-
Medical journals
- Career
Predicting psychological distress after primary oncological treatment in elderly breast cancer survivors: Retrospective study
Authors: Kateřina Skřivanová 1; Tomáš Svěrák 4; Dagmar Brančiková 2; Jiří Jarkovský 3; Klára Benešová 3; Ľubomíra Anderková 4; Nela Elfmarková 4; Hana Peterková 1; Marcela Bendová 5; Luboš Minář 5; Eva Holoubková 1; Jan Nedvěd 1; Markéta Protivánková 2; Ladislav Dušek 3
Authors‘ workplace: Ústav psychologie a psychosomatiky LF MU Brno 1; Interní hematologická a onkologická klinika LF MU a FNB 2; IBA LF MU Brno 3; LF MU Brno, CEITEC, Masaryk University (CEITEC MU) 4; Gynekologicko-Porodnická klinika LF MU a FNB 5
Published in: Prakt Gyn 2015; 19(4): 210-218
Category: Oncogynecology: Original Article
Overview
The aim of this study was to determine the prognosis of psychological distress in breast cancer survivors after primary oncological treatment using biological and psychological variables. The test group consisted of 98 elderly breast cancer survivors (median age was 65 years) who completed the SVF 78 questionnaire (coping styles measures), NEO-FFI questionnaire (personality traits measures), SCL-90 questionnaire (psychopathology measures) completing treatment and another retrospectively at diagnosis. The SAS scale (anxiety measures) was completed by a lower number of patients. Data on tumour-related factors and treatment were obtained from medical records. Within the scope of this study, psychological distress was measured via the SCL-90 method using Global Severity Index (GSI) and Positive Symptom Distress Index (PSDI). Quantification of the relationship between biological and psychological predictors and GSI and PSDI as dependent variables was estimated using both univariate and multivariate linear regression models. C-reactive protein levels were monitored at diagnosis and one year after primary treatment. The best model for the prediction of GSI after treatment was identified by multivariate linear regression as the combination of GSI, CRP level and agreeableness (NEO-FFI subscale) predictors at the time of diagnosis in which R2 = 76.6 %. The best model for predicting PSDI after treatment consisted of PSDI, the self-accusation component of SVF 78 and the stage of the disease (IV vs lower) at the time of diagnosis with R2 = 53.9 %. Incorporating the total raw score of the SAS questionnaire into the multivariate models for prediction of GSI and PSDI caused an increase in R2 (71.5 % to 85.0 % and 46.0 % to 65.1 %), respectively. Both biological and psychological predictors proved significant and suitable for psychological distress prediction in elderly breast cancer survivors after long-term oncological treatment.
Key words:
C-reactive protein – elderly breast cancer survivors – multivariate linear regression – psychoneuroimmunology – psychological distress prediction – retrospective studyIntroduction
Distress in cancer patients is defined as “a multifactorial unpleasant emotional experience of a psychological, social, and/or spiritual nature that may interfere with the ability to cope effectively with cancer, its physical symptoms, and its treatment”, according to National Comprehensive Cancer Network (NCCN) guidelines. Distress, as defined by the NCCN, extends along a continuum, ranging from normal feelings of vulnerability, sadness, and fears, to problems that can become disabling, such as depression, anxiety, panic, social isolation, and existential and spiritual crises. The term “distress” was chosen by the NCCN to minimize the stigma attached to a term such as “psychiatric” [1].
Breast cancer represents a significant stress burden when the level of psychological distress shows the patients’ capability to adapt to this new situation [2]. The results of Donovan-Kicken et al [3] indicated that cancer-related topic avoidance may decrease a patient’s use of emotional support and increase self-blame, each of which may lead to higher levels of psychological distress among breast cancer patients. While factors such as time since diagnosis and treatment are assumed to mitigate the stress effects of cancer, some potential stressors may persist over the long term. Women with breast cancer, in addition to the psychological distress associated with the loss of one or both breasts, may experience swelling (lymphoedema) from the excision of lymph nodes [4]. High levels of comorbid symptoms of anxiety and depression have been reported and genetic risk factors for both have been shown to correlate strongly [5]. However, symptoms of anxiety and depression may also occur independently and progress quite differently after a cancer diagnosis. This process remains almost entirely unexplored in cancer survivors [6]. E. M. Bleiker [7] described the intrusive thoughts related to the illness and anxiety as very important factors. N. Ando et al. [8] concluded that trait anxiety assessed prior to breast cancer diagnosis was the significant predictor of psychological distress after diagnosis.
Chronic stress can suppress several aspects of adaptive immune system function; it also appears to induce a chronic and systemic state of mild inflammation [9,10,11]. This is manifested by elevated blood levels of inflammatory biomarkers: C-reactive protein (CRP) and interleukin 6 (IL6). Inflammation is a key pathogenic mechanism which can lead to many infectious, cardiovascular and neoplastic diseases [12,13]. There is a link between inflammation and clinical as well as subclinical depression, which has been reported in chronically ill populations including cancer survivors, and in patients with acute coronary syndrome (ACS) [14,15]. Fatigue, depression, and sleep disturbance are common adverse effects of cancer treatment and frequently co-occur. The possibility that inflammatory processes may underlie this constellation of symptoms has been examined by Julia Bower et al. [16]. The authors explained the possible mechanisms of complex interactions and examined the relationships between psychological states (anxiety, depression) and the inflammation processes in breast cancer patients. The results of this cross-sectional study indicate a relationship between Tumour Necrosis Factor (TNF) and the level of tiredness of breast cancer patients after chemotherapy.
The aim of our study was to determine the predictive potential of both psychological and biological types of variables. To our knowledge, this is the first study to incorporate a biological variable such as C-reactive protein (CRP) level into a multivariate regression model that combines both psychological and biological types of variables for distress prediction in elderly breast cancer patients in the long-term survival phase. CRP was chosen as an inflammatory marker for our purposes because it is more stable than cytokines [17]. We hypothesized that CRP levels and its change both at the time of diagnosis and one year after were good long-term predictors of psychological distress. Moreover, we hypothesized that psychological variables such as coping styles and personality traits contributed to subjective distress experience in elderly breast cancer survivors. Potential physiologic mechanisms for prognostic effect were not investigated.
Patients and methods
Population Assembly
For this study, 198 women diagnosed with breast cancer were enrolled at the participating Faculty Hospital Brno, Czech Republic, between May 2013 and May 2015. They represent women recruited during the 4 years of a larger cohort study which examined the prognostic effect of a number of life-style related factors [18]. The eligible criteria for the patient group were breast cancer diagnosis, age 30–90, Czech language proficiency, and completion of primary oncological treatment. Women provided informed consent to participate in this study as approved by Ethical Committee at University Hospital Brno. The refusal rate was less than 1 %.
Measurement
Women completed self-assessment questionnaires after breast cancer treatment. Initial semi-structured interviews were conducted by psychologists and were aimed at collecting social and demografic data as well as promoting good personal contact with patients. Each interview took about 30 minutes. The study was designed to be retrospective and patients were asked to fill in the questionnaires two times: first to answer the questions based on their current situation and second to answer the same questions retrospectively, i.e. what their situation was like prior to treatment. The interview was also designed to collect socio-demographic characteristics.
Psychological measurement
Coping style measures
Patients completed the standardized Czech version of the Stress Coping Style Questionnaire [19]. The SVF-78 is a self-report questionnaire of German origin, consisting of 78 items, on which subjects rate on a 5-point Likert scale how much each statement represents their reaction in situations where they are “disturbed, irritated or upset by something or someone”. According to the authors of the SVF-78, the basic theoretical assumption is that coping strategies develop a certain stability over time and situations; therefore, individuals are sufficiently aware of them to be able to self-report them accurately on a questionnaire. Items are grouped into 13 subscales: play down, guilt denial, distraction from situation, satisfaction substitution, situation control, reaction control, positive self-instruction, need for social support, active avoidance, flight tendency, rumination, resignation and self-accusation. The first seven categories are considered by the authors to be positive ones and the last four (flight tendency, rumination, resignation, self-accusation) to be negative ones. The subscales need for social support and active avoidance can be both positive and negative, depending on the context. The internal consistency in Czech standardization varies between 0.77 and 0.94, Cronbach’s alpha.
Personality traits measures
We used the NEO-FFI, a 60-item short form of the 240-item NEO-PI-R [20]. It had been translated and tested in Czech population [21]. The scales of NEO-Five Factor Inventory (NEO-FFI) are Neuroticsm, Extraversion, Openness to Experience, Agreeableness and Consciousness.
Anxiety measures
The Zung Self-Rating Anxiety Scale (SAS) is a method of measuring levels of anxiety in patients who have anxiety-related symptoms [22]. The scale focuses on the most common general anxiety disorders; coping with stress typically causes anxiety. The SAS test is self-administered, with each response posited on a 4-point scale, from “none of the time” to “most of the time”. There are 20 questions with 15 increasing anxiety level questions and 5 decreasing anxiety questions.
Distress measures
Symptom Checklist-90 (SCL-90) is a self-report inventory with ninety items for assessing the 9 basic dimensions of symptoms (psychoticism, paranoid thoughts, obsessive-compulsive symptoms, interpersonal sensitivity, hostility, anxiety, phobias, depression, and somatization). A symptom index (GSI) is created by averaging all 90 items and is considered a sensitive indicator of general psychological distress, and Positive Symptom Distress Index (PSDI) was designed to measure symptom intensity [23]. SCL-90 works on the basis of a five-point Likert scale, where the participant assesses whether and how much he suffers from the mentioned symptoms (0 = never, 4 = strongly). Participants were given the Czech version of this checklist [24,25]. Within the scope of this study, psychological distress was measured with the SCL-90 method using the Global Severity Index (GSI) and Positive Symptom Distress Index (PSDI).
Tumour-related measures and variables
Data on tumour-related factors and treatment were obtained from medical records. Case history data contained information on the presence of comorbidities, including depression. Disease and treatment data contained information about the stage of the disease (I–IV), type of treatment (surgery, chemotherapy, radiotherapy, biological therapy, and hormone therapy), progression, relapse, and treatment response (complete, partial, stable, and progressive). CRP was measured two separate times (at diagnosis and one year after primary treatment completion) by a high-sensitivity assay using Cobas Integra 400 plus with an interassay variation of 0–5 mg/L.
Statistical methods
Standard descriptive statistics were applied in the analysis: absolute and relative frequencies for categorical variables and mean with standard deviation or median with minimum-maximum range for continuous variables. Quantification of the relationship between biological and physical predictors and Global Severity Index (GSI) and Positive Symptom Distress Index (PSDI) as dependent variables was estimated using both univariate and multivariate linear regression models. Statistical analysis was computed using SPSS 22.0.0.1 (IBM Corporation, 2015).
Results
Study sample
The dataset consisted of 98 women with data available on the majority of clinical characteristics and SCL-90, SVF 78 and NEO-FFI subscales at the diagnosis. The median follow-up time was 41.4 months. A sub-analysis was conducted that incorporated the total raw score of the SAS questionnaire in 39 patients. The median age was 65 years, median BMI 27, 92 % of women had children and 84 % were postmenopausal at the time. The primary stage of the disease was 0–I in 37 %, II–III in 53 % and IV in 10 % of patients. Surgery treatment was applied in 96 %, chemotherapy in 65 %, radiotherapy in 85 %, biological therapy in 18 % and hormonal therapy in 81 % of patients. Nearly half (48 %) of patients had hypertension and 18 % depression disorder. A relapse episode occurred in 18 % of patients. 65 % of patients reported experiencing a stressful situation in the last 5 years, excluding stress directly connected to the oncological diagnosis and treatment. The median level of CRP at diagnosis was 5.1 mg/L. This value is higher than the baseline CRP median in population, but is nevertheless still within the range of normal values of the general population [26]. For a detailed description of patients’ characteristics, see Tab. 1a and Tab.1b.
Tab. 1a. Basic characteristics of patients (N = 98) 
Tab. 1b. Characteristics of patients in follow up 
Psychological distress prediction
A statistically significant positive relationship was found between the GSI after treatment and all SCL-90 components at the time of diagnosis. In addition, we found a statistically significant positive relationship between GSI and the need for social support, flight tendency and all three components of overall negative strategy (i.e. rumination, designation and self-accusation), and a negative relationship with the play down strategy of the SVF 78 questionnaire. Concerning NEO-FFI, a statistically significant positive relationship with neuroticism, as well as a negative relationship with agreeableness was shown. Among clinical predictors, only the CRP level before and after treatment exhibited a statistically significant positive association with the GSI (Tab. 2).
1. Prediction of global severity index (GSI) after treatment by univariate linear regression (N = 98) 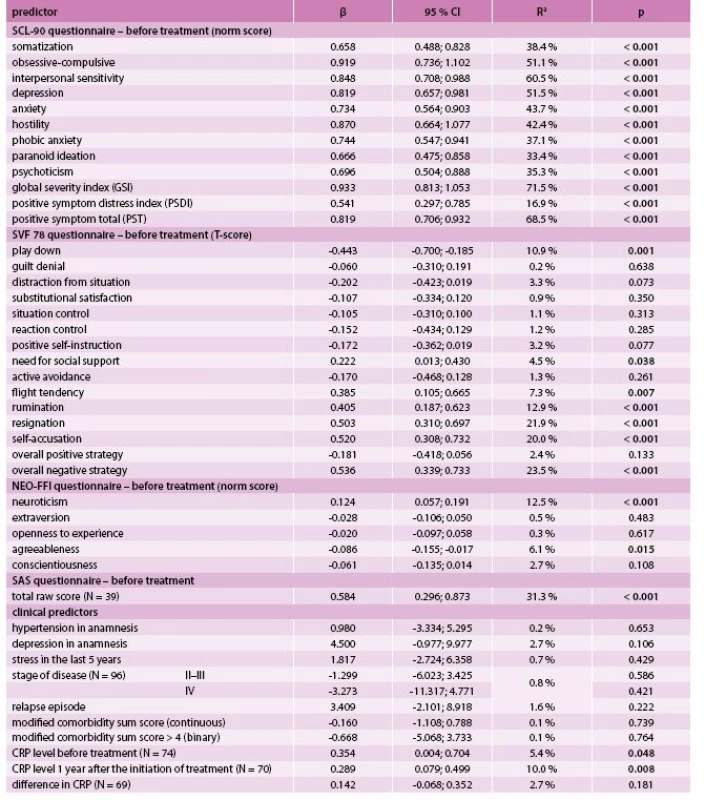
As for the PSDI, a statistically significant positive association was found to all SCL-90 components, an overall negative strategy of SVF 78 (as well as to its three subscales), and to the stage IV, CRP after treatment and its change over time (Tab. 3). Both GSI and PSDI showed a statistically significant and strong positive relationship to the total raw SAS score (Tab. 2, Tab. 3).
2. Prediction of positive symptom distress index (PSDI) after treatment by univariate linear regression (N = 98) 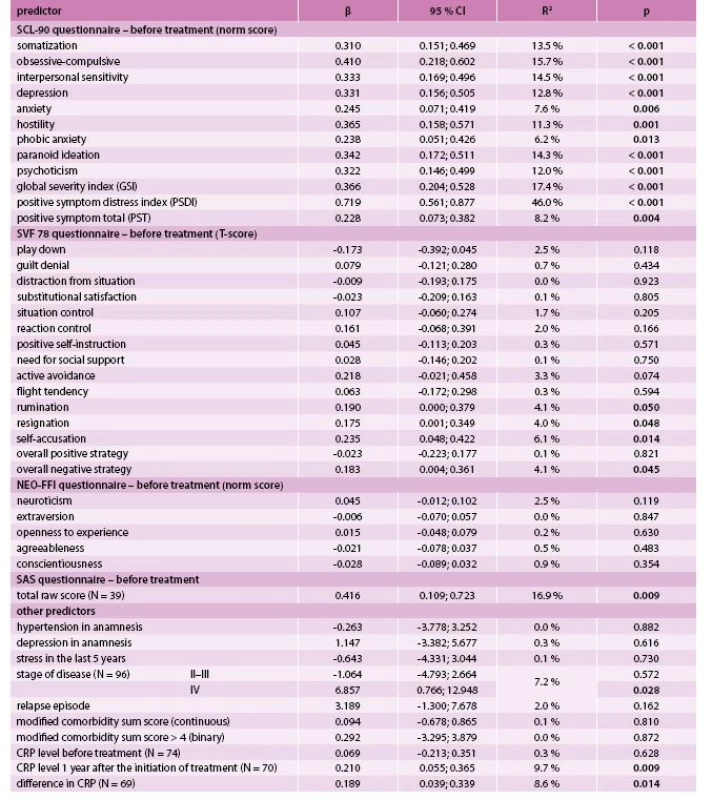
The best model for the prediction of the GSI after treatment was identified by multivariate linear regression as the combination of predictors GSI, CRP level and agreeableness (NEO-FFI subscale) at the time of diagnosis with R2 = 76.6 % (Tab. 4). The best model for predicting PSDI after treatment consisted of PSDI, the self-accusation component of SVF 78 and the stage of the disease (IV vs lower) at the time of diagnosis with R2 = 53.9 % (Tab. 5). Incorporating the total raw score of the SAS questionnaire into the multivariate models for GSI and PSDI prediction caused an increase in R2 (71.5 % to 85.0 % and 46.0 % to 65.1 %, respectively); however, this interpretation is limited by the low number of patients who took the SAS questionnaire. The change in GSI was statistically significantly positively related to CRP both before and after treatment, as well as its change (Tab. 6); the change in PSDI was statistically significantly positively related to CRP level after treatment (Tab. 7).
3. Prediction of GSI after treatment (N = 74) – multivariate linear regression 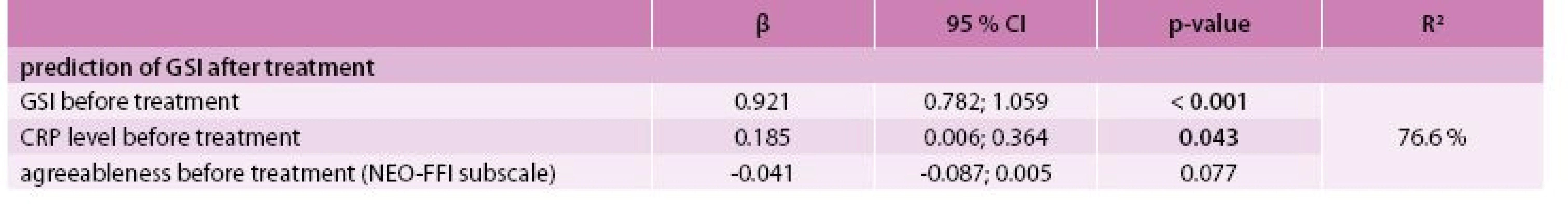
4. Prediction of PSDI after treatment (N = 96) – multivariate linear regression 
5. Association between CRP level and change in GSI – univariate linear regression 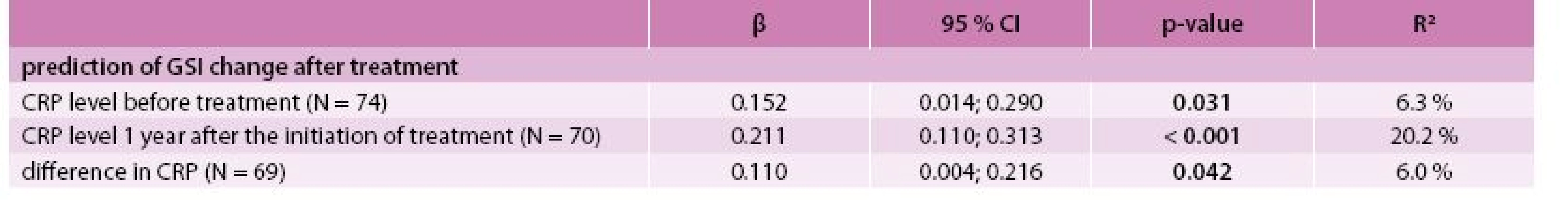
6. Association between CRP level and change in PSDI – univariate linear regression 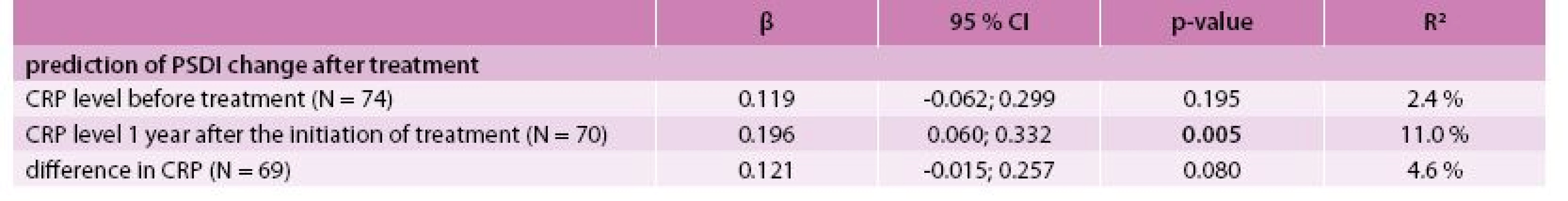
Discussion
A self-report questionnaire was used to identify psychological distress. The instrument used here – SCL-90 – identifies both the extent or depth of the individual’s distress disturbance (GSI) and the intensity of symptoms (PSDI). The selected linear regression model is a standard approach for the identification of potential predictors of quantitative endpoints; the available data allowed for the computation of both univariate and multivariate models which assess the relationship between combinations of predictors and evaluated endpoints. The CRP values were included in the model as a value before and after treatment and also as a difference between these two time points; this approach allows the inclusion of both absolute value and the dynamics of CRP levels over time.
We examined the role of personality traits, coping styles, previous psychological distress experience, and anxiety states for distress prediction after oncological treatment. Psychological variables such as distress at diagnosis, coping style related to feelings of guilt and an overall negative strategy (e.g. rumination, designation and self-blame), neuroticism and anxiety states showed a positive significant relation to distress experiences in survival phase in breast cancer patients, and agreeableness showed a negative one Henselmans et al. [27] found that 15.2 % of breast cancer patients assessed in the short-term survivorship phase (2 and 6 months after the end of treatment) reported a distress experience, and 15.2 % of patients experienced chronic distress. Mastery was the only unique distress level predictor in this study. Illness perception cluster membership and positive focus type coping were the most important and consistent predictors of lower psychological distress at diagnosis and 6 months following diagnosis [28]. In our study, self-accusation as a negative coping strategy was associated with a higher intensity of experienced distress. Donovan-Kicken et al [3] also considered patients’ self-blame as a factor that could lead to higher levels of psychological distress among breast cancer patients. We also found a statistically significant positive relationship between GSI and the need for social support and flight tendency. Patients who tend to escape from unpleasant situations, do not solve their problems, and seek social support typically feel more psychologically stressed later.
Neuroticism is characterized by a general disposition to the experience of distressing emotions such as fear, guilt, and frustration [20]. Costa and McCrae [20] distinguish six aspects of neuroticism: vulnerability to stress, impulsiveness, self-consciousness, depression, hostility, and anxiety. Our results related to neuroticism’s role were in line with the study of Golden-Kroetz et al [29]. Agreeableness represents the inclination toward interpersonal trust and consideration of others. Persons scoring high on agreeableness tend to be warm, sympathetic, and cooperative, and are inclined to avoid conflict [20]. An explanation for our current findings could be that persons who score high on agreeableness tend to have enough social support or perceive their actual social support as adequate [30].
Bleiker et al [7] did not find a significant association between biomedical variables and distress prediction two years after breast cancer diagnosis. Our group of patients reported distress experiences in the long term survivorship phase (4.5 year after diagnosis, MD) and for this design both the psychological and biological sorts of predictors proved significant. Moreover, symptoms should not be viewed in isolation, but rather as part of a cluster of interrelated symptoms [6]. Our results generally linking CRP level as an inflammatory marker and chronic distress in cancer survivors correspond with the findings previously published in other studies [14,15]. Moreover, one interesting finding seems to be the significance of CRP change (at diagnosis and one year after) for change prediction of intensity of distress experiences measured by PSDI (at diagnosis and 4.5 years after). These findings could reflect an individual’s capability to deal with disease and its treatment, connected with a subjective evaluation of distress experience.
In conclusion, both sorts of predictors – biological and psychological, proved significant and suitable for psychological distress prediction in elderly breast cancer survivors after long-term oncological treatment. The retrospective design of the study represents a serious study limitation. Moreover, CRP data were obtained from medical records, and we have no data about the influence of cancer treatment on the inflammatory process. According to our results, inflammatory processes seem to play a role in distress experience of breast cancer survivors. The question is the causality of the studied phenomenon – prolonged inflammation and psychological distress experience. Further research is needed to better understand the related psychoneuroimmunology mechanisms, and a prospective study design to confirm our results.
The results of this retrospective study were presented at the 19th Symposium on Breast Healthcare in Prague on 20 November 2015.
Declaration
The authors indicated no potential conflicts of interest.
Acknowledgement
We thank to all of the patients who participated in our study. The study was supported by the Czech Science Foundation as a Project P407/12/0607.
Doručeno do redakce 3. 12. 2015
Přijato po recenzi 14. 12. 2015
Mgr. et Mgr. Kateřina Skřivanová, Ph.D.
kskrivan@med.muni.cz
Ústav psychologie a psychosomatiky LF MU Brno
www.med.muni.cz
Sources
1. The National Comprehensive Cancer Network. Distress Management Clinical Practise Guidelines in Oncology. Version 2.2013, 2014. Dostupné z WWW: <http://www.nccn.org/professionals/physician_gls/f_guidelines.asp>.
2. Ridner SH. Psychological distress: concept analysis. J Adv Nurs 2004; 45(5): 536–545.
3. Donovan-Kicken E, Cauglin EP. Breast cancer patients’ avoidance and psychological distress: The mediating role of coping. J Health Psychol 2011; 16(4): 596–606. Dostupné z DOI: <http://dx.doi.org/10.1177/1359105310383605>.
4. Deimling GT, Kahana B, Bowman KF et al. Cancer survivorship and psychological distress in later life. Psycho-Oncology 2000; 11(6): 479–494. Dostupné z DOI: <http://dx.doi.org/10.1002/pon.614>.
5. Kendler KS, Gardner M, Gatz NL et al. The sources of co-morbidity between major depression and generalized anxiety disorder in a Swedish national twin sample. Psychol Med 2007; 37(3): 453–462.
6. Aaronson NK, Mattioli V, Minton O et al. Beyond treatment: Psychosocial and Behavioral issues in cancer survivorship research and practice. EJC Suppl 2014; 12(1): 54–64.
7. Bleiker EM, Pouwer F, van der Ploeg HM et al. Psychological distress two years after diagnosis of breast cancer: frequency and prediction. Patient Educ Couns 2000; 40(3): 209–217.
8. Ando N, Iwamitsu Y, Kuranami M et al. Predictors of psychological distress after diagnosis in breast cancer patients and patients with benign breast problems. Psychosomatics 2011; 52(1): 56–64. Dostupné z DOI: <http://dx.doi.org/10.1016/j.psym.2010.11.012>.
9. Kiecolt-Glaser JK, Preacher JK, MacCallum RC et al. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci U.S.A. 2003; 100(15): 9090–9095.
10. Miller GE, Chen E, Sze J et al. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry 2008; 64(4): 266–272.
11. Ranjit N, Diez-Roux AV, Shea S et al. Socioeconomic position race/ethnicity and inflammation in the multi-ethnic study of atherosclerosis. Circulation 2007; 116(21): 2383–2390.
12. Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420(6917): 860–867.
13. Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis 2005; 5(11): 718–725.
14. Jehn CF, Kuehnhardt D, Bartholomae A et al. Biomarkers of depression in cancer patients. Cancer 2006; 107(11): 2723–2729.
15. Musselman DL, Miller AH, Porter MR et al. Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. Am J Psychiatry 2001; 158(8): 1252–1257.
16. Bower JE, Ganz PA, Irwin MR et al Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol 2011; 29(26): 3517–3522.
17. Alfano CM, Imayama I, Neuhouser LM et al. Fatigue, Inflammation, and Omega-3 and Omega-6 Fatty Acid Intake Among breast Cancer Survivors. J Clin Oncol 2012; 30(12): 1280–1287. Dostupné z DOI: <http://dx.doi.org/10.1200/JCO.2011.36.4109>.
18. Skřivanová K, Brančíková D, Bendová M et al. Evaluating Disease and Patient Characteristics which Predict (Retrospectively) Changes in Quality of Life after Breast Cancer Diagnosis and Treatment. Psycho-Oncology 2014; 23(Suppl 3): 396–397.
19. Janke W, Erdmannova G. Strategies for stress coping [questionnaire in Czech]. Testcentrum: Praha 2003. ISBN 80‐86471‐24‐1.
20. Costa PT, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) professional manual. Psychological Assessment Resources: Odessa (FL) 1992.
21. Hřebíčková M, Urbánek T. NEO-FFI according Costa PT and McCrae RR. Testcentrum: Praha 2001.
22. Zung WK. A Rating Instrument for Anxiety Disorders. 12(6): Psychosomatics 1971; 12(6): 371–379.
23. Degoratis LR, Savitz KL. The SCL–90–R and Brief Symptom Inventory (BSI) in primary care. In: Maruish ME. Handbook of psychological assessment in primary care settings. Lawrence Erlbaum Associates Publishers: Mahwah, US 2000. ISBN 978–0805829990.
24. Filip V. Praktický manuál psychiatrických posuzovacích stupnic. Psychiatrické centrum: Praha 1997. ISBN 80–85121–06–9.
25. Bieščad M, Szeliga P. Overenie konštruktovej validity sebaposudzovacej škály Symptom Checklist-90 (SCL-90). Československá psychologie 2005; 49(4): 342–356.
26. Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest 2003; 111(12): 1805–1812
27. Henselmans I, Helgeson VS, Seltman H et al. Identification and prediction of distress trajectories in the first year after a breast cancer diagnosis. Health Psychol 2010; 29(2): 160–168. Dostupné z DOI: <http://dx.doi.org/10.1037/a0017806>.
28. McCorry NK, Dempster M, Quinn J et al. Illness perception clusters at diagnosis predict psychological distress among women with breast cancer at 6 months post diagnosis. Psychooncology 2013; 22(3): 692–698. Dostupné z DOI: <http://dx.doi.org/10.1002/pon.3054>.
29. Golden-Kreutz DM, Andersen BL. Depressive symptoms after breast cancer surgery: relationships with global, cancer-related, and life event stress. Psychooncology 2004; 13(3): 211–220.
30. Den Oudsten BL, Van Heck GL, Van der Steeg AF et al. Predictors of depressive symptoms 12 months after surgical treatment of early‐stage breast cancer. Psychooncology 2009; 18(11): 1230–1237.
Labels
Paediatric gynaecology Gynaecology and obstetrics Reproduction medicine
Article was published inPractical Gynecology

2015 Issue 4-
All articles in this issue
- Minimally invasive surgery in the treatment of uterine fibroids
- Hypertension in females
- Anaemia in gynecology and perinatology
- Pharmacotherapy of rheumatic diseases in pregnancy
- Predicting psychological distress after primary oncological treatment in elderly breast cancer survivors: Retrospective study
- Practical Gynecology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Anaemia in gynecology and perinatology
- Minimally invasive surgery in the treatment of uterine fibroids
- Pharmacotherapy of rheumatic diseases in pregnancy
- Hypertension in females
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

