-
Medical journals
- Career
Breast Hypertrophy and Asymetry: A Retrospective Study on a Sample of 344 Consecutive Patients
Authors: S. Tenna; A. Cogliandro; B. Cagli; M. Barone; P. Delle Femmine; P. Persichetti
Authors‘ workplace: lastic and Reconstructive Surgery Unit, Campus Bio-Medico University of Rome, Rome, Italy
Published in: ACTA CHIRURGIAE PLASTICAE, 54, 1, 2012, pp. 9-12
INTRODUCTION
Breast hypertrophy is a clinical condition that affects many women; it is defined by Lalardrie and Jouglard as an increase in breast volume exceeding 250–300 cm3 (1). The etiology of the disease is different: in young women it is mainly related to obesity, endocrine abnormalities and virginal hypertrophy, while in adult and older patients it tends to be a benign postmenopausal effect (2).
Large breasts can cause functional, psychological and aesthetic discomfort, and awareness that a solution is possible has caused a dramatic increase in requests for surgical correction. In recent decades, reductive mammaplasty has become one of the most popular operations in plastic surgery. Many techniques have been described according to nipple and areola complex (NAC) pedicle, type of skin incision, or both (3, 19, 25, 26, 27). The goal is a natural-looking, symmetrical breast, with traditional proportions and limited scars. Of course all these features should be long-lasting.
In all bilateral procedures careful evaluation of pre-existing differences is essential in order to decide the correct surgical strategy. Normally breast hypertrophy presents itself as a bilateral clinical picture, but no study in the literature refers to the incidence of weight asymmetry.
This study aims to analyze, on a selected sample of Caucasian women, weight differences between the right and left breast, and to consider whether the incidence of the disease is more represented on one side.
PATIENTS AND METHODS
All patients who underwent reductive mammaplasty and/or mastopexy between January 2005 and April 2010 at our Plastic and Reconstructive Surgery Unit were evaluated for this retrospective study. Main inclusion criteria were primary procedure, age, degree of hypertrophy, BMI < 28 and good post-op symmetry at 1 year follow-up. Patients with a history of endocrine or neoplastic diseases, obesity or secondary procedures were excluded.
According to age we divided the population into three groups: patients under the age of 30 years, patients between 31 and 49 and patients older than 50.
The degree of hypertrophy was classified according to Lalardrie and Jouglard’s system (1) into mild (0–300 g), moderate (300–500 g) and severe hypertrophy (> 500 g), considering both breasts (only in cases of severe asymmetry was the degree scored according to the heaviest breast).
All patients underwent operations performed by the same team, with the same surgical technique: breast reduction and/or mastopexy using a self-modified technique according to the Pitanguy-Ribeiro description.
In this technique the nipple areola complex (NAC) is vascularized by a superior pedicle while an inferior dermo-adipose flap is harvested on Cooper’s ligament. After glandular resection the inferior flap is advanced and anchored up to the 3rd intercostal space. The pillars and glandular-subcutaneous skin flaps are advanced and the suture is in an “inverted T” fashion. Finally, the NAC is repositioned at a distance between 3.5 and 5 cm from the groove (14, 24, 28).
Patients were followed up at 3, 6 and 12 months, and only those who maintained good symmetry – as evidence by lack of any difference in the cup of the bra – were considered.
In every case glandular resection per breast were weighed and sent for histological checks, to exclude microscopically benign and malignant tumors. Measurements of all weights were extracted from the operating records and registered. Then patients were grouped according to the weight excised for each breast, using the senior author’s classification (20) for breast asymmetry: Grade A: difference < than 200 g (Fig. 1); Grade B: difference between 200 and 400 g (Fig. 2); and Grade C: difference > than 400 g (Fig. 3). Side predominance was also classified according to age and degree of hypertrophy. We used the μ2 test for non-parametric data in order to produce a statistical analysis of these results and to assess the prevalence of side.
Fig. 1. Frontal pre and post operative view, asymmetry grade A (74 g) 
Fig. 2. Frontal pre and post operative view, asymmetry grade B (367 g) 
Fig. 3. Frontal pre and post operative view, asymmetry grade C (440 g) 
RESULTS
From January 2005 to April 2010 we operated on approximately 483 women diagnosed with breast hypertrophy bilaterally. 139 women were excluded from the study because they had one of the exclusion criteria mentioned earlier; 344 patients were enrolled for this study. The age of the patients was between 17 and 74 years, with a mean age of 42, and they were distributed into the three groups as shown in Graph 1. Classification of degree of hypertrophy established that 75% of patients has moderate to severe hypertrophy, as summarized in Graph 2. Glandular resection per breast was on average 592.7 g on the right side and 590.3 g on the left. Distribution according to the amount of glandular tissue excised (left side compared to right side) is represented in Graph 3.
Graph 1. Distribution of patients by age into 3 groups 
Graph 2. Distribution of patients according to degree of hypertrophy showing 24.13% with mild and moderate degree, and 51.74% with severe excess 
Graph 3. Distribution of patients according to weight asymmetry showing 278 patients (Grade A), 48 patients (Grade B) and 18 patients (Grade C) 
167 cases showed hypertrophy with right predominance, 168 showed hypertrophy with left predominance, and in 9 cases the results were symmetrical. The distribution of breast hypertrophy on the left and right is summarized according to age and degree of hypertrophy in Graph 4 and 5.
Graph 4. Distribution of patients by age showing the side predominance of breast hypertrophy 
Graph 5. Distribution of patients by breast hypertrophy showing the side predominance 
Statistical analysis of these results, as shown in Table 1, demonstrated no significant differences between the right and left side (p > 0.05).
1. Statistical analysis of side prevalence for age and degree of hypertrophy (p >0.05) 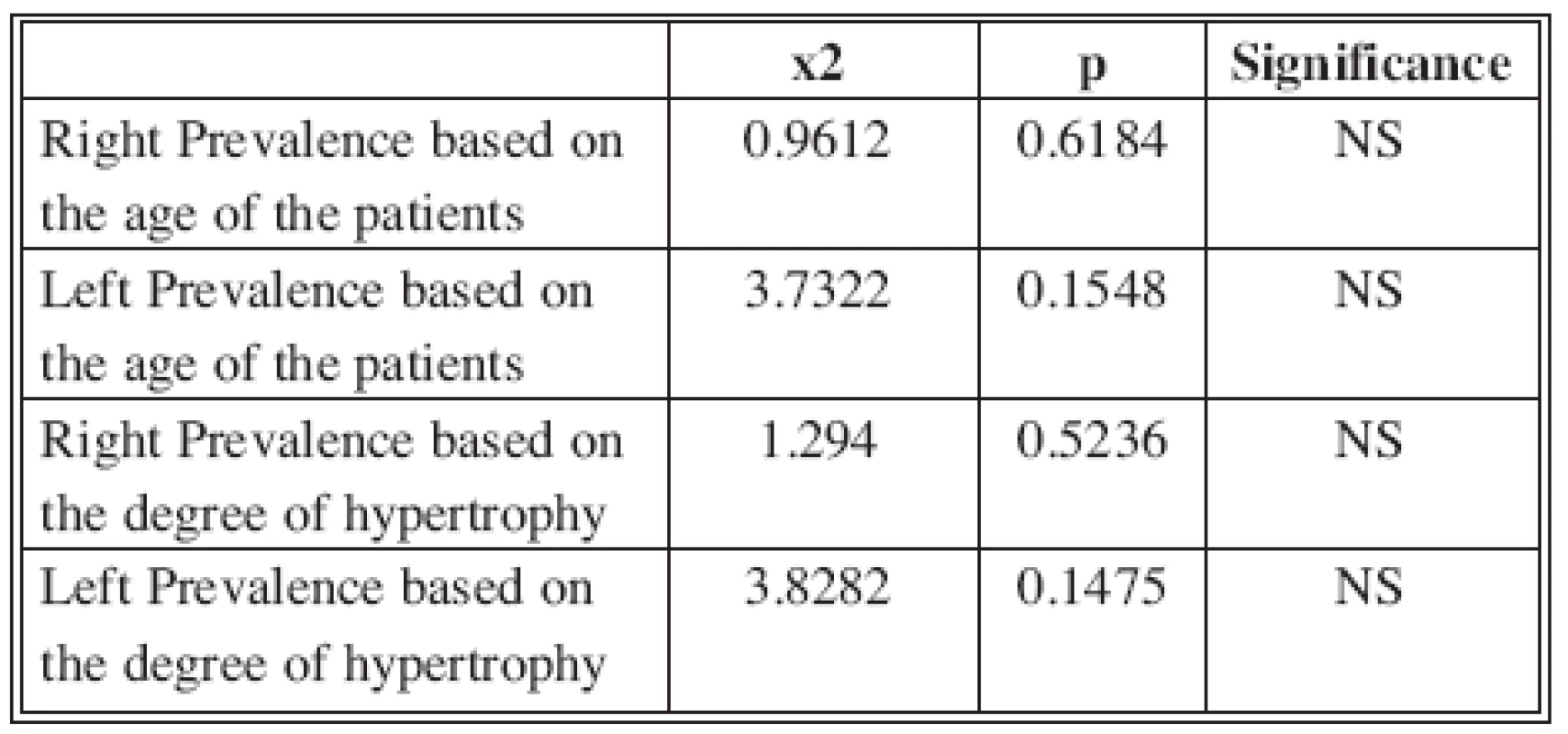
DISCUSSION
Breast reduction is one of the most popular procedures performed by plastic surgeons, with the highest patient satisfaction rate among plastic surgery operations, as 93 percent of women report they would undergo the surgery again (21) and claim great postoperatively improvement of body self-image (22). This disease has a bilateral expressiveness, although it often presents itself with various degrees of asymmetry in shape, weight and position of the breast. Many studies have been published evaluating the asymmetry of shape and position of the breast with special reference to cases of tuberous and tubular breasts (20, 23), but the literature does not provide studies that specifically analyze the breast weight asymmetry.
The review of our patient population over 5 years confirmed that breast hypertrophy is essentially a disease with bilateral expression with no prevalence of side. Statistical analysis, in fact, did not observe a prevalence of hemisoma, nor correlations between asymmetry and degree of hypertrophy or age. However, a weight difference of more than 200 g was found in about 20% of patients. This amount of tissue is frequently associated with different representation of the breast quadrants, which inevitably results in a different surgical approach for the two breasts.
In these cases surgical strategy must be adapted to the anatomical variation in order to achieve an adequate correction. Our approach does not use a predefined pattern but prefers a modified Pitanguy-Ribeiro mammaplasty which proved to be more versatile. All breasts have been reshaped according to appropriate aesthetic proportions, while also varying the preoperative draft in cases of mild intra-operative asymmetry, so to fit the glandular resection to an appropriate removal of skin.
In our experience a self-tailored modification even of a predefined pattern such the Wise pattern may be required in asymmetric breast reduction, to avoid insufficient removal of tissue which could lead to unsatisfactory results later.
CONCLUSION
Breast hypertrophy is confirmed as a substantially bilateral pathology, but in 1 out of 5 patients a difference of more than one quadrant can be present. A careful preoperative evaluation of weight is then advisable in order to adapt surgical strategy and maintain good, long-lasting symmetry.
ACKNOWLEDGMENT The Authors declare that they have no conflict of interest.
Address for correspondence:
Stefania Tenna M.D., PhD.
Plastic and Reconstructive Surgery Unit
Via Alvaro del Portillo, 200
00128 Rome, Italy
E-mail: s.tenna@unicampus.it
Sources
1. Lalardrie J., Jouglard JP. La chirurgie plastique du sein. Paris: Masson et Cie, 1974.
2. Regnault P. Breast ptosis: Definition and treatment. Clin. Plast. Surg., 3(2), 1976, p. 193–203.
3. Benelli L. A new periareolar mammaplasty: The “round block” technique. Aesth. Plast. Surg., 14(2), 1990, p. 93–100.
4. Dufourmentel C., Mouly R. Mammaplasty by the oblique method. Ann. Chir. Plast., 6, 1961, p. 45–58.
5. French Schatten WE., Hartley JH. Jr., Hamm WG. Reduction mammaplasty by the Dufourmentel-Mouly method. Plast. Reconstr. Surg., 48(4), 1971, p. 306–310.
6. Georgiade NG., Serafin D., Morris R., et al. Reduction mammaplasty utilizing an inferior pedicle nipple-areolar flap. Ann. Plast. Surg., 3(3), 1979, p. 211–218.
7. Hall-Findlay EJ. A simplified vertical reduction mammaplasty: Shortening the learning curve. Plast. Reconstr. Surg., 104(3), 1999, p. 748–759.
8. Lalardrie JP., Jouglard JP. Reduction mammoplasty: general approach and basic considerations. Aesth. Plast. Surg., 6(2), 1982, p. 81–83.
9. Lassus C. A 30-year experience with vertical mammaplasty. Plast. Reconstr. Surg., 97(2), 1996, p. 373–380.
10. Lejour M., Abboud M., Declety A., et al. Reduction of mammaplasty scars: from a short inframammary scar to a vertical scar. Ann. Chir. Plast. Esthet., 35(5), 1990, p. 369–379.
11. McKissock PK. Reduction mammaplasty. Ann. Plast. Surg., 2(4), 1979, p. 321–331.
12. Pitanguy I. Mammaplasty. Study of 245 consecutive cases and presentation of a personal technic. Rev. Bras. Cir., 42, 1961, p. 201–220.
13. Planas J., Mosely LH. Improving breast shape and symmetry in reduction mammaplasty. Ann. Plast. Surg., 4(4), 1980, p. 297–303.
14. Ribeiro L. A new technique for reduction mammaplasty. Plast. Reconstr. Surg., 55(3), 1975, p. 330–334.
15. Robbins TH. A reduction mammaplasty with the areola-nipple based on an inferior dermal pedicle. Plast. Reconstr. Surg., 59(1), 1977, p. 64–67.
16. Skoog T. A new instrument to be used in mammaplasty. Plast. Reconstr. Surg., 5(6), 1950, p. 507–511.
17. Strömbeck JO. Reduction mammaplasty. Surg. Clin. North. Am., 51(2), 1971, p. 453–469.
18. Thorek P. Reduction mammaplasty for pendulous breasts. GP, 27, 1963, p. 95–102.
19. Courtiss E., Goldwyn RM. Reduction mammaplasty by the inferior pedicle technique. Plast. Reconstr. Surg., 59(4), 1977, p. 500–507.
20. Persichetti P., Cagli B., Tenna S., et al. Decision making in the treatment of tuberous and tubular breasts: volume adjustment as a crucial stage in the surgical strategy. Aesth. Plast. Surg., 29(6), 205, p. 482–488. Epub 2005 Dec 23.
21. Davis GM., Ringler SL., Short K., et al. Reduction mammaplasty: Long-term efficacy, morbidity, and patient satisfaction. Plast. Reconstr. Surg., 96(5), 1995, p. 1106–1110.
22. Glatt BS., Sarwer DB., O’Hara DE., et al. A retrospective study of changes in physical symptoms and body image after reduction mammaplasty. Plast. Reconstr. Surg., 103(1), 1999, p. 76–82.
23. Muti E. Personal approach to surgical correction of the extremely hypoplastic tuberous breast. Aesth. Plast. Surg., 20(5), 1996, p. 385–390.
24. Champaneria MC., Wong WW., Hill ME., Gupta SC., et al. The evolution of breast reconstruction: A historical perspective. World J. Surg., 36(4), 2012, p. 730–742.
25. Coriddi M., Koltz PF., Gusenoff JA., et al. Reduction mammaplasty, obesity, and massive weight loss: temporal relationships of satisfaction with breast contour. Plast. Reconstr. Surg., 128(3), 2011, p. 643–650.
26. Gulcelik MA., Dogan L., Camlibel M., Karaman N., Kuru B., Alagol H., Ozaslan C., et al. Early complications of a reduction mammoplasty technique in the treatment of macromastia with or without breast cancer. Clin. Breast. Cancer, 11(6), 2011, p. 395–399. Epub 2011 Oct 10.
27. Tarallo M., Cigna E., Fino P., Lo Torto F., Scuderi N. et al. Macromastia surgical therapy. Ann. Ital. Chir., 82(3), 2011, p. 191–195.
28. Ribeiro L., Accorsi A., Buss A., Marcal Pessoa M. Creation and evolution of 30 years of the inferior pedicle in reduction mammaplasties. Plast. Reconstr. Surg., 110(3), 2002, p. 960–970.
Labels
Plastic surgery Orthopaedics Burns medicine Traumatology
Article was published inActa chirurgiae plasticae

2012 Issue 1-
All articles in this issue
- Reconstruction of the Hand in Apert Syndrome: Two Case Reports and a Literature Review of Updated Strategies for Diagnosis and Management
- Successful Replantation of a Completely Amputated Ear on a Child
- Middle Phalangeal Distal Condylar Fracture Remodelling in Children: A Case Report
- Comparison of Otoplasty Results Using Different Types of Suturing Techniques
- Breast Hypertrophy and Asymetry: A Retrospective Study on a Sample of 344 Consecutive Patients
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Comparison of Otoplasty Results Using Different Types of Suturing Techniques
- Breast Hypertrophy and Asymetry: A Retrospective Study on a Sample of 344 Consecutive Patients
- Reconstruction of the Hand in Apert Syndrome: Two Case Reports and a Literature Review of Updated Strategies for Diagnosis and Management
- Middle Phalangeal Distal Condylar Fracture Remodelling in Children: A Case Report
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

