-
Medical journals
- Career
Immunohistochemistry and serum values of S-100B, glial fibrillary acidic protein, and hyperphosphorylated neurofilaments in brain injuries
Authors: D. Vajtr 1,2; M. Filip 3; O. Benada 4; P. Linzer 3; F. Šámal 3; D. Springer 5; P. Strejc 1; M. Beran 6; R. Průša 2; T. Zima 5
Authors‘ workplace: Department of Forensic Medicine and Toxicology, Charles University 1st Medical Faculty , Prague. 1; Department of Clinical Biochemistry and Pathobiochemistry, Charles University 2nd Medical Faculty and University Hospital Motol, Prague. 2; Department of Neurosurgery, KNTB Zlin. 3; Laboratory of Molecular Structure Characterization, Institute of Microbiology, Academy of Sciences of the Czech Republic, Vídeňská 1083, CZ-142 20 Prague, Czech Republic 4; Department of Clinical Biochemistry and Laboratory Diagnostics, Charles University 1st Medical Faculty and General Teaching Hospital, Prague. 5; Department of Forensic Medicine, Charles University 2nd Medical Faculty and University Hospital Motol, Prague. 6
Published in: Soud Lék., 57, 2012, No. 1, p. 7-12
Category: Original Article
Overview
Introduction:
Traumatic brain injury (TBI) triggers a series of reactions resulting in cytoskeletal-related changes varying between focal and diffuse injuries. Methods: The patients (n=38) were divided into group of diffuse axonal injuries (DAI, n=10) and focal (n=28) injuries. Serum hyperphosphorylated neurofilaments (NF-H) and glial fibrillary acidic protein (GFAP) were measured by Biovendor immunoassay, and serum S-100B protein was measured by Cobas e411 (Roche) by immunoassay. Immunohistochemistry was performed with monoclonal antibodies (Chemicon, USA).Results:
The median serum S-100B concentration was higher in patients with focal mass lesions (1.72±0.4 μg/l vs. 0.37±0.1 μg/l, p<0,05) compared to patients with DAI during 10 days of hospitalisation. With respect to all patients, the highest peak of serum S-100B values (4.21±1.1 μg/l) and GFAP (8.58±2.4 μg/l) were found in expansive lesions. The median serum NF-H was higher in DAI compared to focal TBI (0.625±0.14 vs 0.139±0.02 ng/l, p<0.05) during all 10 days after admission. Further, immunohistochemical investigation, in deceased patients with DAI , using NF-H antibody proved positive varicose and waving axons, and retraction balls. Time-dependent profile of serum NF-H demonstrated the increase of values within 4th up to 10th day in both groups. Values ranged from 0.263 up to 1.325 ng/l in DAI, and from 0.103 up to 1.108 ng/l in focal injuries. Patients with expansive contusions had similar levels of serum NF-H as patients without expansive lesions. Immunohistochemistry of cytoskeletal proteins presented strong positive staining of vinculin, vimentin in vessels, GFAP, and S-100B in DAI compared to weak staining in expansive lesions.Conclusion:
The time-profile kinetics of all markers may reflect different types of pathophysiological changes of the BBB or axonal damage in focal and diffuse injuries.Keywords:
brain contusions – diffuse axonal injury – S-100B protein – GFAP – hyperphosphorylated neurofilaments.The outcome of patients with traumatic brain injury (TBI) is substantially dependent upon the development of the secondary brain injury. Secondary TBI is, in part, caused by blood brain barrier (BBB) impairment, manifested by astroglial or endothelial failure. Astroglial endfeet surrounding capillary lumen influence the tightness, permeability, and trafficking function of the BBB (1,2). The opening of BBB is caused by ischemia (3,4), by trauma (5,6), by astroglial disorders (7,8,9,10), and by inflammatory response following injury (11).
Specific proteins released to the circulation after trauma can reflect the severity of TBI, and allowed to predict the outcome of patients follow-up 6 months (12,13). An calcium binding astroglial protein S-100B is marker of TBI, and several studies established the relevance of S-100B in blood as a marker of brain damage after traumatic brain injury (13). Glial fibrillary acidic protein (GFAP) is a structural glial protein with a higher molecular weight (52 kDa) than protein S-100B (20 kDa) and the release of GFAP from brain to the circulation may be different from S-100B (14). GFAP and vimentin, an intermediate filament protein vimentin found by many authors in pathology of the brain, who noted the role of vimentin in the formation of glial scars (13), reflect reactive gliosis. The astrogliosis is accompanied by microglial activation, evaluated by CD-68 staining (15), in the TBI. Microglia might support the onset of astrogliosis, but might delay or reduce subsequent glial scar formation (16).
In terms of clinical use, Pelinka (17) reported lower serum GFAP concentrations in diffuse injury than in mass lesions, and higher for Glasgow outcome scale (GOS) of 1 or 2 than for GOS of 3, 4 (p<0.05), or 5 (p<0.0005). After TBI, GFAP levels in blood were found higher in nonsurvivors when compared to survivors, however after multiple trauma without TBI, GFAP remained normal (17). S-100B was higher in non-survivors (p<0.0005) than survivors. S-100B was lower in focal lesions of less than 25 ml volume than in non-evacuated mass lesions (p<0.0005) and lower in swelling than in midline shifts of more than 5 mm (p<0.005). Both GFAP and S-100B were accurate for mortality prediction (18). S-100B cutoff value between 2.0 and 2.5 μg/l predicted unfavorable outcome (19,20).
Onaya M investigated diffuse brain injuries (21), and whether there was a widespread axonal disruption of cerebral white matter in patients with diffuse axonal injury (DAI). The experimental DAI in animals revealed mild oedema, significant axonal injury, reactive astrogliosis, and localized midline cerebellar hemorrhage (22). Pittella proved in focal TBI axonal injuries in 89.5 % cases at intensive care unit (23).
Immunoassay for measuring serum hyperphosphorylated neurofilaments (NF-H) can monitor axonal disruption in patients with DAI (24), and the levels of serum proteins S-100B, GFAP and NF-H can reflect BBB permeability (5,6,25). Kovesdi summarized the influence of clinical use of biomarkers NSE, S-100B, and GFAP (26).
In progressing expansive contussions the P-selectin facilitates tethering of circulating leukocytes and did their migration into the contusion tissues. The mechanism of upregulation of adhesion molecules such as intercellular adhesion molecule (ICAM), E-selectin, and P-selectin contributes to promotion of cell death (27) under the hypoxic conditions.
Aim of the study was to analyze the ultrastructural and immunohistochemical BBB impairment and axonal damage in focal and diffuse brain injuries related to monitoring of diffuse and focal brain trauma by measuring of serum S-100B, NF-H, and GFAP concentrations.
SUBJECTS AND METHODS
Group of patients: The enrolled patients (n=38) were divided into group of diffuse axonal (DAI, n=10) and focal (n=28) brain injuries. The patients with diffuse axonal injuries were diagnosed with signs of DAI using nuclear magnetic resonance (NMR) performed 2–3 weeks after injury. All patients were also examined by cranial computerized tomography (CT). The patients with focal TBI were divided into two subgroups according to the following CT-scan findings: (1) patients showing predominantly expansive cortical contusions (n=12) submitted to neurosurgery; (2) patients (n=16) without expansive process. The CT scan classification utilizes the degree of midline shift in millimeters and presence or absence of intracerebral haematoma. Patients submitted to neurosurgery for expansive contusion were diagnosed with midline shifts more than 10 mm, mydriasis, and an intracranial pressure (ICP) elevation more than 10 torr. 5 pacients with focal TBI died. Samples (n=7) from autopsy of patients with diagnosies of DAI were obtained with agreement of Department of Forensic Medicine.
Informed consent: The ethics committee at each institution approved this study, and written informed consent was obtained from surviving patients.
Biochemical investigation: Blood samples were collected each day for 10 days after admission to the hospital. Serum S-100B protein concentrations were measured on immunoassay analyzer Roche Cobas e411 by electrochemiluminescence immunoassay (Roche, Switzerland). The reference range of S-100B serum concentrations of healthy subjects is reported to be less than 0.12 μg/l. GFAP was measured by Biotrak activity assay system (GE Healthcare, USA). Serum NF-H was measured by ELISA immunoassay (Biovendor). Serum concentrations of NF-H in healthy subjects is reported to be negative.
Electron microscopy: Cortical biopsies were carried out in 10 patients with craniocerebral trauma submitted to surgery for expansive lesion. The tissue samples from the pericontusional zone on the border line of necrotic contusion tissue were fixed in 3% glutaraldehyde in cacodylate buffer and postfixed with 2% osmium tetroxide. After dehydration in alcohol series, the samples were embedded into Epon resin in accordance with standard procedure. The stained ultrathin sections were examined in Philips CM100 electron microscope (FEI, formerly Philips EO, the Netherlands) and selected areas were digitally recorded using MegaViewII slow-scan camera. The digital images were processed in Analysis 3.2 Pro software.
Immunohistochemistry: Formalin-fixed cortical biopsies and also tissues obtained during autopsies of patients who died and patients submitted to neurosurgery were cut coronally in a way as to make paraffin-embedded sections, which were labeled with anti-GFAP, anti-vimentin, anti-NF-H, anti-vinculin, anti-S100, anti-VEGF, anti-CD68, and anti-P-selectin glycoprotein ligand antibodies (Chemicon). The colour was developed using a chromogenic substrate di-amino-benzidine (DAB).
Statistical methods: Differences in S-100B, GFAP, and NF-H concentrations between groups were tested by the unpaired t-test. Differences at p<0.05 were considered statistically significant. ROC test was based on the Mann-Whitney statistic according to DeLong ER, 1988. Receiver operating characteristic curves (ROC) were applied for the analysis of predictive power of expansive progresive contusion. S-100B value was taken as endpoint for the predictive analyses.
RESULTS
Biochemical results of S-100B, GFAP, and NF values in focal and diffuse TBI
Serum values of S-100B, GFAP, and NF-H over 10 days of sampling of 38 included patients in this study are summarized in Table 1. The cut-off levels for the proteins were clearly higher in focal mass lesions than the reference value of healthy subjects (reference value for S-100B 0.12 μg/l and for GFAP 0.49 μg/l reported by Vos et al.). Table 1 shows the release patterns of the proteins under study in patients with different cerebral trauma pathology according to the time profile. For all patients, the peak value was found in expansive cerebral contusions for S-100B and GFAP proteins, and the highest value of NF-H was found in DAI and non-expansive contusions. The decrease of S-100B and GFAP value is showed in both groups. The median serum S-100B and GFAP concentrations were higher in patients with focal mass lesions (1.72±0.4 vs. 0.37±0.1 μg/l for S-100B, p<0.05, and 4.21±1.1 vs. 0.49±0.3 μg/l for GFAP, p<0.05) compared to patients with DAI. Similarly, patients with expansive contusions had higher concentrations of S-100B and GFAP than did patients with non expansive contusions treated without neurosurgery. The S-100B and GFAP levels increased in focal mass lesions during the first 3 days after admission, but during the 4th to 10th days after admission they decreased dramatically. ROC analysis of S-100B protein and GFAP for expansive contusion revealed that the cut-off value of S-100B >1.82 μg/l had sensitivity 93 % and specificity 55 %, and the cut-off value of GFAP >3.24 μg/l had sensitivity 58 % and specificity 83 % for expansive contusions. The median serum NF-H was higher in DAI compared to focal TBI (0.625±0.14 vs. 0.139±0.02 ng/l, p<0.05) with respect to all data collected within 10 days. We did not established any significant differences between the groups of 1–3 and 4–10 day. The time-dependent profile of serum NF-H revealed an increase from the 4th to the 10th days in both groups. Values ranged from 0.263 ng/l up to 1.325 ng/l in DAI, and from 0.103 ng/l up to 1.108 ng/l in focal brain injuries. Patients with expansive contusions, and non-expansive contusions had similar levels of serum NF-H.
Ultrastructural findings of BBB in progressive expansive contusions:
Ultrastructural changes of BBB were studied by electron microscopy in patients submitted to neurosurgery due to expansive contusion, in order to analyze whether focal lesions are associated with the brain barrier failure. In the early stages (patients submitted to neurosurgery up to 24–48 hours after admission) an ultrastructural investigation of astroglial cells revealed the loss of proteins in their endfeet surrounding the capillary wall. Cytotoxic oedema of astroglial cells was formed, as well as swelling of astrocytic endfeet. The loss of fibrillary proteins was confirmed by immunohistochemistry. Glycogen-rich and glycogen-depleted end-feet were observed and tight junction were found to be disconnected (Fig.1) in patients without GCS improvement). The mean arterial blood flow of brain contusions tissues kept supplied by oxygen (Fig.2) were monitored by transcranial color-coded Doppler sonography (TCCD).
Fig. 1. 33 years old man suffered TBI as a car driver who was brought to stop from high speed by striking of his head on the windscreen frame. After admission in the hospital GCS was 5 points, during 4 days he developed progresive expansive contusion in frontal lobe, and was submitted to neurosurgery. Photomicrograph of the surgical specimens shows a tight junctions (asterisk) disconnection between endothelial cells (see zonulae occludens between endothelial cells in detail (arrowhead). Changes of the BBB appeared within 96 hours. BBB failure allowed Red cells to pass through capillary into the surrounding stroma (magnification 20000x). 
Fig. 2. Repeated vasospasm of cerebral arteries refractery to calcium chanel blocator drugs was proved by way of postoperative transcranial Doppler sonography (TCCD). 
Ultrastructural changes of axons and synaptic clefts in expansive contusions:
Axonal changes (Fig.3) in areas adjacent to expansive cortical contusions appeared within 24–48 hours including the accumulation of multivesicular bodies (Fig.4). Axonal changes and synaptic alterations were demonstrated. The disappearing synaptic vesicules were observed in synaptic clefts, and an increase in synaptic densities at the postsynaptic membranes was proved. Neuroaxonal degenerations of mitochondrial were monitored inside button-like enlargements.
Fig. 3. Photomicrograph of the injured axon (The same case as reported in Fig.1) shows a neuroaxonal degenerations. Lesions in axon were evaluated by electron microscopy (magnification 20000x) proving mitochondrial degenerations (asterisk) inside button-like enlargements with numerous presynaptic vacuoles (arrowhead). 
Fig. 4. Numerous enlarged wave-like axons (magnification 15000x) were filled with accumulated multivesicular bodies (MVB see asterisk) and were crowded by vacuoles (arrowhead). Axoplasmic transport was locally altered by injury, or hypoxia damaged tissues related to vasospasms (see fig.2). 
Immunohistochemical changes of cytoskeletal proteins and S-100 in DAI and expansive contusions:
Immunohistochemical investigation proved the loss of immunopositivity of cytoskeletal proteins (GFAP, vinculin and vimentin) in expansive cortical contusions in patients submitted to neurosurgery within 24–48 hours in contrast to the local strong immunoreactivity of anti-GFAP and anti-vimentin antibodies in astroglial cells in DAI adjacent to the gliding contusions. GFAP (+) astrogliosis was found in small non-expansive contusions of patients with DAI, and lower immunoreactivity was also revealed in patients with focal lesions. Immunopositivity of anti-S100 protein was particularly found in cases of DAI compared to loss of immunopositivity of anti-S100 antibody in expansive contusions. Immunohistochemistry of white matter in DAI demonstrated the immunopositivity of anti-NF (Fig.5) in both wave-like and varicose axons. Vinculin distribution as a junctional molecule was particularly observed in DAI.
Fig. 5. Immunoreactivity with anti-NF antibody was demonstrated in the injured varicose and wave-like axons (asterisk) and the forming of retraction ball was revealed (arrowhead). Lesion in corpus callosum, in patients with DAI, was haemorrhagic called as gliding contusions (magnification 400x). 
P-selectin glycoprotein ligand, CD68 expression and inflammatory response in diffuse and focal TBI:
P-selectin glycoprotein ligand (PSGL) immunopositivity (Fig.6) was proved in activated microglial cells in diffuse and focal TBI. PSGL facilitates neurotrophils transmigration across the BBB. Endothelial cells displayed Weibel-Palade body (Fig.7) proved by electron microscopy. Ultrastructural investigation demonstrated tethering and rolling of neutrophils in brain capillaries of patients with expansive contusions. The process of neuronophagy of broken up neurons by neutrophiles was proved in early stages (patients submitted to neurosurgery up to 24–48 hours after admission). CD68-related local immunoreactivity demonstrated fagocytic activity of microglial cells in patient with focal and diffuse injuries. We proved strong CD68 immunopositivity of microglial cells surrounding capillaries in patients with expansive contusions.
Fig. 6. Immunoreactivity with anti-NF antibody was demonstrated in the injured varicose and wave-like axons (asterisk) and the forming of retraction ball was revealed (arrowhead). Lesion in corpus callosum, in patients with DAI, was haemorrhagic called as gliding contusions (magnification 400x). 
Fig. 7. Electron microscopy of the surgical specimen showed an endothelial cell bearing the specialized organelles called Weibel-Palade bodies (arrowhead) exposed in patients with expansive contusion (magnification 15000x). Cappilary lumen contained red cells (asterisk). 
Hypoxic ultrastructural transformation of expansive contusions, and the VEGF expression:
Focal ischaemic transformation of injured tissue (Fig.8) occured in areas with broken cytoskeletal proteins was proved by immunohistochemistry. An early structural change in ischaemic tissue was mitochondrial bulging, dilatation of endoplasmic reticulum bearing ribosomes, and Golgi apparatus. We observed the increase amount of telolysosomes wrapping round endoplasmic reticulum. VEGF was expressed in patients with expansive contusions (Fig.9). The immunohistochemistry demonstrated a strong labelling of anti-VEGF antibodies in astroglial and endothelial cells around contusions.
Fig. 8. A case of knocked down pedestrian during trafic accident who suffered brain contusions, haemocephalus, subdural haematoma, and skull fractures. 3 days after neurosurgery patient developed the GCS improvement up to 13–15 points. Photomicrograph (magnification 20000x) shows a dilatation of rough endoplasmic reticulum (asterisk) closed nucleus, and mitochondrial bulging (arrowhead). Cells had been rendered ischemic as a consequence of vasospasms monitored by TCCD. 
Fig. 9. Photomicrograph of the surgical specimen shows a injured tissue of patient who suffered TBI caused by falling downstairs in alcohol intoxication. Patient was submitted to neurosurgery due to expansive contusion. Specimen was labelled with anti-VEGF antibody. The immunohistochemistry demonstrated a moderate labelling of anti-VEGF antibodies in astroglial cells (in detail), and pericelular oedema was ascertained (magnification 400x). 
DISCUSSION
This study of 38 patients with severe traumatic brain injury demonstrated the essential diference between focal and diffuse injuries. We discussed the S-100B, GFAP blood values, and ROC characteristics in focal and diffuse TBI. Immunohistological and ultrastructural changes were correlated with some of clinical symptoms (such as uncounsciousness, or functional alterations such as the progressive perifocal oedema in expansive contusions) in focal TBI. An increase in serum NF-H in patients with focal TBI might be explained by axonal changes in expansive contusions. We established a relation between the releasing of S-100B and GFAP into blood, and a loss of cytoskeletal proteins in blood vessels. The alteration to the synaptic clefts can correlate with a long term uncounsciousness or the venous vasoregulation disorders (28). The connection between hypoxic transformation of contusion tissue, and the finding of the highest values of S-100B and GFAP in expansive contusions was discussed (29).
The highest values of S-100B and GFAP found in expansive progressive cerebral contusions (see Tab.1) showed the association between degree of the BBB failure demonstrated by immunohistochemistry and releasing of glial-related proteins. Some authors (2,3) reported, that serum GFAP concentrations were lower in diffuse injury than in mass lesions, and higher for Glasgow outcome scale (GOS) of 1 or 2 than for GOS of 3, 4 (p<0.05), or 5 (p <0.0005). In our froup of enrolled patient the cut-off value of S-100B higher than 1.82 μg/l had sensitivity of 93 % and specificity of 55 %, and the cut-off value of GFAP higher than 3.4 μg/l had sensitivity 58 % and specificity 83 % for the development of progresive expansive contusions. We confirmed higher S-100B and GFAP values in focal compared diffuse TBI, as well as reported Pelinka LE, 2004 (17,18). Recently, the passage of S-100B from BBB was critically revised by Kleindienst et al. In order to decrease the non-specifity of S-100B in neurosurgery trial, we recommended to use the combination of S-100B (26, 30), NSE, GFAP, and NF-H in relation with immunohistochemistry. We observed a relation between the releasing of S-100B and GFAP into circulation, and a loss of cytoskeletal proteins in blood vessels. The regional differences in anti-GFAP, anti-vinculin, and anti-vimentin immunoreactivity could result from the degree of the BBB impairment. In our study the immunohistochemistry demonstrated the loss of GFAP and vimentin reactivity of astroglial end-feet localised inside the cerebral contusion areas. In contrast, GFAP and vimentin immunoreactivity were well documented in small cerebral contusions of patients who died due to diffuse axonal injury. Local immunoreactivity of vinculin was higher in endothelial cells of DAI than in expansive contusions as a junctional molecule (31). Vos et al. proved that serum levels of S-100B and GFAP were higher in patients with hypoxia (3.2 vs. 1.8 μg/l for S100B, and 1.9 vs. 1.9 μg/l for GFAP) and hypotension (3.1 vs. 1.9 μg/l for S100B, and 2.5 vs. 1.9 μg/l for GFAP) compared to other patients, but the differences were not significant (29). With respect to enrolled patients in our study, those patients with expansive contusion had higher levels of S-100B and GFAP during the first 3 days (4.21±1.1 μg/l for S100B, and 8.58±2.4 μg/l for GFAP).
1. Data of diffuse and focal brain injuries with respect to the time-profiling (1–3 days, 4–10 days) during 10 days after admission to the hospital (mean ±SD, μg/l for S-100B and GFAP, ng/l for NF). N.A. not available data. Time-profille kinetics of S-100B and GFAP showed a decrease in values within 10 days, and an NF-H value increase from the 1st up to 10th day. The highest values of S-100B and GFAP were found in expansive contusions. 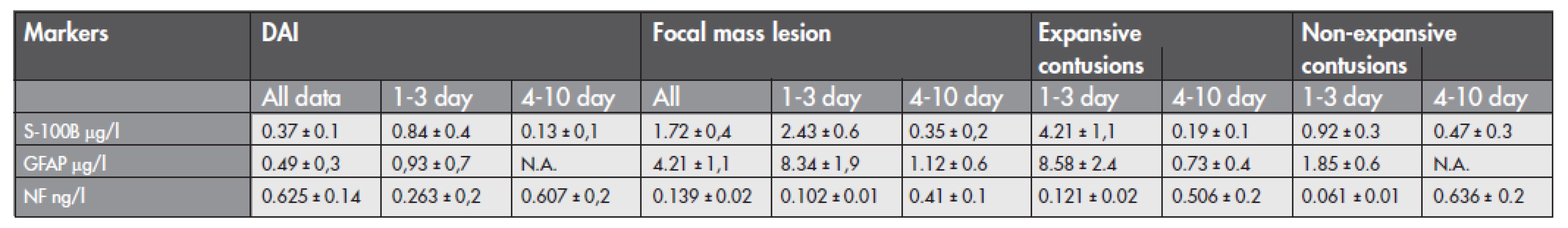
Axonal injury was often occured in focal brain injuries, and in our study, demonstrating time-dependent profile of serum NF-H, there was suggested an increase of NF-H values from the 4th to the 10th days in both groups. Observed axonal changes (wave-like axons filled with accumulated multivesicular bodies and crowded by vacuoles) in expansive contusions might also explain an increase in serum NF-H in patients with focal TBI by way of hypoxia-related neuroaxonal degeneration proved by electron microscopy in focal TBI (see Fig.3,4). Oehmichen M. described axonal injuries in the brain in cases of non-traumatic death due to cerebral hypoxia by way of beta-APP expression in secondary brain damage (32). We confirmed hypoxic transformation of progresive expansive contusions in cases where TCCD revealed vasospasms (see Fig.2) of cerebral vessels (33).
The inflammatory response following TBI was demonstrated by ultrastructural and immunohistochemical analyses in patients with expansive contusions. In progressing expansive contusions the P-selectin facilitates tethering of circulating leukocytes and did theirs migration into the contusions tissues (27) leading to deterioration of pericontusional oedema under hypoxic conditions. Weibel-Palade bodies, stored P-selectin (34), were demonstrated in endothelial cell in our surgical speciment. The hypoxia (documented in histological analysis and related to vasospasms) leads to an increase in hypoxia induced factor HIF synthesis (35) promoting VEGF production. VEGF increases the permeability of BBB via the synthesis and release of NO (7), and plays a significant role in angiogenesis after brain injury (36). Our immunohistochemical study revealed a strong labelling of anti-VEGF antibodies in astroglial and endothelial cells around progressive expanssive contusions.
Based on the present results, we suppose that the kinetics of neurobiochemical markers may reflect different types of pathophysiological changes of the BBB in focal and diffuse injury.
CONCLUSSION
The time-profile kinetics of neurobiochemical markers may reflect different types of pathophysiological changes of the BBB in focal and diffuse injuries. Inflammatory response following injury, hypoxic transformation of contusions, and cytoskeletal proteins dissorders were observed in progresive expansive contusions by electron microscopy and immunohistochemistry.
Acknowledgements:
Our work was supported by IGA CR, reg.no. NR/8793-3/2006 and by Institutional Research Concept (AV0Z50200510).
Sources
1. Persidsky Y, Ghorpade A, Rasmussen J, et al. Microglial and astrocyte chemokines regulate monocyte migration through the blood-brain barrier in human immunodeficiency virus-1 encephalitis. Am J Pathol. 1999; 155(5): 1599–1611.
2. Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004; 16(1): 1–13.
3. Hayashi K, Nakao S, Nakaoke R, Nakagawa S, Kitagawa N, Niwa M. Effects of hypoxia on endothelial/pericytic co-culture model of the blood-brain barrier. Regul Pept. 2004; 123(1–3): 77–83.
4. Coles JP. Regional ischemia after head injury. Curr Opin Crit Care. 2004; 10(2): 120–125.
5. Kukacka J, Vajtr D, et al. Blood metallothionein, neuron specific enolase, and protein S100B in patients with traumatic brain injury. Neuro Endocrinol Lett. 2006; 27(Suppl2): 116–120.
6. Vajtr D, Benada O, Kukacka J, et al. Correlation of ultrastructural changes of endothelial cells and astrocytes occuring during blood brain barrier damage after traumatic brain injury with biochemical markers of BBB leakage and inflammatory response. Physiol Res. 2009; 58(2): 263–268.
7. Lafuente JV, Bulnes S, Mitre B, Riese HH. Role of VEGF in an experimental model of cortical micronecrosis. Amino Acids. 2002; 23(1–3): 241–245.
8. Willis CL, Nolan CC, Reith SN, et al. Focal astrocyte loss is followed by microvascular damage, with subsequent repair of the blood-brain barrier in the apparent absence of direct astrocytic contact. Glia. 2004; 45(4): 325–337.
9. Hamm S, Dehouck B, Kraus J, et al. Astrocyte mediated modulation of blood-brain barrier permeability does not correlate with a loss of tight junction proteins from the cellular contacts. Cell Tissue Res. 2004; 315(2): 157–166. Epub 2003 Nov 13.
10. Fischer S, Wobben M, Kleinstück J, Renz D, Schaper W. Effect of astroglial cells on hypoxia-induced permeability in PBMEC cells. Am J Physiol Cell Physiol. 2000; 279(4): C935–944.
11. Persidsky Y. Model systems for studies of leukocyte migration across the blood - brain barrier. J Neurovirol. 1999; 5(6): 579–590.
12. Korfias S, Stranjalis G, Boviatsis E, et al. Serum S-100B protein monitoring in patients with severe traumatic brain injury. Intensive Care Med. 2007; 33(2): 255–260.
13. Lin J, Cai W. Effect of vimentin on reactive gliosis: in vitro and in vivo analysis. J Neurotrauma. 2004; 21(11): 1671–1682.
14. Nylén K, Ost M, Csajbok LZ, et al. Increased serum-GFAP in patients with severe traumatic brain injury is related to outcome. J Neurol Sci. 2006; 240(1–2): 85–91. Epub 2005 Nov 2.
15. Engel S, Wehner HD, Meyermann R. Expression of microglial markers in the human CNS after closed head injury. Acta Neurochir Suppl. 1996; 66 : 89–95.
16. Röhl C, Lucius R, Sievers J. The effect of activated microglia on astrogliosis parameters in astrocyte cultures. Brain Res. 2007; 1129(1): 43–52. Epub 2006 Dec 13.
17. Pelinka LE, Kroepfl A, Schmidhammer R, et al. Glial fibrillary acidic protein in serum after traumatic brain injury and multiple trauma. J Trauma. 2004; 57(5): 1006–1012.
18. Pelinka LE, Kroepfl A, Leixnering M, Buchinger W, Raabe A, Redl H. GFAP versus S100B in serum after traumatic brain injury: relationship to brain damage and outcome. J Neurotrauma. 2004; 21(11): 1553–1561.
19. Woertgen C, Rothoerl RD, Holzschuh M, Metz C, Brawanski A. Comparison of serial S-100 and NSE serum measurements after severe head injury. Acta Neurochir (Wien). 1997; 139 : 1161–1164; discussion 1165.
20. Woertgen C, Rothoerl RD, Wiesmann M, Missler U, Brawanski A. Glial and neuronal serum markers after controlled cortical impact injury in the rat. Acta Neurochir Suppl. 2002; 81 : 205–207.
21. Onaya M. Neuropathological investigation of cerebral white matter lesions caused by closed head injury. Neuropathology. 2002; 22(4): 243–251.
22. Adelson PD, Jenkins LW, Hamilton RL, Robichaud P, Tran MP, Kochanek PM. Histopathologic response of the immature rat to diffuse traumatic brain injury. J Neurotrauma. 2001; 18(10): 967–976.
23. Pittella JE, Gusmčo SS. Cerebral contusion in victims of fatal traffic accidents. Frequency and association with other craniocerebral lesions. Arq Neuropsiquiatr. 1999; 57(3B): 848–852.
24. Shaw G, Yang C, Ellis R, et al. Hyperphosphorylated neurofilament NF-H is a serum biomarker of axonal injury. Biochem Biophys Res Commun. 2005; 336(4): 1268–1277.
25. Ding M, Haglid KG, Hamberger A. Quantitative immunochemistry on neuronal loss, reactive gliosis and BBB damage in cortex/striatum and hippocampus/amygdala after systemic kainic acid administration. Neurochem Int. 2000; 36(4–5): 313–318.
26. Kövesdi E, Lückl J, Bukovics P, et al. Update on protein biomarkers in traumatic brain injury with emphasis on clinical use in adults and pediatrics. Acta Neurochir (Wien). 2010; 152(1): 1–17.
27. Wen YD, Zhang HL, Qin ZH. Inflammatory mechanism in ischemic neuronal injury. Neurosci Bull. 2006; 22(3): 171–182.
28. Martin NA, Patwardhan RV, Alexander MJ, et al. Characterization of cerebral hemodynamic phases following severe head trauma: hypoperfusion, hyperemia, and vasospasm. J Neurosurg. 1997; 87(1): 9–19.
29. Vos PE, Lamers KJ, Hendriks JC, et al. Glial and neuronal proteins in serum predict outcome after severe traumatic brain injury. Neurology. 2004; 62(8): 1303–1310.
30. Kleindienst A, Schmidt C, Parsch H, Emtmann I, Xu Y, Buchfelder M. The Passage of S100B from Brain to Blood Is Not Specifically Related to the Blood-Brain Barrier Integrity. Cardiovasc Psychiatry Neurol. 2010; Epub 2010 Jul 8.
31. Mazzetti S, Librizzi L, Frigerio S, de Curtis M, Vitellaro-Zuccarello L. Molecular anatomy of the cerebral microvessels in the isolated guinea-pig brain. Brain Res. 2004; 999(1): 81–90.
32. Oehmichen M, Meissner C, Schmidt V, Pedal I, König HG. Pontine axonal injury after brain trauma and nontraumatic hypoxic-ischemic brain damage. Int J Legal Med. 1999; 112(4): 261–267.
33. Steiger HJ, Aaslid R, Stooss R, Seiler RW. Transcranial Doppler monitoring in head injury: relations between type of injury, flow velocities, vasoreactivity, and outcome. Neurosurgery. 1994; 34(1): 79–85; discussion 85–86.
34. Barkalow FJ, Goodman MJ, Gerritsen ME, Mayadas TN. Brain endothelium lack one of two pathways of P-selectin-mediated neutrophil adhesion. Blood. 1996; 88(12): 4585–4593.
35. Perrin JS, Araneda S, Catteau J, Autran S, Denavit-Saubié M, Pequignot JM. Glial vascular endothelial growth factor overexpression in rat brainstem under tolerable hypoxia: evidence for a central chemosensitivity. J Neurosci Res. 2009; 87(1): 79–85.
36. Nag S, Eskandarian MR, Davis J, Eubanks JH. Differential expression of vascular endothelial growth factor-A (VEGF-A) and VEGF-B after brain injury. J Neuropathol Exp Neurol. 2002; 61(9): 778–788.
Labels
Anatomical pathology Forensic medical examiner Toxicology
Article was published inForensic Medicine

2012 Issue 1-
All articles in this issue
- Evaluation of childhood medicolegal autopsies in Bratislava and Trnava regions, Slovakia
- The importance of the detail forensic-neuropathological examination in the determination of the diffuse brain injuries
- Immunohistochemistry and serum values of S-100B, glial fibrillary acidic protein, and hyperphosphorylated neurofilaments in brain injuries
- Forensic Medicine
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Immunohistochemistry and serum values of S-100B, glial fibrillary acidic protein, and hyperphosphorylated neurofilaments in brain injuries
- The importance of the detail forensic-neuropathological examination in the determination of the diffuse brain injuries
- Evaluation of childhood medicolegal autopsies in Bratislava and Trnava regions, Slovakia
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

