-
Medical journals
- Career
Matrix metalloproteinases and their inhibitors in correlation to proliferative and classical tumour markers during surgical therapy of colorectal liver metastases
Authors: V. Liska 1; V. Treska 1; A. Sutnar 1; L. Holubec jr. 2,3; T. Skalický 1; M. Pesta 3; S. Kormunda 3; J. Fínek 2; M. Rousarova 2; O. Topolcan 3
Authors‘ workplace: Department of Surgery, Teaching Hospital and Medical School Pilsen, Charles University Prague, 2Department of Oncology, Teaching Hospital and Medical School Pilsen, Charles University Prague, 3Central Isotopic Laboratory, Teaching Hospital and Medical Sch 1
Published in: Gastroent Hepatol 2012; 66(1): 44-50
Category: Gastrointestinal Oncology: Original Article
Overview
Introduction:
A proliferative tumour markers and matrix metalloproteinases and their tissue inhibitors reflect the features of malignancy and they have a role in prognosis prediction in patients with colorectal metastases (CLM) in the liver. Their physiological functions during liver regeneration is not comptelety known.Methods:
Only patients those had been operated in our department between 11/2002 and 12/2004 were included. They underwent the following surgical procedures: Group (control) A: 22 patients with groin hernias, Group B: 26 patients with benign liver lesions, Group C: 30 CLM patients were treated by radiofrequency ablation, Group D: 41 patients with CLM those underwent radical liver surgery and Group E: 22 patients with inoperable CLM those underwent explorative laparotomy only. The preoperative and postoperative serum levels of CEA, CA19-9, K, TPA, TPS, MMP-2, MMP-9, TIMP-1 and TIMP-2 were measured.Results:
We proved a asignificat differences of serum levels MMP-2, -9; TIMP-2 and TPS, TK, MMP-2, TIMP-2 in a preoperative and postoperative settings respectively between control Group A and Group B. The comparison of control Group A and Group C displayed a significant differences in the preoperative serum levels of TPA, TPS, MMP-2, TIMP-2, CEA and CA19-9 and postoperative serum levels of TK, TPA, TPS, TIMP-1, -2, CEA, CA19-9 respectively. The comparison of control Group A and Group D demonstrated a significant diferences in the preoperative serum levels of TPA, TPS, MMP-2 and -9, TIMP-1, -2, CEA, CA19-9 and postoperative serum levels of TK, TPA,TPS, MMP-9, TIMP-1, -2, CEA, CA19-9 respectively. The comparison of control Group A with Group E proved a significant differences in the preoperative serum levels of TK, TPA, TPS, MMP-2 and -9, TIMP-1, -2, CEA, CA19-9 and postoperative serum levels of TK, TPA, TPS, MMP-2, TIMP-1, -2, CEA, CA19-9 respectively.Conclusion:
The retrospective analysis of serum matrix metalloproteinases and their tissues inhibitors in differenet group of patients has shown that serum levels of tumour markers are influenced significantly due to the regeneration and remodelling of healing tissues. The study findings support a critical view how to use these tumour markers for disease prognosis prediction in patients after surgical procedure for CLM.Key words:
tumour markers – liver surgery – behaviour of tumour markers –liver resection – radiofrequency ablation – prognostic factors – colorectal liver metastases – early recurrence – benign liver diseasesColorectal liver metastases (CLM) are the main secondary malignancy of the liver that liver surgery focuses on today. The main problem is not the operative technique or preoperative detection of malignant lesions but preoperative judgement of the planned operative procedure with regard to the patient's benefit, which is expressed by the disease-free interval, survival rate and quality of life. These parameters are confronted in all accessible treatment strategies: surgical vs. oncological, radical vs. palliative vs. symptomatic. The remaining and open question for each preoperatively discussed patient is early recurrence, which could shorten all the named parameters and so move our patients with regard to the benefit from a radically operated patient to palliative. All the undergone surgical procedures with their complications, duration of hospital stay and subsequent rehabilitation, morbidity and mortality have in the case of early recurrence very poor benefit! The question for today is: Is there any possibility to predict early recurrence with high accuracy?
The aim of the study was to assess the relation between tumour markers (MMP, TIMP, classical and proliferative tumour markers) and prediction of the recurrence and survival rate after liver surgery for CLM. The behaviour of particular tumour markers was studied in benign liver lesions during their surgical treatment and healthy patients without any malignancy or complicated comorbidity undergoing hernioplasty for groin hernias without affecting the abdominal cavity. The comparison of separate tumour markers in the studied cohorts of patients and the relevance between them should clear up their role in the recurrence and mechanism of tumour progression. The aim was not to study each tumour marker alone but in relation to other tumour markers especially with their other supposed function and mechanism of behaviour. Last but not least, the factor taken into account in this study was to study all these named tumour markers in the same patients and so uncover their relation in each patient. Only CLM patients were enrolled in this study so as to eliminate the influence of diverse malignant diseases (primary or secondary) with different behaviour.
Material and methods
In the presented study were included patients that were operated on at the Department of Surgery, University Hospital Pilsen, Charles University Prague between 11/2002 and 12/2004 and underwent subsequent surgical procedures for CLM, benign liver lesions and groin hernias.
The patients were divided into four groups for statistical analysis as follows:
Group A: patients with groin hernias, where classical hernioplasty was performed with usage of their own tissue without opening of the abdominal cavity (laparotomy or laparoscopy), without usage of mesh – prolene or goretex, and without any complication. The patients included in this group have no malignant or degenerative diseases in anamnesis. They also have no extensive polymorbidity including liver diseases and no inflammatory diseases six months before operation. This group was designed as manifesting the physiological function and expression of studied tumour markers under primary healing of the wound. 22 patients (11 men and 11 women) were included. Mean age was 56 years (16–78 years).
Group B: patients with benign liver lesions (cysts, focal nodular hyperplasia, hemangiomas, adenomas etc.). This contains 26 patients (8 men and 18 women) with mean age 54 years (33–83 years). The performed surgical procedures ranged from enucleation of lesion, fenestration of cysts, resection of maximally four segments).
Group C: 30 patients (22 men and 8 women) with CLM that were treated by radiofrequency ablation (RFA) with mean age 64 years (48–78 years).
Group D: 41 (29 men and 12 women) patients with CLM that underwent radical surgical therapy – resection. Mean age in this group was 61 years (45–82 years).
Group E: 22 patients (10 men and 12 women) with inoperable CLM that underwent explorative laparotomy without any surgical procedure. Mean age in this group was 63 (29–85 years).
The serum samples were obtained before (maximally 14 days before) and after the operation (maximally 14 days after). The performed surgical therapy was absolutely independent on the cohort membership – the patients with CLM were classified in the study cohort retrospectively.
All the blood samples for assessments of tumour markers, matrix metallo proteinases and their tissue inhibitors were taken in standard conditions during the morning hours from the cubital vein. The serum for assessment of routine tumour markers acquired through centrifugation was stored until laboratory analysis at a temperature of –20 °C. The serum for assessment of matrix metalloproteinases and their tissue inhibitors acquired through centrifugation was stored until laboratory analysis at a temperature of –75 °C. Tumour markers were assessed at the Dept. of Nuclear Medicine, Faculty Hospital Pilsen with commercial laboratory kits, in accordance with the manufacturers’ recommendations. The following tumour markers were evaluated: carcinoembryonic antigen (CEA - ng/mL, IRMA, Immunotech, CR), carbohydrate antigen 19-9(CA 19-9 - IU/L, Shering-CIS France), cytokeratines: tissue-specific polypeptide antigen (TPS - IU/L, IRMA, IDL Sweden), tissue polypeptide antigen (TPA-IU/L, IRMA, DiaSorin, Italy). Thymidine kinase (TK-IU/L) was measured by radioreceptor analysis (REA) using Immunotech (Prague) assay kits. Matrix metalloproteinases (MMP-2 - ng/mL, MMP-9-ng/mL) and their tissue inhibitors (TIMP-1 - ng/mL, TIMP-2-ng/mL) were assessed by ELISA methods (Chemicon - Millipore, USA). Serum levels of tumour markers were correlated with the clinical diagnosis of the patient.
Statistical analysis was performed with the use of CRAN statistical software. The statistical description parameters were used: average value, median, standard deviation, dispersion, interquartile interval, minimum value, maximum value. Non-parametrical Kruskal-Wallis and Wilcoxon tests were used for the statistical comparison of the distribution of particular parameters in the studied groups with regard to the distribution of these values.
Results
First, we estimated the normal serum levels of the studied tumour markers. For this, group A was used – patients with groin hernias. The optimal value of normal serum levels was taken as the 95th percentile of the particular serum level values. All the following groups were studied in this group A in the comparison of changes in serum levels evoked either by the disease or by surgical procedure. The results are presented in tab. 1–4.
Group B: the comparison of control group (A) with the group of benign liver lesions demonstrated the differences in the preoperative serum levels of MMP-2 and -9 and TIMP-2 (p-value < 0.016, 0.0002, 0.0001 respectively) and the postoperative serum levels of TPS, TK, MMP-2 and TIMP-2 (p-value < 0.018, 0.0012, 0.013 and 0.0001 respectively) as statistically significant (tab. 1).
1. The comparison of control group (A) with the group of benign liver lesions (group B). Tab. 1. Srovnání kontrolní skupiny A se skupinou B (benigní jaterní ložiskové léze). 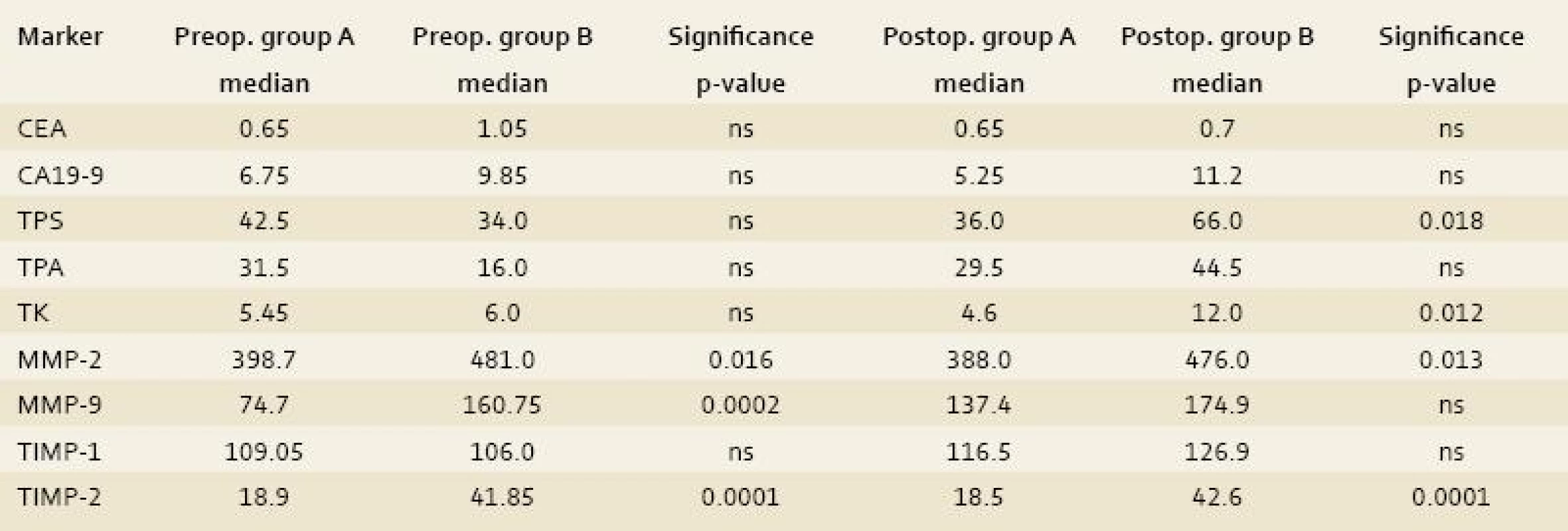
Group C: the comparison of the control group with the group of patients that underwent radiofrequency ablation of CLM demonstrated differences in the preoperative serum levels of TPA, TPS, MMP-2 ,TIMP-2, CEA and CA19-9 (p-value < 0.0018, 0.0246, 0.007, 0.001, 0.001 and 0.0219 respectively) and the postoperative serum levels of TK, TPA, TPS, TIMP-1 and -2, CEA and CA19-9 (p-value < 0.0082, 0.0018, 0.0001, 0.002, 0.001 and 0.001, 0.0007 respectively) as statistically significant (tab. 2).
2. The comparison of the control group (A) with the group of patients that underwent radiofrequency ablation of CLM (group C). Tab. 2. Srovnání kontrolní skupiny A se skupinou pacientů, kteří podstoupili radiofrekvenční ablaci CLM (C). 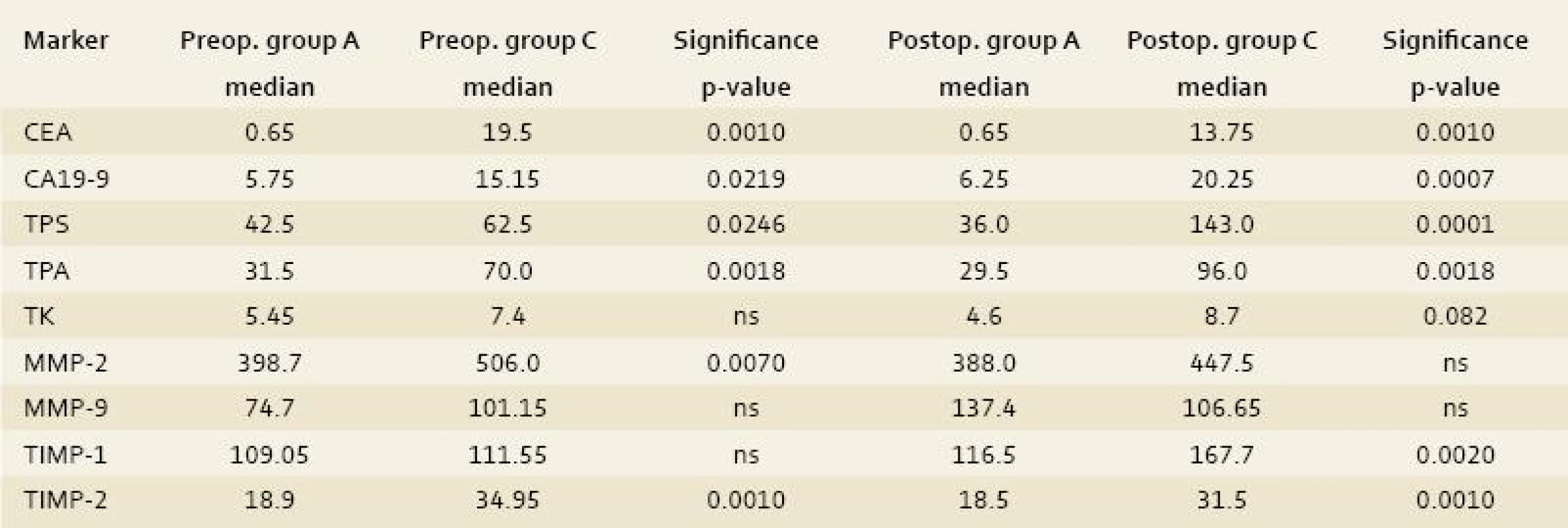
Group D: the comparison of the control group with the group of patients that underwent radical surgical procedure for CLM demonstrated differences in the preoperative serum levels of TPA, TPS, MMP-2 and -9, TIMP-1 and -2, CEA and CA19-9 (p-value < 0.0007, 0.0007, 0.02, 0.003, 0.04, 0.001, 0.001 and 0.0012 respectively) and the postoperative serum levels of TK, TPA, TPS, MMP-9, TIMP-1 and -2, CEA and CA19-9 (p-value < 0.0001, 0.0014, 0.0001, 0.001, 0.003, 0.001, 0.001 and 0.008 respectively) as statistically significant (tab. 3).
3. The comparison of the control group (A) with the group of patients that underwent radical surgical procedure for CLM (group D). Tab. 3. Srovnání kontrolní skupiny A se skupinou pacientů, kteří podstoupili radikální chirurgický výkon pro CLM (D). 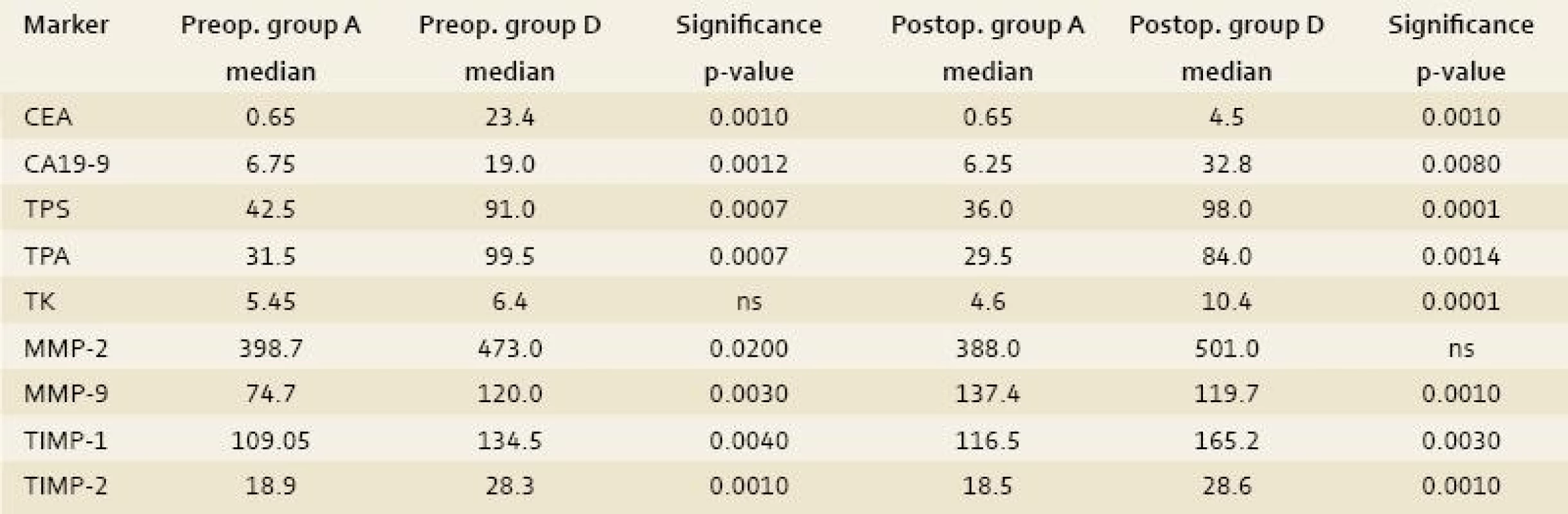
Group E: the comparison of the control group with the group of patients that underwent explorative laparotomy for inoperable CLM displayed differences in the preoperative serum levels of TK, TPA, TPS, MMP-2 and -9, TIMP-1 and -2, CEA and CA19-9 (p-value < 0.0032, 0.0001, 0.0001, 0.004, 0.007, 0.0007, 0.001, 0.0011 and 0.0001 respectively) and the postoperative serum levels of TK, TPA, TPS, MMP-2, TIMP-1 and -2, CEA and CA19-9 (p-value < 0.0119, 0.0016, 0.0052, 0.008, 0.0003, 0.0002, 0.0008 and 0.0001 respectively) as statistically significant (tab. 4).
4. The comparison of the control group with the group of patients that underwent explorative laparotomy for inoperable CLM (group E). Tab. 4. Srovnání kontrolní skupiny A se skupinou pacientů, kteří podostupili explorativní laparotomii pro inoperabilní CLM (E). 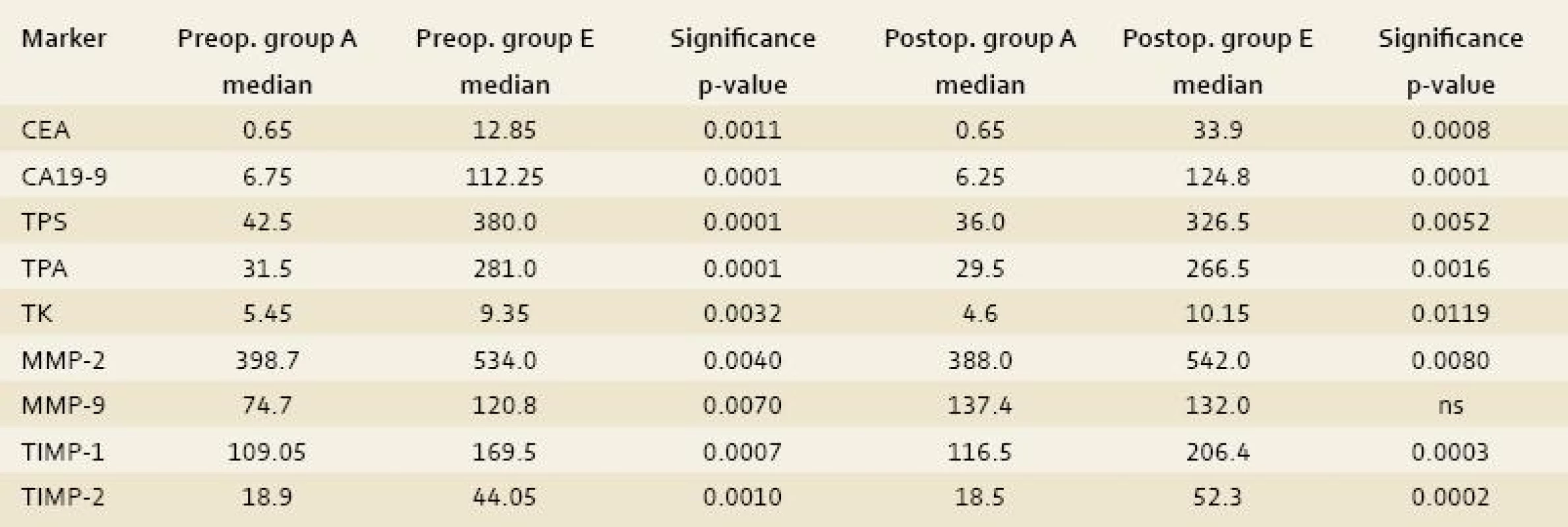
Discussion
Matrix metalloproteinases (MMP) and Tissue inhibitors of metalloproteinases (TIMP) have been implicated not only in tumour invasion but also in tissue remodelling, especially in rebuilding the extracellular matrix and in regeneration processes and inflammatory responses [1]. The levels of MMPs and TIMPs have also been proven to correlate to tumour aggression and progression [2–4]. The activity of MMP-2 and -9 and TIMP-1 is overexpressed in the tumour mass of colorectal cancer contrary to surrounding healthy colon tissue [5]. Contrary TIMP-2 levels are controversial. Baker observed significantly greater levels in normal colon tissue [5] but Murashige did not [6]. The upregulated levels of MMPs and TIMPs also correlate to aggression or recurrence in the metastatical process of colorectal cancer especially in colorectal liver metastases (CLM) [7,8]. Elevation of MMP-9 is connected with the invasivity of colorectal cancer [9]. Successive increase of tissue expression of MMP-2, -7 and -9 relates with an increase in malignancy – from mucous lesions and adenomas to carcinomas [9].
MMP-2 and -9 have been shown to be overexpressed by the stroma surrounding the tumour [10,11]. This could be an explanation of the mechanism of the invasivity of the malignancy. The same mechanism of degradation of the extracellular matrix (basement membrane) occurs in the initiation of angiogenesis, which is crucial for growth of the tumour mass [9].
Expression of TIMP-1 is tied with invasivity [12] or extension [13] of colorectal cancer. TIMP-1 is produced by fibroblast-like cells in the invading cancer. The tumour surrounding mucosis has almost no production of TIMP-1. TIMP-1 is not secreted by benign or malignant cells, cells of vessels or muscle cells [12]. MMPs reflect not only the penetration of malignancy into the surrounding healthy tissue through the increased destruction of matrix and new synthesis of tumour stroma but also influence the tumour growth secondary by releasing cytokines (transforming growth factor alpha and Insulin-like growth factor – II) bound in extracellular matrix in the inactive form and activated just through release [14]. MMPs participate in other processes influencing primary tumour growth, angiogenesis and invasion, intra - and extravasation of metastatical cells and growth of the metastatical process [15]. The synchronous determination of the levels of MMP-9 in portal and peripheral blood is useful for selecting colorectal cancer patients at high risk of hepatic recurrence [16]. TIMP-1 and -2 and MMP-2 were expressed during tissue regeneration in the rat while MMP-3,-9, -10, -13 and -14 were not [17]. Hanke et al conclude that serum MMP-2 appears to reflect tumour resorption, while serum TIMP-1 may mirror tumour expansion [23]. The findings of Mroczko et al suggest greater usefulness of serum TIMP-1 than MMP-9 in the diagnosis of CRC, especially in the assessment of Duke’s classification of tumour stage, survival of cancer patients, and resectability of tumour [24]. Groblewska et al proved in patients with CLM the highest diagnostic sensitivity of TIMP-2 among the biomarkers tested and increased in combined use with CEA-79% at 95% specificity [25]. Dragutinovic et al proved that the overexpression of MMP-2 and MMP-9 strongly suggests its association with colorectal adenocarcinoma. Detection of MMP-2 and MMP-9 in serum might be useful for the identification of patients with higher risk of colorectal cancer recurrence [26].
The role of classical tumour markers is generally accepted in the prediction of relapse or survival rate in patients after liver surgery for colorectal cancer [18,19]. The serum levels of proliferative tumour markers (TPS, TPA, TK) have been used as good prognostic factors for the aggression of tumour and recurrence of CLM after liver surgery. Their relation to survival rate is more unsure [20–22].
In our study, we focused on the influence of the type of surgical procedure at the serum level of the studied tumour markers during operations.
In group A: the estimated serum levels of studied tumour markers in control group A were used for analysis of other cohorts of patients with benign or malignant liver diseases. The surgical procedure (incisional groin hernia repair) had to serve as the physiological background of normal wound healing. We studied the influence of serum levels of the studied tumour markers to differentiate the changes during other more extensive surgical procedures in liver parenchyma. Some elevations or depressions during the operation in serum levels in group A were interpreted through the pleiotropic function of the named tumour markers during the initial period of wound healing and regeneration of non-liver tissues.
In group B: the differences in preoperative and postoperative changes between the group of benign liver lesions and the control group could be explained by the activity of tissue of benign lesions, which could express some features of precancerosis and sometimes malignant inversion (liver adenomas) or increased proliferative activity (focal nodular hyperplasia) could be observed. Inconsiderable is also the influence of the large active surface of endothelia in hemangiomas, especially cavernomatous. These differences between group A and B are not comparable with increases in the groups with liver malignancy. In the case of benign liver diseases we could also hypothesize about the influence of the activity of liver parenchyma in the immediate surroundings of the benign lesion. This could be irritated by insufficient biliary drainage and underlies ischemia as a consequence of the pressure of the benign lesion (cyst, hemangioma). This could contribute to remodelling of the tissue or changes in functions and elevations of the named tumour markers compared to the control group. These first two discussed groups (A and B) serve as essential cohorts, which had to eliminate the influence of changes in serum levels during surgical procedures, which reflect the physiological function of metalloproteinases, their inhibitors and other studied tumour markers. These differences prove our hypothesis that also in benign liver lesions, the regulated serum levels of the studied tumour markers could be raised.
In group C: the statistical analysis of the studied tumour markers in group C (radiofrequency of CLM) brought valuable results. These were influenced by the retained destroyed tumour tissue, which could release tumour markers in blood circulation not only during its thermic destruction but also in the postoperative period during remodelling of this destroyed lesion and the creation of scarring. For this hypothesis a nearly unchanged serum level of CEA is crucial. In the studied metalloproteinases, their inhibitors and proliferative tumour markers an absolute elevation in their serum levels in the postoperative period is observed. This could be explained by their release from tissue deposits or by fibrogenesis and proliferation in scarring lesion and their immediate surroundings.
In group D: the classical tumour markers reacted in the elimination of malignant lesion by important reductions in their serum levels. This confirms the relation of classical tumour markers to the volume of the tumour mass. The metalloproteinases and their inhibitors or proliferative tumour markers, which reflect the aggression, invasivity and lateness of CLM [20], were elevated after radical liver surgery. In the case of resection, we could also hypothesize the persisting activity of the named tumour markers in serum. We could also not omit the participation of these tumour markers in the regeneration and remodelling of liver parenchyma after resection as a reaction to changes in the functional reserves of the remaining liver parenchyma. The liver parenchyma in the immediate surroundings of the resection surface could also increase the expression of these tumour markers as a reflection of its remodelling because of changes in its blood supply and biliary drainage.
In group E: most of the studied tumour markers continued in the same expression of their serum activity after explorative laparotomy for inoperable CLM. This could be explained by the minimal influence of the performed laparotomy, which is a standard component of other surgical procedures (RFA, liver resections). We could confirm the gain that changes in groups C and D result from surgical procedure in liver parenchyma. The group of patients with performed explorative laparotomy serves as another comparative group, this time with tumour without any intervention in this mass [21,22].
Conclusion
These results reflect the influence of the type of surgical procedure on the serum level of the studied tumour markers during operation. The authors tried to negate the influence of the physiological activity of these tumour markers, which is supposed from their pleiotropic functions in the regeneration and remodelling of healing tissues. It was first described by using these types of comparison of all metalloproteinases, their inhibitors, proliferative and classical tumour markers. It could help us to find the critical relation of these tumour markers for the prognosis of disease-free survival or overall survival in patients after surgical procedure for CLM.
This article was supported by grants IGA MZ CR NR 10230, 9731, 10240 and VZ MSM 0021620819.
Abbreviations
CA 19-9 – carbohydrate antigen 19-9
CEA – carcinoembryonic antigen
CLM – colorectal liver metastases
DFI – disease-free interval
MMP – matrix metalloproteinase
RFA – radiofrequency ablation
ROC – receiving operative curves
TIMP – tissue inhibitor of metalloproteinases
TPA – tissue polypeptide antigen
TPS – tissue specific polypeptide antigen
TK – thymidine kinaseAutoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
The Editorial Board declares that the manuscript met the ICMJE „uniform requirements“ for biomedical papers.Doručeno/Submitted: 10. 1. 2011
Přijato/Accepted: 25. 11. 2011
Václav Liška M.D.
Department of surgery
University Hospital Pilsen
Alej svobody 80, 304 00 Pilsen
Vena.Liska@seznam.cz
Sources
1. Curran S, Murray GI. Matrix metalloproteinases: molecular aspects of their roles in tumour invasion and metastasis. Eur J Cancer 2000; 36 (13 Spec No): 1621–1630.
2. Curran S, Murray GI. Matrix metalloproteinases in tumour invasion and metastases. J Pathol 1999; 189(3): 300–308.
3. Matsuyama Y, Takao S, Aikou T. Comparison of matrix metalloproteinases expression between primary tumours with or without liver metastases in pancreatic and colorectal carcinomas. J Surg Oncol 2002; 80(2): 105–110.
4. Zeng ZS, Huang Y, Cohen AM et al. Prediction of colorectal cancer relapse and survival via tissue RNA levels of matrix metalloproteinases-9. J Clin oncol 1996; 14(12): 3133–3140.
5. Baker EA, Bergin FG, Leaper DJ. Matrix metalloproteinases, their tissue inhibitors and colorectal cancer staging. Br J Surg 2000; 87(9): 1215–1221.
6. Murashige M, Miyakara M, Shiraishu N et al. Enhanced expresssion of tissue inhibitors of metalloproteinases in human colon tumours. Jpn J Clin Oncol 1996; 26(5): 303–309.
7. Bird NC, Mangnall D, Majeed AW. Biology of colorectal liver metastases: A review. J Surg Oncol 2006; 94(1): 64–80.
8. Yukawa N, Yoshikawa T, Akaike M et al. Plasma concentration of tissue inhibitor of matrix metalloproteinases 1 in patients with colorectal cancer. Br J Surg 2001; 88(12): 1596–1601.
9. Heslin MJ, Yan J, Johnson MR et al. Role of matrix metalloproteinases in colorectal carcinogenesis. Ann Surg 2001; 233(6): 786–792.
10. Pyke C, Ralfkiaer E, Tryggvason K et al. Messenger RNA fo two type IV collagenases is located in stromal cells in human colon cancer. Am J Pathol 1993; 142(2): 273–282.
11. Wagenaar-Miller R, Gorden L, Matrisian LM. Matrix metalloproteinases in colorectal cancer: Is it worth talking about? Cancer and Metastatic Reviews 2004; 23(1–2): 119–135.
12. Holten-Andersen MN, Stephens RW, Nielsen HJ et al. High preoperative plasma tissue inhibitor of metalloproteinase-1 levels are associated with short survival of patients with colorectal cancer. Clin Cancer Res 2000; 6(11): 4292–4299.
13. Ishida H, Murata N, Hayashi Y et al. Serum levels of tissue inhibitor of metalloproteinases-1 in colorectal cancer patients. Surg Today 2003; 33(12): 885–892.
14. Chau I, Rigg A, Cunningham D. Matrix metalloproteinases inhibitors – an emphasis on gastrointestinal malignancies. Cri Rev Oncol Hematol 2003; 45(2): 151–176.
15. Chambers AF, Matrisian LM. Changing views of ther role of matrix metalloproteinases in metastasis. J Natl Cancer Inst 1997; 89(17): 1260–1270.
16. Ishida H, Murata N, Tada M et al. Determining the levels of matrix metalloproteinases-9 in portal and peripheral blood is useful for predicting liver metastases of colorectal cancer. Jpn J Clin Oncol 2003; 33(4): 186–191.
17. Knittel T, Mehde M, Grundmanc A et al. Expression of matrix metalloproteinases and their inhibitors during hepatic tissue repair in the rat. Histochem Cell Biol 2000; 113(6): 443–453.
18. Duffy MJ, van Dalen A, Haglund C et al. Clinical utility of biochemical markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines. Eur J Cancer 2003; 39(6): 718–727.
19. Liska V, Holubec L Jr., Treska V et al. Dynamics of serum levels of tumour markers and prognosis of recurrence and survival after liver surgery for colorectal liver metastases, Anticancer Res 2007; 27(4C): 2861–2864.
20. Liska V, Holubec L, Treska V et al. Tumor markers as useful predictors of survival rate after explorative laparotomy for liver malignancies. Anticancer Res 2007; 27(4A): 1887–1892.
21. Sutnar A, Pesta M, Liska V et al. Clinical relevance of the expression of mRNA of MMP-7, MMP-9, TIMP-1 and TIMP-2 tissue samples from colorectal liver metastases. Tumor Biol 2007; 28(5): 247–252.
22. Kijima M, Togo S, Ichikawa Y et al. Clinical significance of serum CEA protein and CEA mRNA after resection of colorectal liver metastases. Anticancer Res 2005; 25(2B): 1327–1332.
23. Hanke B, Wein A, Martus P et al. Serum markers of matrix turnover as predictors for the evolution of colorectal cancer metastasis under chemotherapy. Br J Cancer 2003; 88(8): 1248–1250.
24. Mroczko B, Groblewska M, Okulczyk B et al. The diagnostic value of matrix metalloproteinase 9 (MMP-9) and tissue inhibitor of matrix metalloproteinases 1 (TIMP-1) determination in the sera of colorectal adenoma and cancer patients. Int J Colorectal Dis 2010; 25(10): 1177–1184.
25. Groblewska M, Mroczko B, Gryko M et al. Matrix metalloproteinase 2 and tissue inhibitor of matrix metalloproteinases 2 in the diagnosis of colorectal adenoma and cancer patients. Folia Histochem Cytobiol 2010; 48(4): 564–571.
26. Dragutinović VV, Radonjić NV, Petronijević ND. Matrix metalloproteinase-2 (MMP-2) and -9 (MMP-9) in preoperative serum as independent prognostic markers in patients with colorectal cancer. Mol Cell Biochem 2011 May 4. Electronic publication.
Labels
Paediatric gastroenterology Gastroenterology and hepatology Surgery
Article was published inGastroenterology and Hepatology
2012 Issue 1-
All articles in this issue
- Journal at the beginning of 66th annual volume: the current state and perspectives
- News from the IBD world
- A word to new subscribers to the journal – members of the Czech Hepatological Society ČLS JEP
- Guidelines for the administration of biological therapy in patients with inflammatory bowel diseases: 2nd edition
- Gastrocolic fistula
- Endoscopic submucosal dissection of early gastric cancer
- Bowel preparation for colonoscopy
- Václav Havel – a look back
- What did Václav Havel for us and what can we do for his heritage?
- Serum levels of infliximab and antibodies to infliximab, clinical using
- The role of vitamin D for inflammatory bowel diseases
-
How important is the multidisciplinary approach to the colorectal cancer patients?
The 1st National Colorectal Cancer Congress/1. Postgraduate Course of SGO report - New standards in Crohn’s disease treatment
- Recollection from Gastrofórum
- Low serum deoxyribonuclease I activity is associated with antiTNF-alpha induced skin adverse events in patients with inflammatory bowel disease
- Matrix metalloproteinases and their inhibitors in correlation to proliferative and classical tumour markers during surgical therapy of colorectal liver metastases
- Gastroenterology and Hepatology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Bowel preparation for colonoscopy
- Gastrocolic fistula
- Serum levels of infliximab and antibodies to infliximab, clinical using
- The role of vitamin D for inflammatory bowel diseases
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

