-
Medical journals
- Career
New approaches in the follow-up of patients suffering from inflammatory bowel diseases
Authors: I. Romanko 1; M. Lukas 2; M. Bortlík 2,3
Authors‘ workplace: Joint Admission of the General University Hospital for Persons with Medical Problems, Prague 1; IBD Clinical and Research Centre, ISCARE Lighthouse and 1st Medical Faculty of Charles University, Prague 2; Department of Internal Medicine, 1st Medical Faculty, Charles University and Central Military Hospital, Prague 3
Published in: Gastroent Hepatol 2015; 69(5): 441-448
Category: IBD: Review Article
doi: https://doi.org/10.14735/amgh2015441Overview
Inflammatory bowel diseases (IBD) are chronic, presently incurable conditions, meaning a lifetime struggle for patients. They are characterised by episodes of remissions and relapses, which affect the patients’ well-being and quality of life (QoL). Disease activity (DA) and QoL are important aspects of IBD follow-up and should be properly assessed during outpatient visits. Long-term follow-up is necessary, but can be complicated for some patients due to a limited number of IBD centres, long distance travelling, or lack of appointments with physicians. The use of information technology in medicine (telemedicine) could help with IBD surveillance. DA and QoL tools in the form of online or mobile phone questionnaires could be completed by patients from home and a visit to a physician recommended according to the results, thereby allocating health care to more complicated cases. Such a method of remote monitoring could save time and money for physicians and patients who are in clinical remission. The article summarises invasive (endoscopy) and non-invasive tools (questionnaires, faecal calprotectin) for DA and QoL assessment and highlights possible implications of telemedicine in routine care.
Key words:
Crohn’s disease – ulcerative colitis – disease activity – quality of life – faecal calprotectin – telemedicineIntroduction
Inflammatory bowel diseases (IBD) are chronic diseases comprising ulcerative colitis (UC) and Crohn’s disease (CD), which result from a disregulated immune response in genetically susceptible individuals [1]. Both conditions are characterised by periods of remission and flare-ups. Medications targeting the immune system locally or systematically are used for the treatment of both diseases. However, CD and UC seem to be separate entities [2,3]. UC stays limited to the colon, which is affected from the rectum proximally and inflammation does not exceed the mucosa of the intestinal wall in the majority of cases. On the other hand, CD can affect the entire digestive tract in patchy lesions with transmural involvement of the intestinal wall, but typical cases start as terminal ileitis [3]. Both conditions could have numerous extraintestinal manifestations (e.g. primary sclerosing cholangitis, ankylosing spondylitis, decreased bone mineral density, psoriasis, etc.) [4].
Loftus states that in Europe, incidence rates range from 1.5 to 20.3 cases per 100,000 person-years for UC and from 0.7 to 9.8 cases per 100,000 person-years for CD. Both diseases are most commonly diagnosed in late adulthood or early adolescence and there is a slight female predominance in CD, but male predominance in UC [5]. North to south and west to east gradient of IBD incidence in European countries have been recognised recently [6].
As IBD has a chronic course, affecting mostly young individuals, there is a need for being able to assess disease activity to distinguish the occurrence of relapse and remission.
Many patients have to travel a long distance to seek proper care at specialised IBD centres. This often complicates the availability of immediate treatment necessary for appropriate management of disease relapse, or other complications. Telemedicine is emerging as a new tool creating an interface for long-distance, real-time communication between patient and specialist doctor without the need for direct contact. This review summarises new approaches, possibilities and insight into the outpatient follow-up of patients with IBD.
Assessment of disease activity and quality of life in IBD
Activity index
Activity indexes mostly offer non-invasive options for how to assess disease progression based on clinical symptoms. However, some include laboratory parameters (e.g. albumin or hematocrit) or clinical signs (e.g. abdominal mass) and do not rely on patient - -reported information only. Best et al. published a first scoring system to stratify CD progress into remission and mild, moderate or severe activity of disease – the CD Activity Index (CDAI) [7]. It had been later simplified by Harvey and Bradshaw, hence the Harvey-Bradshaw Index (HBI, tab. 1) was created with good correlation with CDAI [8]. The clinical activity indexes for CD patients are used in clinical trials, but rarely in clinical practice.
1. Harvey-Bradshaw Index (HBI). Tab. 1. Harvey-Bradshaw Index (HBI). 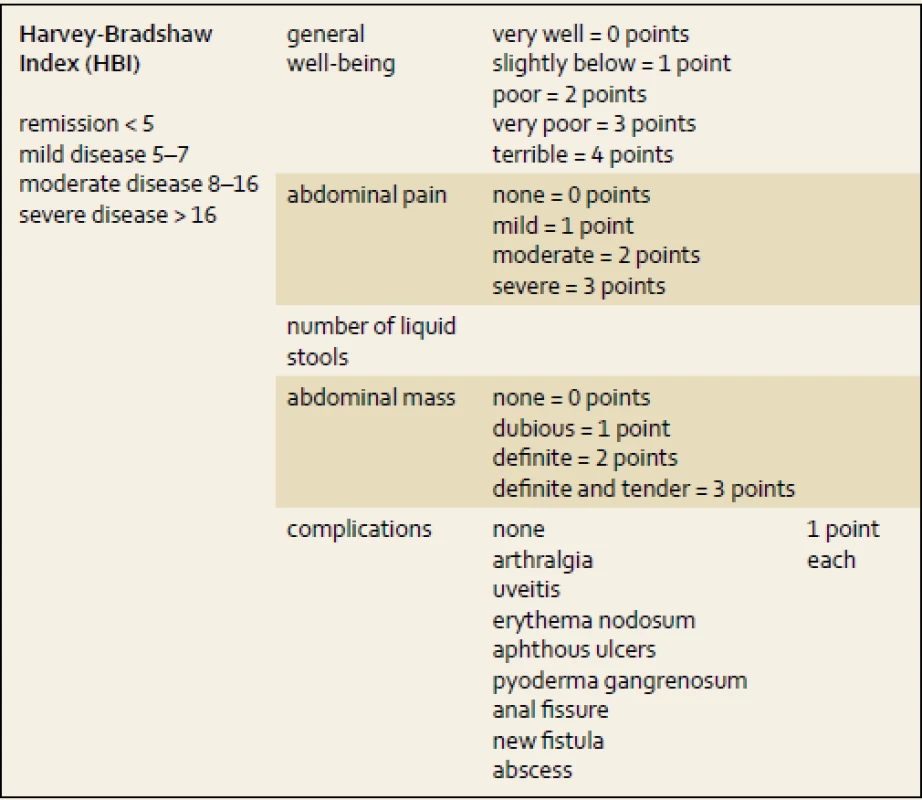
On the contrary, for UC patients, the Truelove and Witts index (tab. 2) still remains a very helpful tool for distinguishing patients with severe relapses of colitis. Bloody stools six or more times a day plus one of the clinical symptoms or signs of a systemic inflammatory reaction (heart rate of more than 90/min; temperature higher than 37.5 degrees; hemoglobin of less than 110 g/l and elevation of C-reactive protein (CRP) or erythrocyte sedimentation rate of more than 30 mg/l or 30 mm/hour, resp.) [9]. In those patients intensive care, including hospital admission and intravenous corticosteroids, is needed.
2. Truelove and Witts severity index for ulcerative colitis. Tab. 2. Truelove a Witts severity index ulcerózní kolitidy. 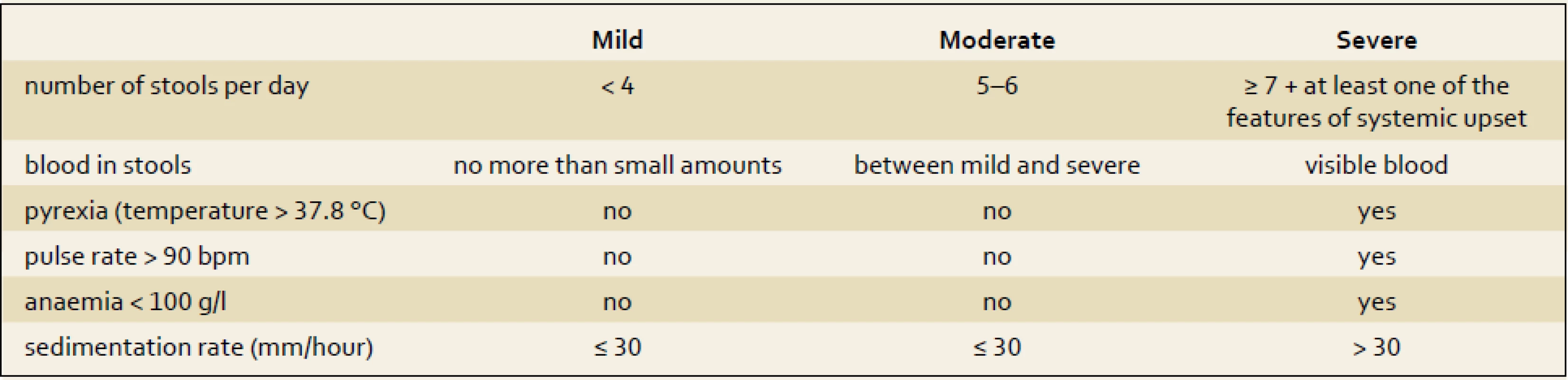
Other clinical activity indexes comprising a Powell-Tuck St Mark’s index are rarely used in clinical practice [10]. A simplified version of the Powell-Tuck index was developed by Walmsley et al. in 1998 and named the Simple Clinical Colitis Index (SCCAI, tab. 3) [11], based on the clinical parameters (number of stools, general well-being, blood in stools). There are many other activity indexes for UC widely used in clinical trials, such as the Sutherland index (UC-DAI), or the Travis index which was developed recently. In clinical trials with UC, the Mayo index is frequently used. This index evaluates clinical and endoscopic aspects of the disease course. In the last 15 years many new drugs, including biologicals, have been evaluated with these indexes [12–14].
3. Simple Clinical Colitis Activity Index (SCCAI). Tab. 3. Jednoduchý klinický index aktivity kolitidy (SCCAI). 
Endoscopy and imaging
Ileocolonoscopy is the golden standard for the diagnosis and therapeutic monitoring of IBD. According to the European Crohn’s and Colitis Organisation guidelines, the golden standards for endoscopy assessment are Crohn’s disease Endoscopic Severity Index (CDEIS) and the Ulcerative Colitis Endoscopic Index of Severity or Mayo score. The use of Simple Endoscopic activity Score for Crohn’s Disease (SES-CD) is recommended as well [15]. In clinical practice, the application of CDEIS or SES-CD is quite difficult due to the complicated composition of indexes. The mucosal healing in CD is characterised by the disappearance of deep mucosal ulceration and a decrease of SES-CD or CDEIS of more than five points is required. In UC patients, endoscopial remission is characterised by a Mayo score of 0 points. In CD patients after ileocolonic resection, Rutgeerts index is used to determine the risk of relapse. Apart from ileocolonoscopy, there are other endoscopic methods, such as capsule endoscopy or enteroscopy, which are important in cases, where ileocolonoscopy is inconclusive. Other imaging modalities, such as CT or MRI, play important role in disease extend and progression assessment as well [16].
Quality of life
In some patients, even though remission is achieved through therapy, their QoL may be diminished due to emotional or social problems. QoL can be low in spite of low scoring in activity indexes, which are aimed at clinical symptoms and not the patient’s emotions, feelings and social interactions [17]. In these cases, a multidisciplinary approach with a psychologist or psychiatrist might be needed.Guyatt et al. published an Inflammatory Bowel Disease Questionnaire (IBDQ) developed for QoL assessment of IBD patients [18]. Its disadvantage was its length – 36 questions, which could be time consuming. In 2001 Jowett et al. published a simplified version of the IBDQ named the Short inflammatory disease questionnaire (SIBDQ) consisting of just 10 questions from the original IBDQ which used the same scoring system. It has been validated and showed correlation with IBDQ [19]. The short IBDQ is frequently used in many IBD centres at the moment, enabling complex evaluation of treatment efficacy. Up to now, a variety of different tools have been used, as discussed in the section about patient reported-outcome measures.
Faecal calprotectin as non-invasive biochemical marker of disease activity in IBD
Faecal calprotectin is a heterocomplex calcium-binding protein abundant in cytoplasm of leucocytes [20,21]. During active inflammation of mucosa, leucocytes infiltrate the intestinal wall and undergo degradation. This results in the release of calprotectin into the intestinal lumen, therefore being present in the stool. The more active the inflammation is, the more leucocytes infiltrate the mucosa and the level of calprotectin in the stool rises [21,22]. In a healthy bowel, calprotectin stool levels are almost undetectable, levels of up to 50 µg/g in the stool are considered physiological [23]. Its stability gives calprotectin a great advantage in diagnostic use, as it keeps preserved in stool samples for up to seven days if kept under room temperature [24]. Calprotectin is one of the stool inflammatory markers derived from leucocytes, such as lactoferrin, M2-pyruvate kinase, S100A12 protein or PMN-elastase [25]. Among these, most attention is paid these days to calprotectin as an appropriate candidate for a biomarker of inflammation [20]. Besides calprotectin, lactoferrin and PMN-elastase may have possible roles as markers for predicting relapses and differential diagnosis of IBD, resp. [18,25–27].
It has been proven by several authors that calprotectin and lactoferrin are suitable markers for disease activity assessment both for CD and UC, moreover correlating with endoscopic findings in both diseases [28,29]. Sipponen showed that faecal calprotectin and lactoferrin levels correlated better with endoscopic findings than with the CDAI. The severity of endoscopic findings was evaluated using CDEIS and correlation coefficient with calprotectin was 0.77. Elevated calprotectin levels showed more sensitivity in detecting the active disease than an increase in CDAI. Levels of calprotectin below 100 µg/g in the stool were considered normal [30]. Another endoscopic evaluation score SES-CD has been proven to correlate with faecal calprotectin with coefficient of 0.75, while with CDAI the correlation was weaker, coefficient being only 0.38, although still considered statistically significant [31].
Unfortunately, calprotectin is not 100%, neither specific nor sensitive. It can be elevated during other non-IBD intestinal inflammatory processes or during nonsteroidal anti-inflammatory drug (NSAID) use. Sometimes patients in clinical remission can have high levels of calprotectin despite a lack of symptoms [30].
As calprotectin significantly correlates with endoscopic severity, a decrease of calprotectin levels could point to ongoing mucosal healing. Roseth showed a decrease of calprotectin correlated with remission due to endoscopy confirmed by histology [32]. In other work of Sipponen, it has been shown that both calprotectin and lactoferrin decreased with therapy-achieved mucosal healing confirmed by endoscopy using SES-CD score for grading. A decrease in SES-CD correlated with a decrease in stool biomarkers in patients responding to the therapy. In non-responders, there was no endoscopic improvement and a calprotectin decrease was not as significant as in therapy responders [33].
Faecal calprotectin can therefore be used as a diagnostic marker, biomarker of mucosal inflammation and healing, and a predictor of relapses in IBD [34,35]. It is suitable to assess disease activity in symptomatic, asymptomatic patients and to predict a relapse in patients following surgery [16,36].
Determining appropriate cut-off values for calprotectin still remains a question open for discussion. In the work of Rogler a consensus has been suggested regarding calprotectin semiquantitative point-of-care test cut-offs (tab. 1) stratifying the disease activity into active disease, flare-up and remission. A similar scale has been suggested for differential diagnosis of irritable bowel syndrome (IBS) and IBD. For patients with diagnosed UC, Rogler suggests levels below 400 µg/g are considered as remission, above 400 as flare-up and over 1,000 as active disease [25]. Values suggested by Rogler are 250 µg/g higher than those proposed by D’Haens who found calprotectin being 60.4%, 71% sensitive and 79.5%, 100% specific for detection of severe endoscopic lesions in CD and UC, resp. [37].
Volatile organic compounds in IBD diagnostics
Volatile organic compounds (VOCs) are emerging as a new possible non-invasive IBD diagnostic method. The VOC in exhaled breath as a diagnostic marker are still being investigated and are not yet used in clinical practice, as they remain to be identified. On the other hand, there is growing evidence that VOC might become biomarkers capable not only of determining IBD disease activity, but also to diagnose IBD. Differences in VOC between affected and healthy individuals are most likely due to changes in gastrointestinal microbiota [38]. Bodelier et al. presented a study of exhaled breath from CD patients (in remission and with active disease) and healthy controls. Samples were analysed by gas chromatography and mass spectrometry. They identified 6 (remission) and 10 discriminatory (active disease) VOCs capable of correct prediction of healthy controls vs. CD patients and CD-remission patients vs. active-disease patients, resp. [39].
Patient-reported outcome measure for assessment of disease activity
As already mentioned, disease activity of IBD can be measured based on clinical symptoms by using activity indexes. Laboratory markers and imaging are also used to confirm the severity of clinical symptoms. Indexes used today are a valid and quick way for physicians to distinguish remission, flare-up and active disease. However, they have been validated against physicians’ global assessments and not against endoscopy findings. It is known that the severity of clinical symptoms does not have to correlate with an endoscopy image. For this reason Pieriks’ group at Maastricht University Medical Centre decided to develop a questionnaire based on patient-reported clinical symptoms, but validated against the endoscopy image using SES-CD and Mayo for scoring. Questions had been chosen from existing activity indexes according to expert clinical opinion and the questionnaire does not include any laboratory parameters. Final score gives a dichotomous answer if there is a mucosal inflammation present or not and is calculated due to logistic regression.
The validation study is ongoing in the Netherlands and Czech Republic and results are not yet available. Authors believe this questionnaire will be a patient-reported outcome measure (PROM) to assess the presence of mucosal inflammation, which correlates with endoscopy better then recent activity indexes.
Recently, several articles have been published proposing new patient-reported outcomes for IBD, combining QoL and disease activity measurements.
Surti et al. suggested the Numeric Rating Scale (NRS), a simple tool similar to the visual analogue scale, where patients respond to questions about general well-being on a scale from 0 to 10 (0 is worst, 10 is the best) as well as questions about being in remission (yes or no, in their subjective view). The same questions were posed to the physician and results compared with IBDQ, HBI, CDAI, SCCAI, calprotectin and CRP. Authors found good and significant correlations of NRS with IBDQ, CDAI and HBI, while poor correlations with SCCAI, CRP and calprotectin [40].
Bodger proposed an IBD-Control questionnaire. A quick and simple tool consisting of 13 questions derived from IBDQ, EQ-5D-3L and Hospital Anxiety and Depression Scale (HADS) aiming towards physical, social, and emotional aspects of IBD and therapy compliance. The idea was to focus on a patient’s opinion of their general well-being, and QoL questions important for patients, not just individual gastrointestinal symptoms. The questionnaire has proven to be valid and reliable. It has been validated against SCCAI, HBI and a physician’s global assessment, but not against endoscopic image [41].
Keefer et al. designed and validated the IBD Self-Efficacy-Scale (IBD SES) questionnaire in 2011 [42]. It comprises 29 questions divided into four domains: stress management, therapy adherence, symptoms management, and remission management. IBD SES focuses on patient-reported outcomes and each question is scored on a scale of 1–10 according to how confident the patient feels in dealing with tasks asked by tge questionnaire. It is aimed at a patient’s self-management highlighting if patients are actively fighting the disease through lifestyle changes and adjustments.
Recently Alrubayi published a review article comparing and summarising several available patient-reported outcome measures for QoL assessment in IBD [43]. The reported IBD patient questionnaire looks, in the opinion of authors, very promising for its high internal consistency, construction and content validity and reliability compared to other measures. Unfortunately, this review did not include new measures as mentioned above.
Telemedicine
Telemedicine – the use of electronic information and communication in providing and supporting health care when distance separates the participants [44] – is a contemporary example of technology where health care can be provided by lesser trained personnel, thereby rationalising the need for a physician for those patients who have more serious problems [45].
For IBD, telemedicine seems to be a very suitable approach for patients. The length of clinical remission can vary between individuals and some patients can be in remsion for years without a flare-up. As they have to be checked regularly in outpatient clinics, it is desirable to have a tool for checking patients’ conditions and inviting for a face-to-face consultation only those who are deteriorating or need changes in therapy. This approach an save time and money both for physicians and patients and seems to be satisfying for patients as well [46].
In 2010 Elkjaer et al. published results of a study where UC patients with mild to moderate activity, treated with aminosalicylates, tried a new web-based educational/self-management programme called Constant care [47]. The study took place in Denmark and Ireland with a duration of 12 months. Patients were randomised into a control group with a classical follow-up and web-based group using the Constant care-browser programme where a health check could be done by answering a specifically designed questionnaire based on existing disease activity indexes. Out of 333 patients, 88% preferred this new approach. Authors showed that although there was no difference in relapse rates, hospitalisation, surgery and adverse events between groups, the median relapse duration was 18 days in the web-based group compared to 77 days in the control group. The Constant care programme proved to be cost effective as well by reducing the number of acute and routine visits by patients.
A recent meta-analysis conducted by Huang reviewed several studies and trials comparing distance management and standard clinic follow-up in adult IBD patients. Out of the six trials included in the systematic review, in three the telemedicine approach was studied. Distance management in general showed to significantly increase QoL due to IBDQ and reduce the number of patient visits. There was no difference detected in the relapse rate or hospital admission rate [48].
Evidence of feasibility and validity of telemedicine e-health tools is growing. There is also a tendency to adjust the activity scores version of PROM, which can be completed by patients remotely using web-based programmes. In Korea a team lead by Kim designed Crohn’s Disease Symptom Diary (CDSD) based on five variables derived from HBI. Patients were asked to complete this diary online through the CDSD web page and they also kept a CDAI symptom diary for seven days prior to the appointment. The Crohn’s Disease Symptom Diary showed a good correlation (Spearmen r = 0.720; p < 0.001) and a cut-off value of five points had a positive and negative predictive value for remission of 91.7% and 88.5%, resp. [49].
The e-health approach could not only help physicians to monitor patients remotely with validated tools, but it may also help to increase compliance and self-management by providing patients with more information about the disease course, complications or possible treatment options by educational portals included in web-based interfaces. Patients could become better educated, which means they might cope more easily with flare-ups and complications, their QoL might be significantly increased and their dependency on doctors decreased [47].
The aim of telemedicine is not to prevent a patient from seeing a doctor. On the other hand, it should help to allocate medical care more efficiently, concentrating more on individuals with serious trouble and relapses, while saving time for those in long-term remission. Using a telemedicine tool does not mean a patient will lose the option to contact a doctor directly in an emergency.
In Maastricht University Medical Centre (MUMC) group led by Dr. Pierik designed a new telemedicine tool for IBD patients named My IBD coach, which allows physicians to follow-up patients remotely regularly or in urgent cases via a series of questionnaires derived from CDAI, HBI, SCCAI, HADS, SIBDQ according to expert clinical opinion. A part of the questionnaire is a new tool developed by MUMC specialists to assess mucosal inflammation due to patient-reported symptoms only. A broader validation study of this new PROM has been taking place recently in the Netherlands and the Czech Republic. In the Czech Republic we are also working on the Czech version of MyIBDcoach in co-operation with MUMC, while in the Netherlands a randomised control trial with 1,000 IBD patients from three IBD centres has already started. Patients are being randomised into groups using MyIBDcoach and a control group with classic follow-up. Both CD and UC patients are included. Data are not available yet, as the study is designed to take one year to evidence changes in adherence, QoL, relapse, hospital admissions and complication rates. Prior to the launched trial, a smaller feasibility study with MyIBDcoach had run, showing good satisfaction amongst patients who used this telemedicine tool [50].
A similar project has started in the USA. Authors have designed a platform for mobile phones aimed at remotely monitoring IBD patients, their overall health condition and medication compliance in order to decrease the number of outpatient visits [51]. A randomised control trial will be used to assess the impact of this e-health tool on QoL and quality of care of IBD patients.
Conclusion
IBD is a chronic and (to date) an incurable condition. As patients have to struggle with these diseases in their day-to-day life, it is appropriate to monitor not only the disease activity to detect relapses, but also QoL as well to see if a patient’s condition is improving or worsening. Emotional and social function management is as important as management of the disease activity.
To make long-term monitoring easier both for patients and physicians, telemedicine is emerging as a new approach for patients, where validated clinical indexes and questionnaires are adapted or transformed into versions of patient-reported outcome measures (PROMs). They should be simple, based on patient information only, correlating with activity indexes or endoscopy and not time consuming. Faecal calprotectin appears to be an adequate non-invasive marker of activity, suitable for a long-term follow-up. A combination of remote communication along with calprotectin measurement would be an ideal tool for the home follow-up of patients. Such tools are proving to be feasible and their further development and upgrading is highly likely to save money and time for patients and health care providers in the future.
MUDr. Igor Romanko
Joint Admission of the General University Hospital for Persons with Medical Problems, Prague
U Nemocnice 2094/1
128 08 Praha 2
igor.romanko89@gmail.com
Sources
1. Vora P, Shih DQ, McGovern DP et al. Current concepts on the immunopathogenesis of inflammatory bowel disease. Front Biosci (Elite Ed) 2012; 4 : 1451–1477.
2. Yoshida EM. The Crohn’s Disease Activity Index, its derivatives and the Inflammatory Bowel Disease Questionnaire: a review of instruments to assess Crohn’s disease. Can J Gastroenterol 1999; 13(1): 65–73.
3. Ogorek CP, Fisher RS. Differentiation between Crohn’s disease and ulcerative colitis. Med Clin North Am 1994; 78(6): 1249–1258.
4. van Sommeren S, Janse M, Karjalainen J et al. Extraintestinal manifestations and complications in inflammatory bowel disease: from shared genetics to shared biological pathways. Inflamm Bowel Dis 2014; 20(6): 987–994. doi: 10.1097/MIB.0000000000000032.
5. Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 2004; 126(6): 1504–1517.
6. Burisch J, Munkholm P. The epidemiology of inflammatory bowel disease. Scand J Gastroenterol 2015; 50(8): 942–951. doi: 10.3109/00365521.2015.1014407.
7. Best WR, Becktel JM, Singleton JW et al. Development of a Crohns disease activity index. National Cooperative Crohns Disease Study. Gastroenterology 1976; 70(3): 439–444.
8. Harvey RF, Bradshaw JM et al. A simple index of Crohn’s disease activity. Lancet 1980; 1(8167): 514.
9. Stenke E, Hussey S. Ulcerative colitis: management in adults, children and young people (NICE Clinical Guideline CG166). Arch dis Child Educ Pract Ed 2014; 99(5): 194–197. doi: 10.1136/archdischild-2013-305512.
10. Powell-Tuck J, Brown RL, Lennard-Jones JE. A comparison of oral prednisone given as single or multiple daily doses for active proctocolitis. Scand J Gastroenterol 1978; 13(7): 833–837.
11. Walmsley RS, Ayres RC, Pounder RE et al. A simple clinical colitis activity index. Gut 1998; 43(1): 29–32.
12. Seo M, Okada M, Yao T et al. An index of disease activity in patients with ulcerative colitis. Am J Gastroenterol 1992; 87(8): 971–976.
13. Sutherland LR, Martin F, Greer S et al. 5-Aminosalicylic acid enema in the treatment of distal ulcerative colitis, proctosigmoiditis, and proctitis. Gastroenterology 1987; 92 : 1894–1898.
14. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 1987; 317(26): 1625–1629.
15. Annese V, Daperno M, Rutter MD et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis 2013; 7(12): 982–1018. doi: 10.1016/j.crohns.2013.09.016.
16. Sauter B, Beglinger C, Girardin M et al. Monitoring disease activity and progression in Crohn’s disease. A Swiss perspective on the IBD ahead ’optimised monitoring’ recommendations. Digestion 2014; 89(4): 299–309. doi: 10.1159/000360283.
17. Garrett JW, Drossman DA. Health status in inflammatory bowel disease. Biological and behavioral considerations. Gastroenterology 1990; 99(1): 90–96.
18. Guyatt G, Mitchell A, Irvine EJ et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology 1989; 96(3): 804–810.
19. Jowett SL, Seal CJ, Barton JR et al. The short inflammatory bowel disease questionnaire is reliable and responsive to clinically important change in ulcerative colitis. Am J Gastroenterol 2001; 96(10): 2921–2928.
20. Judd TA, Day AS, Lemberg DA et al. Update of fecal markers of inflammation in inflammatory bowel disease. J Gastroenterol Hepatol 2011; 26(10): 1493–1499. doi: 10.1111/j.1440-1746.2011.06846.x.
21. Desai D, Faubion WA, Sandborn WJ. Review article: biological activity markers in inflammatory bowel disease. Aliment Pharmacol Ther 2007; 25(3): 247–255.
22. Røseth AG, Schmidt PN, Fagerhol MK. Correlation between faecal excretion of indium-111-labelled granulocytes and calprotectin, a granulocyte marker protein, in patients with inflammatory bowel disease. Scand J Gastroenterol 1999; 34(1): 50–54.
23. Moum B, Jahnsen J, Bernklev T. Fecal calprotectin variability in Crohn’s disease. Inflamm Bowel Dis 2010; 16(7): 1091–1092. doi: 10.1002/ibd.21136.
24. Joishy M, Davies I, Ahmed M et al. Fecal calprotectin and lactoferrin as noninvasive markers of pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2009; 48(1): 48–54. doi: 10.1097/MPG.0b013e31816533d3.
25. Rogler G, Aldeguer X, Kruis W et al. Concept for a rapid point-of-care calprotectin diagnostic test for diagnosis and disease activity monitoring in patients with inflammatory bowel disease: expert clinical opinion. J Crohns Colitis 2013; 7(8): 670–677. doi: 10.1016/j.crohns.2013.02.014.
26. Walker TR, Land ML, Kartashov A et al. Fecal lactoferrin is a sensitive and specific marker of disease activity in children and young adults with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2007; 44(4): 414–422.
27. Schröder O, Naumann M, Shastri Y et al. Prospective evaluation of faecal neutrophil-derived proteins in identifying intestinal inflammation: combination of parameters does not improve diagnostic accuracy of calprotectin. Aliment Pharmacol Ther 2007; 26(7): 1035–1042.
28. Røseth AG, Aadland E, Grzyb K. Normalization of faecal calprotectin: a predictor of mucosal healing in patients with inflammatory bowel disease. Scand J Gastroenterol 2004; 39(10): 1017–1020.
29. D’Incà R, Dal Pont E, Di Leo V et al. Calprotectin and lactoferrin in the assessment of intestinal inflammation and organic disease. Int J Colorectal Dis 2007; 22(4): 429–437.
30. Sipponen T, Savilahti E, Kolho KL et al. Crohn’s disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn’s disease activity index and endoscopic findings. Inflamm Bowel Dis 2008; 14(1): 40–46.
31. Schoepfer AM, Beglinger C, Straumann A et al. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn’s disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol 2010; 105(1): 162–169. doi: 10.1038/ajg.2009.545.
32. Røseth AG, Aadland E, Grzyb K. Normalization of faecal calprotectin: a predictor of mucosal healing in patients with inflammatory bowel disease. Scand J Gastroenterol 2004; 39(10): 1017–1020.
33. Sipponen T, Bjorkesten CG, Farkkila M et al. Faecal calprotectin and lactoferrin are reliable surrogate markers of endoscopic response during Crohn’s disease treatment. Scand J Gastroenterol 2010; 45(3): 325–331. doi: 10.3109/00365520903483650.
34. van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ 2010; 341:c3369. doi: 10.1136/bmj.c3369.
35. Costa F, Mumolo MG, Ceccarelli L et al. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut 2005; 54(3); 364–368.
36. Orlando A, Modesto I, Castiglione F et al. The role of calprotectin in predicting endoscopic postsurgical recurrence in asymptomatic Crohn’s disease: a comparison with ultrasound. Eur Rev Med Pharmacol Sci 2006; 10(1): 17–22.
37. D’Haens G, Ferrante M, Vermeire S et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis 2012; 18(12); 2218–2224. doi: 10.1002/ibd.22917.
38. Aggio R, Probert C. Future methods for the diagnosis of inflammatory bowel disease. Dig Dis 2014; 32(4): 463–467. doi: 10.1159/000358153.
39. Bodelier A, Smolinska A, Dalinga J et al. Volatile organic compounds in breath as new test for Crohn's disease. United European Gastroenterology Journal 2013; 1 (Suppl 1): A36.
40. Surti B, Spiegel B, Ippoliti A et al. Assessing health status in inflammatory bowel disease using a novel single-item numeric rating scale. Dig Dis Sci 2013; 58(5): 1313–1321. doi: 10.1007/s10620-012-2500-1.
41. Bodger K, Ormerod C, Shackcloth D et al. Development and validation of a rapid, generic measure of disease control from patient’s perspective: the IBD-control questionnaire. Gut 2014; 63(7): 1092–1102. doi: 10.1136/gutjnl-2013-305600.
42. Keefer L, Kiebles JL, Taft TH et al. The role of self-efficacy in inflammatory bowel disease management: preliminary validation of a disease-specificmeasure. Inflamm Bowel Dis 2011; 17(2): 614–620. doi: 10.1002/ibd.21314.
43. Alrubaiy L, Rikaby I, Dodds P et al. Systematic review of health-related quality of life measures for imflammatory bowel diseases. J Crohns Colitis 2015; 284–292. doi: 10.1093/ecco-jcc/jjv002.
44. Capalbo SM, Heggem CN. Valuing rural health care: issues of access and quality. Am J Agri Econ 1999; 81(3): 674–679.
45. Berman M, Fenaughty A. Technology and managed care: patient benefits of telemedicine in a rural health care network. Health Econ 2005; 14(6): 559–573.
46. Krier M, Kaltenbach T, McQuaid K. Potential use of telemedicine to provide outpatient care for inflammatory bowel disease. Am J Gastroenterol 2011; 106(12): 2063–2067. doi: 10.1038/ajg.2011.329.
47. Elkjaer M. E-health: web-guided therapy and disease self-management in ulcerative colitis. Impact on disease outcome, quality of life and compliance. Dan Med J 2012; 59(7): B4478.
48. Huang VW, Reich KM, Fedorak RN. Distance management of inflammatory bowel disease: systematic review and meta-analysis. World J Gastroenterol 2014; 20(3): 829–842. doi: 10.3748/wjg.v20.i3.829.
49. Kim ES, Park KS, Cho KB et al. Development of a Web-based, self-reporting symptom diary for Crohn’s disease, and its correlation with the Crohn’s disease activity index: web-based, self-reporting symptom diary for Crohn’s disease. J Crohns Colitis 2014; S1873–9946(14)00268–2. doi: 10.1016/j.crohns.2014.09.003.
50. Degens J, Romberg-Camps M, Cilissen M et al. Results from a feasibility study with the telemedicine tool myIBDcoach in the Netherlands. J Crohns Colitis 2014; 8 (Suppl 1): S58. doi: 10.1016/S1873-9946(14)60115-X.
51. Atreja A, Khan S, Rogers JD et al. Impact of the Mobile healthPROMISE platform on the quality of care and quality of life in patients with inflammatory bowel disease: study protocol of a pragmatic randomized controlled trial. JMIR Res Protoc 2015; 4(1): e23. doi: 10.2196/resprot.4042.
Labels
Paediatric gastroenterology Gastroenterology and hepatology Surgery
Article was published inGastroenterology and Hepatology

2015 Issue 5-
All articles in this issue
-
XXIXth Hildebrand Bardejov gastroenterology days
Inflammatory diseases in case reports and well-arranged lectures - The selection from international journals
- Leaving
- A pilot experimental study of oesophageal stenosis after ESD
- Variable endoscopic appearance of early squamous cell carcinoma of the oesophagus
- First experience with digital Spyglass™ DS in Slovakia from the gastroenterology department of the Trnava University Hospital
- Endoscopic histologisation of diminutive colorectal polyps. Are we ready for a change?
- Quality of biopsies in patients with Barrett’s esophagus – jumbo vs. large capacity forceps
- Screening colonoscopy among elderly patients over 70 years
- Home parenteral nutrition – its importance and use in clinical practice
- Standard diagnostic and therapeutic approaches to chronic hepatitis C virus infection
- Ledipasvir/ sofosbuvir – rapid development of knowledge reduces treatment time
- Hands-on training of advanced endoscopic methods – an international workshop in Athens
- New approaches in the follow-up of patients suffering from inflammatory bowel diseases
-
XXIXth Hildebrand Bardejov gastroenterology days
- Gastroenterology and Hepatology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Endoscopic histologisation of diminutive colorectal polyps. Are we ready for a change?
- Screening colonoscopy among elderly patients over 70 years
- Home parenteral nutrition – its importance and use in clinical practice
- First experience with digital Spyglass™ DS in Slovakia from the gastroenterology department of the Trnava University Hospital
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

