcutaneous malignant lesion under 2.5 cm (1 inch), the optimal solution can be covering the excision site with an artificial TSC and sending the patient to a center for further treatment [22]. The incidence of a subcutaneous small malignant tumor is low, between 1 : 100 and 1 : 1 000 benign lesions [1]. Nevertheless, after removing a suspicious tumor, the surgeon should cut it in half and in cases when it is not an obvious benign lesion like lipoma or fibroma,
he/she should consider a possible malignant superficial tumor.
In tumor surgery of STS, wide resection is always the first step and the reconstruction is the second step. In case of any clinical or technical problems, it is always possible to interrupt the surgery after the first step, using a TSC and perform the second stage under safer and more comfortable conditions. Of course, this is an emergency situation, not a standard.
Limitation of the study
The limitations of the study include the fact that nobody has published the indication for TSC in STS of the extremities so far and this topic has been only discussed on medical congresses dealing with mainly surgical treatment of soft tissue sarcomas. No comparison is therefore possible. This is due to the rare indication that can be proved in our cohort of 11 patients out of 594 patients treated for STS. In most cases, we had random and unplanned indications for the use of TSC. In most cases, this occurred in order to solve an unplanned situation. We can only suggest from our personal experience and from the small number of cases, which indications we found appropriate. Statistical evaluation to prove our suggestions is impossible due to the small number of patients and diversity of locations. Further studies and experiences will prove or exclude our initial suggestions.
Conclusion
We have clearly shown that there exist indications, even though rare, for the use of TSC in extremity STS. For the optimal use of TSC, information from the whole surgical (including anesthesiologist) as well as oncological team must be carefully considered and evaluated. Plastic surgery lately improved the results of limb sparing surgeries after large resections on extremities, covering extensive skin and soft tissue defects with large flaps. Nevertheless, the viability of the used flaps can be endangered by comorbidities and previous treatments. In such cases, a two-stage surgery with the use of artificial TSC can be beneficial, especially on the foot or shin areas. The indications can be made by both the plastic as well as tumor surgeons.
The aim of the work was to present our rare indications for the use of TSC in extremity STS, when it can be of good use for the plastic surgeon as well as tumor surgeon for the benefit of the patient. This is adequately discussed with further possible indications that should result from cooperation with other specialists, e.g., the anesthesiologist or oncologist. We additionally presented a brief overview over conditions that can be of importance for the plastic surgeon when he starts tumor surgery and must decide about the type of reconstruction. We proved that in some situations, TSC can be of benefit for the patient. Our main aim was to open the discussion over the indications for TSC in extremity STS and we believe that we have fulfilled it adequately.
Role of authors: Jana Matějovská introduced the COM technique for patients with STS, participated in most of the plastic surgeries, followed the patients, and prepared the manuscript.
Petros Christodoulou performed microsurgical procedures and some final surgeries and checked the sections of the manuscript concerning the flaps.
Vendula Šorelová evaluated and controlled the cohort of patients.
Michaela Frindrichová checked the indications from the oncologic point of view as the oncologist of the sarcoma team, and checked the oncologic part of the manuscript.
Zdeněk Matějovský performed or participated in most of the orthopedic surgeries, and controlled the orthopedic part of the manuscript.
Disclosure: No financial nor non-financial support was used during the preparation of this article and there is no conflict of interest in preparing and presenting of the paper by neither of the authors.
Compliance with ethical requirements: All procedures performed in this study involving human participants were in accordance with ethical standards of the institutional and/or national research committee and with the Helsinki declaration and its later amendments or comparable ethical standards.
Jana Matějovská, MD
Department of Plastic Surgery
Institute for Postgraduate Studies,
1st Medical Faculty, Charles University, Prague
University Hospital Bulovka
Budínova 2
180 81 Prague
Czech Republic
e-mail: janamate@centrum.cz
Submitted: 10. 3. 2022
Accepted: 23. 7. 2022
Sources
1. Quyn AJ., Johnston C., Hall D., et al. The open abdomen and temporary abdominal closure systems-historical evolution and systematic review. Colorectal Dis. 2012, 14(8): e429–e438.
2. Sheridan R. Closure of the excised burn wound: autografts, semipermanent skin substitutes, and permanent skin substitutes. Clin Plast Surg. 2009, 36(4): 643–651.
3. Saffle JR. Closure of the excised burn wound: temporary skin substitutes. Clin Plast Surg. 2009, 36(4): 627–641.
4. Grassner L., Marhold F., Yousif M., et al. Experiences with a temporary synthetic skin substitute after decompressive craniectomy: a retrospective two-center analysis. Acta Neurochir. 2019, 161(3): 493–499.
5. Matějovský Z., Krška Z., Hoskovec D. Kapitola 36. Nádory měkkých tkání. In: Krška Z., Hoskovec D., Petruželka L. Chirurgická onkologie. Praha: Grada 2014.
6. Matějovský Z., Matějovská J. Strategie péče o pacienty s nádory měkkých tkání v České republice a ve světě. Referátový výběr z onkologie. 2007, 24(speciál): 6–13.
7. Zgonis T., Stapleton JJ. Innovative techniques in preventing and salvaging neurovascular pedicle flaps in reconstructive foot and ankle surgery. Foot Ankle Spec. 2008, 1(2): 97–104.
8. Pretzsch E., Bösch F., Renz B., et al. Operative trauma and blood loss – impact on tumor growth and recurrence. Shock. 2021, 55(4): 455–464.
9. Kim HS., Sun X., Lee JH., et al. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv Drug Deliv Rev. 2019, 146 : 209–239.
10. Bickels J., Malawer MM. Adult soft-tissue sarcomas of the extremities. J BoneJoint Surg Am. 2022, 104(4): 379–389.
11. Bussell HR., Aufdenblatten CA., Gruenenfelder C., et al. Comparison of lower extremity fasciotomy wound closure techniques in children: vacuum-assisted closure device versus temporary synthetic skin replacement. Eur J Trauma Emerg Surg. 2019, 45(5): 809–814.
12. Lewis KG., Dufresne RG. A meta-analysis of complications attributed to anticoagulation among patients following cutaneous surgery. Dermatol Surg. 2008, 34(2): 160–164.
13. Kass JL., Lakha S., Levin MA., et al. Intraoperative hypotension and flap loss in free tissue transfer surgery of the head and neck. Head Neck. 2018, 40(11): 2334–2339.
14. Albertsmeier M., Quaiser D., Dossow-Hanfstingl V., et al. Major surgical trauma differentially affects T-cells and APC. Innate Immunity. 2015, 21(1): 55–64.
15. Nakanishi K., Kanda M., Kodera Y. Long-lasting discussion: adverse effects of intraoperative blood loss and allogeneic transfusion on prognosis of patients with gastric cancer. World J Gastroenterol. 2019, 25(22): 2743–2751.
16. Iwasaki M., Edmondson M., Sakamoto A., et al. Anesthesia, surgical stress and „long-term“ outcomes. Acta Anaesthesiologica Taiwanica. 2015, 53(3): 99–104.
17. Cata JP., Hernandez M., Lewis VO., et al. Can regional anesthesia and analgesia prolong cancer survival after orthopaedic oncologic surgery? Clin Orthop Relat Res. 2014, 472(5): 1434–1441.
18. Kumar N., Zaw AS., Khine HE., et al. Blood loss and transfusion requirements in metastatic spinal tumor surgery: evaluation of influencing factors. Ann Surg Oncol. 2016, 23(6): 2079–2086.
19. Cata JP., Gottumukkala V. Blood loss and massive transfusion in patients undergoing major oncologic surgery: what do we know? ISNR Anesthesiology. 2012, 918938 : 1–11.
20. Tan KJ., Lim CT., Lim AYT. The use of muscle flaps in the salvage of infected exposed implants for internal fixation. J Bone Joint Surg Br. 2010, 92(3): 401–405.
21. Spierer MM., Alektiar KM., Zelefsky MJ., et al. Tolerance of tissue transfers to adjuvant radiation therapy in primary soft tissue sarcoma of the extremity. Int J Radiat Oncol Biol Phys. 2003, 56(4): 1112–1116.
22. Yang P., Evans S., Bali N., et al. Malignant bone tumours of the foot. Ann R Coll Surg Engl. 2017, 99(7): 568–572.
Labels
Plastic surgery Orthopaedics Burns medicine Traumatology
Article was published inActa chirurgiae plasticae

2022 Issue 2-
All articles in this issue
- Editorial
- Single-incision endoscopic-assisted temporoparietal fascia harvest for single stage auricular reconstruction
- Temporary skin closure in extremity soft tissue sarcoma – our indications
- Modified harvesting technique for pedicled pectoralis major muscle flap after extended manubrial resection in case of recurrent cervicothoracic junction tumors
- Bilateral latissimus dorsi for breast reconstruction in one stage
- Subcutaneous shoulder hibernoma presenting as an atypical lipomatous tumor – a case report
- Salvage of a large exposed cranial implant on irradiated necrosed scalp using free latissimus dorsi and forehead flaps – a case report
- Single-stage reconstruction of a large lower eyelid defect using a full-thickness bilamellar autograft
- Hook nail treatment – a bulky palmar flap as an alternative to the “antenna” procedure and to the thenar flap for fingertip coverage
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Single-incision endoscopic-assisted temporoparietal fascia harvest for single stage auricular reconstruction
- Salvage of a large exposed cranial implant on irradiated necrosed scalp using free latissimus dorsi and forehead flaps – a case report
- Bilateral latissimus dorsi for breast reconstruction in one stage
- Hook nail treatment – a bulky palmar flap as an alternative to the “antenna” procedure and to the thenar flap for fingertip coverage
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

