-
Medical journals
- Career
CULTURED KERATINOCYTES AND THEIR POSSIBLE APPLICATIONS
Authors: M. Orság; P. Bukovčan; J. Koller
Authors‘ workplace: Department of Burns and Reconstructive Surgery, University Hospital Bratislava, and Central Tissue Bank, Bratislava, Slovakia
Published in: ACTA CHIRURGIAE PLASTICAE, 54, 2, 2012, pp. 67-70
INTRODUCTION
The first successful auto-transplantation of cultured keratinocytes performed on a human was described by OęConnor in 1981. Three years later Gallico applied sheets of cultured keratinocytes to cover 50% of the surface of the wounds in two children who sustained burns of 95% of their body surface area. It was possible to expand epithelial cells from a skin biopsy with a surface area of 2 cm2 a thousand-fold during a period of 3–4weeks. (7).
Very soon after these findings were published many other scientists and institutions discovered the significance of this method, which enables expansion of the epithelium, and began to implement it in extensive burn treatment. (2). The usage of cultured keratinocytes has also spread to other types of wounds, including ulcus cruris, skin defects in epidermolysis bullosa and surgical wounds. Moreover, it has been found that cultured allogeneic kearatinocytes are able to enhance the treatment of extensive superficial and deep dermal burns, skin donor sites, chronic wounds and some recessive types of dystrophic epidermolysis bullosa (7).
MATERIALS AND METHODS
At present the method first described by Rheinwald and Green in 1975 is used for the cultivation of keratinocytes, with several modifications. This technique enables successful cultivation and subsequent passaging of human keratinocytes suspension, with expansion to an extent which permits the production of large amount of epithelium suitable for transplantation.
The skin samples for autologous cultures are obtained from patients with extensive deep burns where the samples need to be retrieved as soon as possible to ensure prevention of infection and retention of optimal cell viability. Allogenic skin is obtained from live donors, most frequently from patients undergoing plastic surgical procedures such as otoplasty, plastic surgery of the torso or breast, or circumcision. It is necessary to obtain informed consent to skin donation from the donor beforehand.
The procured skin is transported in properly labeled sterile containers placed in a transport medium. Appropriate documentation and a blood sample for serological testing (in allogenic donations) are attached and sent to the Central Tissue Bank Cell Culture Laboratory, along with the skin for further processing.
Processing begins in laminar air flow boxes Class A by thorough rinsing of the sample in saline and subsequent incubation in antibiotics solution for one hour at laboratory temperature. We use Penicillin 500 IU/ml and Amphotericin B 1.25 μg/ml. In cases of known bacterial flora of the patient we use targeted antibiotics.
The next step is separation of keratinocytes from the skin sample by incubating it in trypsin solution, which separates the epidermis from the dermis. Subsequently mechanical separation of the epidermis and disaggregation of the epidermis is performed, to obtain a keratinocytes suspension. The keratinocytes are seeded in tissue culture dishes on a feeder layer of mitomycin-treated 3T3 fibroblasts, and keratinocytes culture medium with the content of fetal calf serum and mitogens (epidermal growth factor and cholera toxin) are added.
The cultivation itself is carried out according to a modified Rheinwald and Green method on a support layer of mitotically inhibited 3T3 murine fibroblasts. On one hand the feeder cells possess an initiative function (initiation of keratinocyte reproduction), while on the other hand the fibroblasts reproduction, which would overwhelm the population of keratinocytes, is inhibited by previous exposure to mitomycine. Keratinocytes are cultivated at 37°C. The culture medium is changed regularly as needed, usually three times a week. The cultivation length depends on many factors, mostly the vitality of the keratinocytes related to the donor’s age and physiological status. The mean cultivation length for one passage is 7–10 days. With each passaging we increase the amount of growing cells.
By the use of this method the keratinocytes from neonatal epidermis can undergo up to 100 cell population divisions, and the longevity of adult human keratinocytes represents 40–70 cell generations.
Another method is cultivation of keratinocytes eliminates the use of feeder cells. This requires a special type of culture medium (3). The advantage of this method is that it does not use xenogenic mice cells. For feeder-free cultivation we use the standard method.
As soon as the cells in the culture become confluent (monolayer), they continue to replicate, forming several cell layers (multilayer). In this phase the culture can be enzymatically detached from the cultivation dish (by dispase), clipped to a carrier (mesh gauze) and applied clinically to the patient. (Fig. 1, 2, 3).
Fig. 1. Microscopic picture of keratinocytes 
Fig. 2. Transport boxes with cultures for clinical application 
Fig. 3. Cell culture attached to mesh gauze carrier ready for use 
If it is not possible to use the cell culture after reaching the multilayer phase, the sheets can be preserved with viable cells by controlled rate freezing in cryoprotective media (cryopreservation).
The sheets should be stored in liquid nitrogen vapors with cells remaining viable for up to two years.
The indications for clinical application of cultured keratinocytes were as follows:
- For autologous keratinocytes:
- a) Excised extensive deep burns following wound bed preparation by allogenic dermis,
- b) Residual defects following split thickness skin grafting of deep burns,
- c) Dermabraded postburn scars during reconstructive procedures.
- For allogenic keratinocytes:
- a) Split thickness skin donor sites for acceleration of epithelialisation,
- b) No healing or slow healing in smaller chronic wound following surgical debridement for healing stimulation.
RESULTS
In the years from 2005 to 2009 the production of cultured keratinocytes in our skin bank was 37,566 cm2; consumption also amounted to 37 566 cm2. The total number of recipients was 38 (Table 1).
1. Production and consumption of cultured keratinocytes in Department of Burns and Reconstructive Surgery 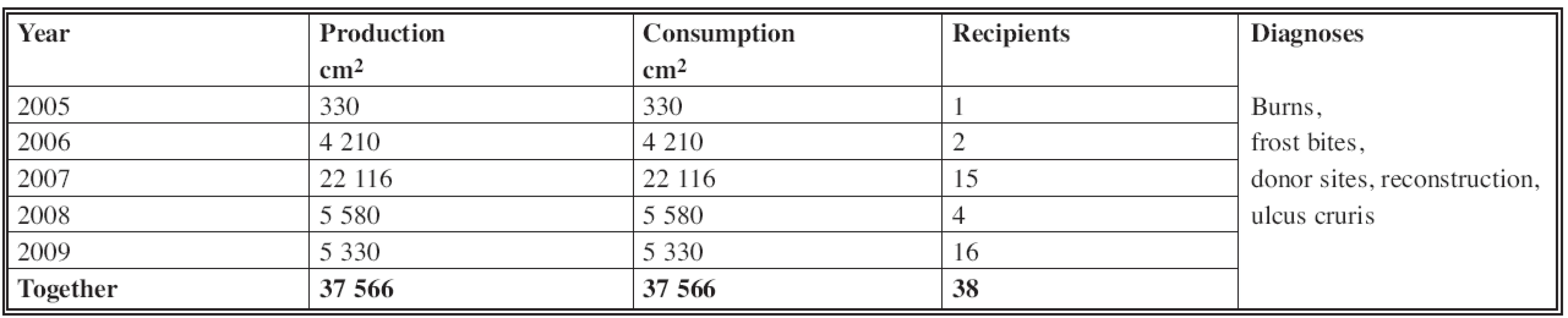
Keratinocytes were mainly used in the treatment of burns. We used them for coverage of residual defects (Fig. 4) and donor sites coverage (Fig. 5) to speed healing. They can also be used for wound closure when applied on top of the healed allogeneic dermis, following previous coverage of excised burned wounds by skin allografts (see Fig. 5). In reconstructive surgery they have been used for coverage/closure of the surfaces after abrasion.
Fig. 4. Residual defects covered by cultured keratinocytes 
Fig. 5. De-epidermised allografted wound bed covered by cultured autologous keratinocytes 
DISCUSSION
Cultured autologous epidermal cells – keratinocytes – represent an alternative to skin substitution by skin autografts in cases of deep extensive burns where shortage of donor sites is a major problem.
Allogeneic cultured keratinocytes are used everywhere where we would like to stimulate significantly the healing of a wound which refuses to heal, and to assure permanent closure of the wound. In clinical use of allogeneic keratinocyte cultures for wound coverage rejection always occurred after a certain time. Keratinocyte culture with a feeder layer contains many cytokines and growth factors which are able to speed healing, eventually inducing the healing process in wounds with slow or compromised healing. They create thin, very fragile and almost transparent membranes with a surface area of between 75 cm2 and 150 cm2 which are fixed to mesh gauze carriers for easier manipulation and submerged in liquid culture medium to avoid dessication. After being removed from the medium, immediate application is essential. They are used for donor sites, whereby the presence of cultured keratinocytes contributes to speed up the healing of these areas; this is particularly advantageous if repeated retrieval of skin autografts is necessary, for example due to lack of donor sites, for partial thickness burns, ulcus cruris and some other types of wounds with problematic healing. In mass burn casualties the technical possibilities and high costs do not allow broader use of epidermis from tissue cultures in clinical practice.
The main advantage of the use of cultured allogeneic keratinocytes is overcoming the “time factor”: there is no need to wait for 3–4 weeks as in the case of cultured autologous keratinocytes, since they are prepared in advance. A further advantage is their capability to stimulate epithelialization and healing of superficial burns due to the presence of growth factors and cytokines. The disadvantage is fragility, the fact that they do not create definitive wound closure, and the rather high cost. Keratinocytes can grow on substrates created not to imitate the dermis. These substrates are based on type I collagen and the content of mesenchymal cells. The resulting keratinocyte cultures are known as skin equivalents, organotypic cultures or composite cultures, and they can be used for transplantation (6). Such products include, for example, the commercially produced Apligraf (Graftskin), Transcyte, Dermagraft, Alloderm, or Laserskin (4, 1).
CONCLUSION
Cultured keratinocytes do not represent a fully viable skin substitute. The cultivation takes 3–4 weeks, and the costs are not insignificant. Allogeneic cultured keratinocytes are used when we would like to stimulate wound healing process, whether in residual defects or donor sites. They can be used on carriers of both biological and synthetic origin.
Address for correspondence:
M. Orság, M.D.
Department of Burns and Reconstructive Surgery
University Hospital Bratislava
Ružinovská 6
826 06 Bratislava
Slovak Republic
E-mail: orsag@ru.unb.sk, miroorsag@hotmail.com
Sources
1. Bar-Meier E, Mendes D, Winkler E. Skin Substitutes. In: IMAJ, 8, March, 2006.
2. Brychta P, Suchanek I, Rihova H, Adler J, Komarkova J. Cultured epidermal allografts for treatment of deep dermal burns. Acta Chir. Plast., 37(1), 1995, p. 20–24.
3. Castro-Munozledo F. Cultivation, serial transfer and differentiation of epidermal keratinocytes in serum-free medium. In: Biochem., Biophys. Res. Commun., 234(1), 1997.
4. Hansbrough JF, Boyce ST, Cooper ML, Foreman TJ. Burn wound closure with cultured autologous keratinocytes and fibroblasts attached to a collagen-glycosaminoglycan substrate. JAMA, 262, 1989, p. 2125–2130.
5. Koller J, Orság M. Skin grafting options at the Burn and reconstructive surgery department of the faculty hospital in Bratislava. Acta Chir. Plast., 48(2), 2006, p. 65–71.
6. Leigh Irene M, Watt FM. Keratinocyte methods.1. vyd. Cambridge: University Press, 1994, 0-521-45013-6.
7. Wai-Sun, H. Skin substitutes: An overview. Ann. Coll. Surg. H.K, 6, 2002, p. 102.
Labels
Plastic surgery Orthopaedics Burns medicine Traumatology
Article was published inActa chirurgiae plasticae

2012 Issue 2-
All articles in this issue
- INDEX Acta Chir. Plast. Vol. 54, 2012
- CLINICAL TRIAL OF THE TEMPORARY BIOSYNTHETIC DERMAL SKIN SUBSTITUTE BASED ON A COLLAGEN AND HYALURONIC ACID NAMED COLADERM H/HM, FIRST PART
- EXTENSION OF OROFACIAL CLEFT SIZE AND GESTATIONAL BLEEDING IN EARLY PREGNANCY
- HETEROLOGOUS RECONSTRUCTION AND RADIOTHERAPY: THE ROLE OF LATISSIMUS DORSI FLAP AS A SALVAGE
- FASHIONING REVERSED AXIAL PATTERN FOREARM TISSUES IN DIFFERENT CHALLENGING CONDITIONS OF THE FOREARM TERRITORY AS A RELIABLE SUBSTITUTE FOR FREE TISSUE TRANSFER
- GIANT MALIGNANT MELANOMA: A CASE REPORT
- OUR EIGHT-YEAR EXPERIENCE WITH BREAST RECONSTRUCTION USING ABDOMINAL ADVANCEMENT FLAP (207 RECONSTRUCTIONS)
- CULTURED KERATINOCYTES AND THEIR POSSIBLE APPLICATIONS
- CONTENTS Acta Chir. Plast. Vol. 54, 2012
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- GIANT MALIGNANT MELANOMA: A CASE REPORT
- HETEROLOGOUS RECONSTRUCTION AND RADIOTHERAPY: THE ROLE OF LATISSIMUS DORSI FLAP AS A SALVAGE
- CLINICAL TRIAL OF THE TEMPORARY BIOSYNTHETIC DERMAL SKIN SUBSTITUTE BASED ON A COLLAGEN AND HYALURONIC ACID NAMED COLADERM H/HM, FIRST PART
- EXTENSION OF OROFACIAL CLEFT SIZE AND GESTATIONAL BLEEDING IN EARLY PREGNANCY
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

