-
Medical journals
- Career
Epidemiology of hepatitis E virus infection
Authors: A. Kasem; K. Azeem; J. Vlčková; S. Zatloukalová; L. Štěpánek; Z. Kyselý; H. Kollárová
Authors‘ workplace: Department of Public Health, Faculty of Medicine and Dentistry, Palacky University Olomouc, Czech Republic
Published in: Epidemiol. Mikrobiol. Imunol. 68, 2019, č. 4, s. 176-182
Category: Review articles, original papers, case report
Overview
Hepatitis E is a major concern to public health, it is estimated that 44,000 people die annually due to hepatitis E. Hepatitis E virus (HEV) is the most common cause of acute viral hepatitis in the world. HEV has been found with 7 genotypes, only 4 can infect humans.
Genotypes 1 and 2 are major causes of the epidemic and endemic acute hepatitis in developing countries. In the poor hygienic conditions, these genotypes are obligate human pathogens transmitted between humans by the fecal-oral route and via contaminated water. On the contrary, in developed countries, genotypes 3 and 4 are of zoonotic origin and they are transmitted by alimentary route and via direct contact with the infected animal. Transmission by blood, blood products and tissues from infected persons or animals and even mother-to-infant transmission is also possible. Hepatitis E clinical course varies from self-limiting disease to fulminant hepatic failure, and extrahepatic manifestations have occurred.
Management to control and prevent this infection is mainly hygienic measures. The provision of clean drinking water and ameliorating the sanitation of human wastes are the most effective preventive approaches as in any infection outbreak transmitted through the fecal-oral route. Nevertheless, dietary habits, distribution of different zoonotic reservoirs and the hygienic level play a crucial role in limiting and preventing the spread of hepatitis E in the developed countries.
Although HEV vaccine was developed, it is still available only in China. It protects against genotype 4 with no clear evidence of protection against other genotypes.
Keywords:
Epidemiology – hepatitis E – HEV – risk factors – prevention
INTRODUCTION
Hepatitis E virus (HEV) infection was documented for the first time in 1955 during an outbreak in New Delhi, India [1]. In 1983, HEV was discovered by Mikhail Balayan during an outbreak of unknown hepatitis among Soviet soldiers in Afghanistan [2].
In the past, HEV was assumed to be endemic only in developing countries with poor sanitation and all cases in developed countries were considered to be imported. But more recent data suggest that HEV is endemic in developed countries too. Nowadays, HEV might be the most common cause of acute viral hepatitis in the world. Over 20 million cases are estimated annually worldwide [3, 4].
This review article presents the current knowledge of the epidemiology of HEV.
CAUSATIVE AGENT
Hepatitis E virus, a small RNA virus, is a member of the genus Hepevirus in the family Hepeviridae. The virions are icosahedral, non-enveloped, spherical particles with a diameter of approximately 27–34 nm. The viral genomes are positive-sense monopartite a single-stranded RNA of about 6.4–7.2 kb containing 3 open reading frames (ORF). Third ORF (ORF3) encodes a small immune reactive protein, which is associated with viral pathogenesis. The replication of HEV is not well-understood but it is known that it occurs in association with endoplasmic reticulum of the host cell [5–7].
HEV is a ubiquitous virus, which causes liver infection. HEV has been found with 7 genotypes, which varies in the host, mode of transmission, clinical course and its geographical distribution. Four of these genotypes can infect humans: HEV1, 2, 3, and 4. Genotypes 5 and 6 are found only in animals. Genotype HEV7 is a new potential genotype which might infect the humans [8–11].
SOURCE, RESERVOIR, AND TRANSMISSION
Source of HEV is an infected human or animal. Recent evidence indicates that hepatitis E is a zoonotic disease, and its reservoir is swine and other potential animals. Both human and swine strains of HEV are genetically closely related and, in some cases, indistinguishable [9]. Several other species of small mammals are designated as potential reservoirs. For instance rat and rabbit hepatitis E virus has been isolated and described [12, 13].
The human can acquire infection both directly and indirectly. HEV is transmitted directly via the fecal-oral route or contact with an infected animal. Indirect transmission occurs mainly through contaminated water or food [14, 15]. Other modes of transmission may include transfusion of infected blood or blood products, transplantation of solid organs and tissues, as well as vertical transmission, via the intrauterine and perinatal routes [16].
Since the infection is transmitted via direct contact with animals, there are some specific groups of workers that could be in higher risk of HEV infection, such as farmers, veterinarians, and workers attending animals [17–19]. People who were in direct contact with pigs, pig wastes and carcasses, for instance, slaughterers, traders, and suppliers, show increased seroprevalence as high as 66% than those who were not in direct contact. HEV infection often runs silently among the workers in slaughterhouses. Their serum tests were anti-HEV IgM positive, the overall prevalence was 13%, the highest seroprevalence was 50% among slaughterers. As this silent infection of HEV is maintained amongst swine abattoir workers, a new occupational risk might be arising as a challenge to the public health systems [19–21]. The same route of transmission is applied at pet owners with their pet pigs [18].
Indirectly HEV is transmitted by contaminated water and food. Contamination of drinking water supplies with human feces usually occurs after disruption of water supplies due to heavy rainfalls, floods, and monsoons [15]. In foodborne transmission, the most important vehiculum is raw or rare meat of domestic pigs, rabbits, deer, and wild boars, that are infected with HEV, as has been intelligibly demonstrated with cogent evidence [22, 23].
In Japan, some surveys showed the infection rates among the domestic pigs had been exceeding 95% [24]. Transmission via consumption both of wild-boar meat and uncooked deer meat has been recorded [25, 26]. Transmissions through the consumption of contaminated food products such as pork have been reported in Japan [27]. In the latest studies, fully infective HEV was detected and isolated from pork liver sausage produced in France, suggesting a new risk factor for HEV infection by the consumption of these products [28], and measures to eliminate the source of this viral infection.
Shellfish can also serve as a potential vehiculum with their capability to concentrate viral particles from the surrounding environment. Generally, these viral infections occur due to the consumption of raw or undercooked shellfish from contaminated water. Moreover, HEV RNA has been found in shellfish worldwide [29].
In developing countries where the sanitation is poor, HEV1 and HEV2 the predominating genotypes, are transmitted between humans by the fecal-oral route. The virus is excreted in the stool of an infected person. In developed countries (including Europe), the most common genotypes are HEV3 and HEV4. They are transmitted zoonotically from animal reservoirs (including pigs, wild boars, and deer), with infrequent cases that have increasingly been reported in recent years [14, 30].
HEPATITIS E CLINICAL COURSE
The pathogenesis of hepatitis E is not well-understood. After the patient gets infected, the virus reaches the liver but the mechanism is unknown. Then, the virus replicates in the liver and other tissues, for example small intestine, lymph nodes and colon. Studies indicate that this replication occurs in the small intestine, lymph nodes or colon. After replication, virions are released in the bloodstream. the virus is shed in the stool approximately 5 days before the onset of jaundice and up to 30 days after its onset. Chronically infected persons shed the virus in the stool as long as infected [14, 30].
The incubation period is 15–60 days. The infection is usually self-limiting and lasts 2–6 weeks until full recovery. Most infections with HEV3 and 4 in developed countries are asymptomatic or unrecognized. Infections with HEV3 and 4 in developed countries have the same clinical features of infections caused by HEV1 and 2 in developing countries. The features of symptomatic cases are common symptoms of acute icteric hepatitis (nausea, fever, malaise, arthralgia, hepatomegaly, vomiting, diarrhea, and abdominal pain). Jaundice occurs in about 75% of symptomatic patients with HEV3 and 4, while it occurs in about 40% of patients with HEV1 and 2. Few patients may have neurological manifestations. Glomerulonephritis is related to infection with HEV3 [31, 32]. Occasionally, a fulminant hepatitis E can occur [40], it was reported in 1–4% of the cases and it was caused mainly by HEV1. However, fulminant hepatitis is more often to occur in those patients with hepatitis E than those ones with hepatitis A [33, 34].
Likewise, chronic HEV infections can develop and lead to chronic hepatitis and cirrhosis particularly in immunocompromised patients with HEV3 [4, 30, 35].
Pregnant women with HEV infection showed a higher maternal mortality rate and their obstetric and fetal outcomes were worse than in pregnant women infected by other types of viral hepatitis [36]. In some endemic zones (northern and central India, Pakistan), the mortality rate due to hepatitis E is up to 25% in pregnant women [37, 38].
In some studies, HEV infection can predict lethal consequences for patients with chronic liver disease (mortality in this subgroup could reach up to 75%) or immunocompromised patients. Severe progress is more common also in immunosuppressed people, due to transplantation, diabetes mellitus, HIV, etc. [39–41].
EPIDEMIOLOGY
HEV is a major public health issue and it is estimated that one-third of the human population (roughly 2.4 billion people) has been already infected worldwide [42]. The World Health Organization (WHO) estimates, that in 2015 the total deaths due to HEV were 44,000, which constitutes 3.3% of the mortality caused by all types of viral hepatitis [4]. Some other references have mentioned even higher annual deaths (approximately 70,000 deaths due to HEV) [43]. More detailed information on the epidemiology of HEV infection is in Table 1 [44].
1. Epidemiology of HEV Infection in Developing and Developed Countries 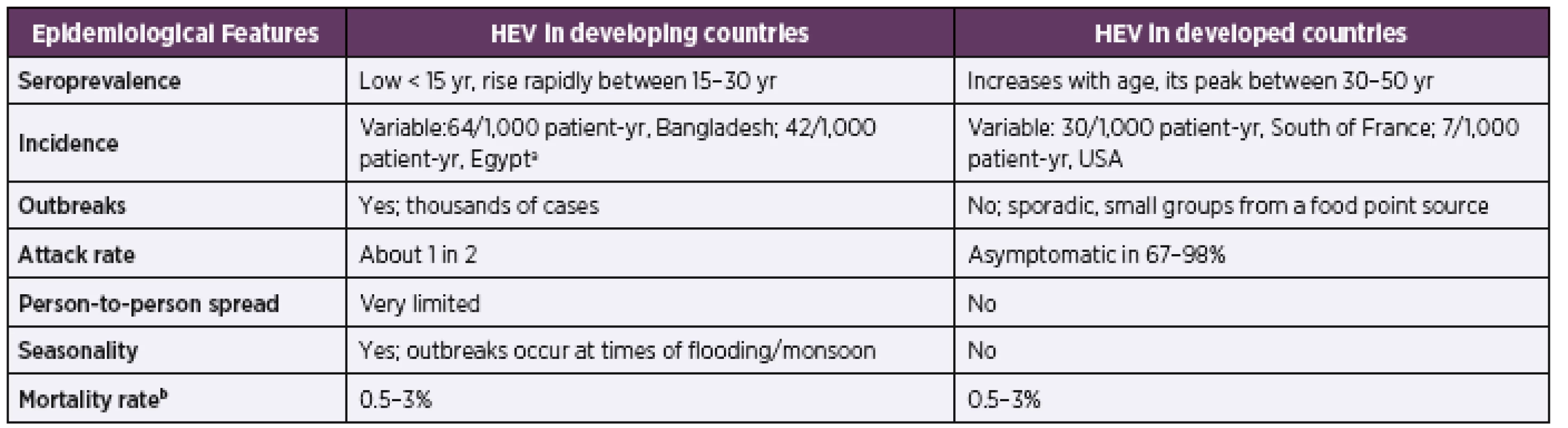
aadapted from the reference [15]
badapted from reference [68]
Adapted from [44]Hepatitis E is a frequent disease in Central and East Asia however several outbreaks in Europe, Central America, and the Middle East have been reported. Most of the outbreaks in high endemic areas occurred due to contamination of drinking water supplies with human feces. These water-borne outbreaks occur in Asia, especially Southeast Asia. Significant outbreaks in the history of HEV were in 1955–1956 in New Delhi, India (30,000 cases) [1], then in Kashmir, India 1978 [45], in Burma between 1976–1977 [46], again in Kanpur, India in 1991 [47], and another striking outbreak was in China 1986–1988 [48]. Outbreaks worldwide in last two decades are summarized in Gideon database [49].
Regions with adequate sanitary conditions and well-controlled water supplies are considered low endemic areas for hepatitis E, such as Europe, Australia, and The Americas. In these regions, the disease is less frequent and mostly occurs as sporadic cases [50]. The prevalence of anti-HEV IgG is ranging from 0.6% to 52.2% [51]. HEV infection of autochthonous cases in these zones appears to be related to zoonotic transmission from domestic animals (most often from pigs) to humans [52], or either through the ingestion of raw or undercooked HEV contaminated food, particularly pork meat and liver containing products [53]. The majority of the autochthonous hepatitis E reported cases were in middle-aged and elderly men [54].
Distribution of HEV genotype varies between countries (Figure 1). The genotypes dominance is influenced by their route of transmission and whether they have a reservoir. Mapping the distribution of HEV genotypes helps to understand the regional differences in prevalence and clinical course, thus, to control and prevent HEV. Both genotypes 3 and 4 are dominating in the developed countries. Genotype 1 dominates in central Asia and the Middle East. Genotype 2 is predominant only in Mexico and relatively frequent in few other countries in Africa. In selected countries, more than one genotype can dominate.
1. The Dominating HEV Genotypes in the World. Data Adjusted According [22, 60, 77]. ![The Dominating HEV Genotypes in the World. Data Adjusted
According [22, 60, 77].](https://www.prolekare.cz/media/cache/resolve/media_object_image_small/media/image_pdf/01c50cf170ceebff713fe93f1557d1aa.jpeg)
Earlier, cases of hepatitis E in Europe were considered to be confined to returners from endemic areas, however, it is now confirmed that HEV is endemic in the European Union/European Economic Area (EU/EEA) [55]. In a surveillance report based on data from 22 countries of the EU/EEA Member States, for over the period 2005–2015, a total of 21,018 confirmed hepatitis E cases were reported. The vast majority (80%) of all confirmed cases were reported from Germany, France, and the United Kingdom. Since at least 2005, these three Member States have had national-level surveillance [55, 56]. Annually, the total number of confirmed cases has been increasing, with a particularly sharp increase between 2011 and 2015, this increase is most probably because of the advancements in the diagnostic techniques and screening possibilities, rather than an increase in its incidence [56]. Also, data on anti-HEV antibody prevalence can be severely affected by the test used because their sensitivity and specificity significantly changed over the time.
In EU/EEA countries, seroprevalences show an increase in seropositivity with age and the highest values were in people aged 50 years and above. As the studies differ in terms of the sampled population and the test used so they also differ in the estimates. Seroprevalence estimates differ even within one country, for instance, the average prevalence in France is around 20% [57]. Frequent studies have been conducted in France, showing variability in the seroprevalence between the southern and northern regions in France. The southern regions in France have shown significantly higher endemicity than the northern regions. For instance, the seroprevalence was the highest in Ariège in the southwest, 86.4% of the tested blood donors were HEV positive [57]. In another study conducted among HIV patients in France, the anti-HEV IgG seroprevalence was remarkably higher in southern patients (9%) in comparison to northern patients (3%) [58].
In Central Europe, the average seroprevalence of anti-HEV IgG among the adult population was 16.8% in Germany, while in the Czech Republic, it was 8.6%. In both countries, seroprevalence rates increase with age and the majority of HEV cases are autochthonous of zoonotic origin with a minority of travel-related infections. Recent studies show HEV infection as an under-reported infection [59–63].
PREVENTION
Prevention measures vary regarding the geographic regions and thus the predominating genotypes and their route of transmission.
In low endemic areas, the zoonotic transmission of hepatitis E dominates, therefore public health concern and the prevention should focus on food safety and zoonotic risk. HEV remains infectious at up to 60°C, proposing the transmission possibility of HEV by consumption of raw or undercooked food contaminated with HEV [64]. In order to inactivate the virus and achieve food safety, the required cooking temperatures should be minimally 71°C for 20 min [65]. The virus RNA has also been found in ready-to-eat food products, including raw sausage and liver sausage at retail level [66, 67].
In high endemic regions, where the outbreaks are more frequent than in other regions, the infection is transmitted through the fecal-oral route, therefore, the provision of clean drinking water and ameliorating the sanitation of human wastes are the most effective preventive approaches. On the contrary, the scarcity of clearly defined risk factors for sporadic hepatitis E makes it more difficult to develop prevention strategies [68]. However, on the individual level, infection risk can be reduced by keeping hygienic habits such as hand-washing with drinking water, avoiding consumption of water of unknown resources; and abiding by WHO safe food procedures.
To introduce HEV screening test for blood products is of significant value in endemic areas and in high-risk population taking into the consideration the influence on the disease course [69–73].
In December 2011, the HEV vaccine was developed and approved by China’s State Food and Drug Administration (SFDA) but is available only in China. Hecolin® is a recombinant vaccine with 3-dose scheme, developed by Xiamen Innovax Biotech Co. and University in China. The annual production capacity of Hecolin® is 200,000 doses/year currently, with a high potentiality to produce 5 million doses a year, in order to meet the world requirements. Hecolin® offers protection against HEV4 infection with no evidence whether it offers protection against other genotypes [74].
The need for a vaccine is associated with the worldwide distribution of HEV. Accordingly, HEV vaccination would be used the most in the endemic areas and in high-risk groups, for instance, food industry workers, immunosuppressed patients and those with chronic liver disease, in addition to the travelers to endemic areas, the elderly above 60 years old, children less than 2 years of age, and women before a planned pregnancy. The vaccine could also be used as a part of HEV outbreaks combat and their control [75, 76].
WHO recommends more researches on vaccines, particularly hepatitis E vaccine, and their safety, immunogenicity, and efficacy. The Advisory Committee on Immunization Practices (ACIP) still does not recommend any emergency immunization with inactive hepatitis E vaccine. The HEV vaccination is still not in the global recommendations [76].
CONCLUSIONS
HEV distribution is not restricted to geographical or political borders. It is confirmed to be endemic not only in developing countries but also in developed ones with variations in genotypes and mode of transmission, regarding the regions and countries.
HEV infection and outbreaks are preventable in developing countries by proper sanitation and providing clean drinking water. While in developed countries HEV infection is a zoonotic infection mainly, thus, animal source food has to be processed and prepared for serving properly. Such measures may include general hygienic measures, proper heating, hygienic handling to avoid any potential contamination. Avoidance of consumption of raw or undercooked pork meat or liver products participates in decreased incidence of the seroprevalence. Elimination of HEV from the entire pork production can be achieved by veterinarian control, regular checkups and tests of the livestock in order to achieve full elimination of the virus.
Transmission of HEV by infected blood, blood products, solid organs, and tissues is not frequent but possible. Therefore, implying screening tests for donors to prevent HEV transmission among people, particularly high-risk patients, would be desirable as well.
Once HEV vaccination is available worldwide, not only in China, it could be used in the endemic areas, where its necessity is the highest. In general, vaccination prior to pregnancy can be an essential preventive measure in ordinary obstetric care in high-incidence countries.
Acknowledgments: This study was financially supported by the Palacky University Internal Financial Support, project no. IGA_LF_2019_022.
Do redakce došlo dne 20. 5. 2019.
Adresa pro korespondenci:
Mgr. Kateřina Azeem, Ph.D.
Ústav veřejného zdravotnictví LF UPOL
Hněvotínská 3
775 15 Olomouc
e-mail: katerina.azeem@upol.cz
Inzerce A191000081 τ
Sources
1. Vishwanathan R. Infectious hepatitis in Delhi (1955–1956): a cri-tical study – epidemiology. Ind J Med Res, 1957;45(Suppl.1):1–29.
2. Balayan MS, Andjaparidze AG, Savin Skaya SS, et al. Evidence for a virus in Non-A, Non-B hepatitis transmitted via the fecal-oral route. Intervirol, 1983;20(1):23–31.
3. European Centre For Disease Prevention And Control. Facts about hepatitis E [online]. 2017 [cit. 2018-12-08]. Dostupné na www: <https://ecdc.europa.eu/en/hepatitis-e/facts>
4. WHO. Hepatitis E: Key facts [online]. 2019 [cit. 2019-09-17]. Dostupné na www: <https://www.who.int/news-room/fact-sheets/detail/hepatitis-e>
5. Nan Y, Zhang YJ. Molecular Biology and Infection of Hepatitis E Virus. Front Microbiol, 2016;7 : 1419.
6. Emerson SU, Purcell RH. Hepatitis E virus. In: Fields virology. 6th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2013. s. 3047–3058. ISBN 1451105630.
7. Purdy MA, Harrison TJ, Jameel S, et al. ICTV Virus Taxonomy Profile: Hepeviridae. J Gen Virol, 2017;98(11):2645–2646.
8. Schlauder GG, Mushahwar IK. Genetic Heterogenecity of Hepatitis E Virus. J Med Virol, 2001;65 : 282–292.
9. Lu L, Li C, Hagedorn CH. Phylogenetic analysis of global hepatitis E virus sequences: genetic diversity, subtypes and zoonosis. Rev Med Virol, 2006;16(1):5–36.
10. Lee GH, Tan BH, Teo ECH, et al. Chronic Infection With Camelid Hepatitis E Virus in a Liver Transplant Recipient Who Regularly Consumes Camel Meat and Milk. Gastroenterol, 2016;150(2):355–357.
11. Kenney SP. The Current Host Range of Hepatitis E Viruses. Viruses, 2019;211(8).
12. Johne R, Plenge-Bonig A, Hess M, et al. Detection of a novel he-patitis E-like virus in faeces of wild rats using a nested broad-spectrum RT-PCR. J Gen Virol, 2010;91(3):750–758.
13. Zhao C, Zhongren MA, Harrison TJ, et al. A novel genotype of he-patitis E virus prevalent among farmed rabbits in China. J Med Virol, 2009;81(8):1371–1379.
14. Hoofnagle JH, Nelson KE, Purcell RH, et al. Hepatitis E. N Engl J Med, 2012;367(13): 1237–1244.
15. Khuroo MS, Khuroo MS, Khuroo NS. Hepatitis E: Discovery, global impact, control and cure [online]. World J Gastroenterol, 2016;22(31):7030–7045.
16. Khuroo MS, Kamili S, Jameel S. Vertical transmission of hepatitis E virus. Lancet, 1995;345(8956):1025–1026.
17. Meng XJ, Wiseman B, Elvinger F, et al. Prevalence of antibodies to hepatitis E virus in veterinarians working with swine and in normal blood donors in the United States and other countries. J Clin Microbiol, 2002;40(1):117–122.
18. Renou C, Cadranel JF, Bourlière M, et al. Possible zoonotic transmission of hepatitis E from pet pig to its owner. Emerg Infect Dis, 2007;13(7):1094–1096.
19. Ukuli AQ, Mugimba KK. Seroprevalence of hepatitis E in swine abattoir workers. Afr Health Sci, 2017;17(4):1022–1028.
20. Drobeniuc J, Favorov MO, Shapiro CN, et al. Hepatitis E virus antibody prevalence among persons who work with swine. J Infect Dis, 2001;184(12):1594–1597.
21. Galiana C, Fernández-Barredo S, García A, et al. Occupational exposure to hepatitis E virus (HEV) in swine workers. Am J Trop Med Hyg, 2008;78(6):1012–1015.
22. Meng XJ. Zoonotic and foodborne transmission of hepatitis E virus. Sem Liver Dis, 2013;33(1):41–49.
23. Lhomme S, Dubois M, Abravanel F, et al. Risk of zoonotic transmission of HEV from rabbits. J Clin Virol, 2013;58(2):357–362.
24. Satou K, Nishiura H. Transmission dynamics of Hepatitis E among swine: Potential impact upon human infection. BMC Veterinary Research, 2007;3.
25. Li TC, Chijiwa K, Sera N, et al. Hepatitis E virus transmission from wild boar meat. Emerg Infect Dis, 2005;11(12):1958–1960.
26. Tei S, Kitajima N, Takahashi K, et al. Zoonotic transmission of he-patitis E virus from deer to human beings. Lancet, 2003;362(9381):371–373.
27. Yazaki Y, Mizuo H, Takahashi M, et al. Sporadic acute or fulminant hepatitis E in Hokkaido, Japan, may be food-borne, as suggested by the presence of hepatitis E virus in pig liver as food. J Gen Virol, 2003;84(Pt9):2351–2357.
28. Berto A, Grierson S, Hakze-van der Honing R, et al. Hepatitis E virus in pork liver sausage, France. Emerg Infect Dis, 2013;19(2):264–266.
29. La Rosa G, Proroga YTR, de Medici D, et al. First Detection of Hepatitis E Virus in Shellfish and in Seawater from Production Areas in Southern Italy. Food Environ Virol, 2018;10(1):127–131.
30. Kamar N, Bendall R, Legrand-Abravanel F, et al. Hepatitis E. Lancet, 2012;6736(11):1–12.
31. Dalton HR, Stableforth W, Thurairajah P, et al. Autochthonous hepatitis E in Southwest England: natural history, complications and seasonal variation, and hepatitis E virus IgG seroprevalence in blood donors, the elderly and patients with chronic liver disease. Eur J Gastroenterol Hepatol, 2008;20(8):784–790.
32. Marrone G, Biolato M, Mercurio G, et al. Acute HEV hepatitis: clinical and laboratory diagnosis. Eur Rev Med Pharmacol Sci, 2019;23(2):764–770.
33. Wedemeyer H, Rybczynska J, Pischke S, et al. Immunopathogenesis of hepatitis E virus infection. Sem Liver Dis, 2013;33(1):71–78.
34. Krawczynski K, Aggarwal R, Kamili S. Hepatitis E. Infect Dis Clin North Am, 2000;14(3):669–687.
35. Adlhoch C, Avellon A, Baylis SA, et al. Hepatitis E virus: Assessment of the epidemiological situation in humans in Europe, 2014/15. J Clin Virol, 2016;82 : 9–16.
36. Patra S, Kumar A, Trivedi SS, et al. Maternal and fetal outcomes in pregnant women with acute hepatitis E virus infection. Ann Int Med, 2007;147(1):28–33.
37. Javed N, Ullah SH, Hussain N, et al. Hepatitis E virus seroprevalence in pregnant women in Pakistan: maternal and fetal outcomes. East Mediterr Health J, 2017;23(8):559–563.
38. Hussaini SH, Skidmore SJ, Richardson P, et al. Severe hepatitis E infection during pregnancy. J Viral Hepatitis, 1997;4(1):51–54.
39. Pas SD, de Man RA, Mulders C, et al. Hepatitis E virus infection among solid organ transplant recipients, the Netherlands. Emerg Infect Dis, 2012;18(5):869–872.
40. Ramachandran J, Eapen CE, Kang G, et al. Hepatitis E su-perinfection produces severe decompensation in patients with chronic liver disease. J Gastroenterol Hepatol, 2004;19(2): 134–138.
41. Kumar AS, Kumar PS, Singh R, et al. Hepatitis E virus (HEV) infection in patients with cirrhosis is associated with rapid decompensation and death. J Hepatol, 2007;46(3):387–394.
42. WHO. Viral hepatitis: Report by Secretariat [online]. 2009 [cit. 2019-09-17]. Dostupné na www: <http://apps.who.int/gb/ebwha/pdf_files/A62/A62_22-en.pdf>
43. Rein DB, Stevens GA, Theaker J, et al. The global burden of he-patitis E virus genotypes 1 and 2 in 2005. Hepatol, 2012;55(4):988–997.
44. Khuroo MS, Khuroo MS. Hepatitis E: an emerging global di-sease – from discovery towards control and cure. J Viral Hepatitis, 2016;23(2):68–79.
45. Khuroo MS. Study of an epidemic of non-A, non-B hepatitis. Am J Med, 1980;68(6):818–824.
46. Reyes G, Purdy M, Kim J, et al. Isolation of a cDNA from the virus responsible for enterically transmitted non-A, non-B hepatitis. Sci, 1990;247(4948):1335–1339.
47. Naik, SR, Aggarwal R, Salunke PN, et al. A large waterborne viral hepatitis E epidemic in Kanpur, India. Bull World Health Organ, 1992;70(5):597–604.
48. Zhuang H, Cao XY, Liu CB, et al. Epidemiology of hepatitis E in China. Gastroenterol Jpn, 1991;26(Suppl3):135–138.
49. Gideon database [online]. 2018 [cit. 2018-12-20]. Dostupné na www: <https://web.gideononline.com/login.php?gdn_form=Y21kPXVuYXV0aCZ0YXJnZXQ9L3dlYi9lcGlkZW1pb2xvZ3kvaW5kZXgucGhw>
50. WHO. The Global Prevalence of Hepatitis E Virus Infection and Susceptibility: A Systematic Review [online]. 2010 [cit. 2019-09-17]. Dostupné na www: <https://apps.who.int/iris/bitstream/handle/10665/70513/WHO_IVB_10.14_eng.pdf?sequence=1>
51. Hartl J, Otto B, Madden RG, et al. Hepatitis E seroprevalence in Europe: A meta-analysis. Viruses, 2016 : 6(8).
52. Wihelm BJ, Rajić A, Greig J, et al. A systematic review/meta-analysis of primary research investigating swine, pork or pork products as a source of zoonotic hepatitis E virus. Epidemiol Infect, 2011;139(8):1127–1244.
53. Renou C, Roque-Afonso AM, Pavio N. Foodborne transmission of hepatitis E virus from raw pork liver sausage, France. Emerg Infect Dis, 2014;20(11):1945–1947.
54. Dalton HR, Bendall R, Ijaz S, et al. Hepatitis E: an emerging infection in developed countries. Lancet Infect Dis, 2008;8(11):698–709.
55. Aspinall EJ, Couturier E, Faber M, et al. Hepatitis E virus infection in Europe: surveillance and descriptive epidemiology of confirmed cases, 2005 to 2015. Eurosurveillance, 2017;22(26).
56. European Centre for Disease Control and Prevention. Hepatitis E in the EU/EEA, 2005–2015: Surveillance report [online]. 2017 [cit. 2018-10-02]. Dostupné na www: <https://ecdc.europa.eu/sites/portal/files/documents/HEV_Surveillance-report-2005-2015.pdf>
57. Mansuy JM, Gallian P, Dimeglio C, et al. A nationwide survey of hepatitis E viral infection in French blood donors. Hepatol, 2016;63(4):1145–1154.
58. Renou C, Lafeuillade A, Cadranel JF, et al. Hepatitis E virus in HIV-infected patients: a prospective sero-virological study in France. AIDS, 2010;24(10):1493–1499.
59. Faber MS, Wenzel JJ, JILG W, et al. Hepatitis E virus seropre-valence among adults, germany. Emerg Infect Dis, 2012;18(10):1654–1657.
60. Pischke, S, Behrendt P, Bock CT, et al. Hepatitis E in Germany – an under-Reported Infectious Disease. Dtsch Arztelb Int, 2014;111(35–36):577–583.
61. Príkazská M, Beneš Č. Virová hepatitida E v ČR [Viral hepatitis E in the Czech Republic]. Zpr Cent Epidemiol Mikrobiol, 2015; 24(2):63–68.
62. Trmal J, Beneš Č, Trnková M. Differences in the incidence of viral hepatitis A and E in the Czech Republic. Epidemiol Mikrobiol Imunol, 2013;62(1):19–25.
63. Němeček V, Butovičová P, Malý M, et al. The prevalence of antibodies against Hepatitis E Virus in the Czech Republic: serological survey. Epidemiol Mikrobiol Imunol, 2017;66(1):3–7.
64. Emerson SU, Arankalle VA, Purcell RH. Thermal stability of hepatitis E virus. J Infect Dis, 2005;192(5):930–933.
65. Barnaud E, Rogée S, Garry P, et al. Thermal inactivation of infectious hepatitis E virus in experimentally contaminated food. Appl Environ Microbiol, 2012;78(15):5153–5159.
66. Moor D, Liniger M, Baumgartner A, et al. Screening of Ready-to-Eat Meat Products for Hepatitis E Virus in Switzerland. Food Environ Virol, 2018;10(3):263–271.
67. Szabo K, Trojnar E, Anheyer-Behmenburg H, et al. Detection of hepatitis E virus RNA in raw sausages and liver sausages from retail in Germany using an optimized method. Int J Food Microbiol, 2015;215 : 149–156.
68. Teshale EH, Hu DJ. Hepatitis E: Epidemiology and prevention. World J Hepatol, 2011;3(12):285–291.
69. Colson P, Coze C, Gallian P, et al. Transfusion-associated hepatitis E, France. Emerg Infect Dis, 2007;13(4):648–649.
70. Kumar N, Sarin SK. Hepatitis E - Is it a risk to transfusion safety? Asian J Transfus Sci, 2013;7(1):1–3.
71. Mirazo S, Ramos N, Mainardi V, et al. Transmission, diagnosis, and management of hepatitis E: an update. Hepat Med, 2014;6 : 45–59.
72. Kamp C, BlümeL J, Baylis SA, et al. Impact of hepatitis E virus testing on the safety of blood components in Germany – results of a simulation study. Vox Sang, 2018;113(8):811–813.
73. Arankalle VA, Chobe LP. Retrospective analysis of blood transfusion recipients: evidence for post-transfusion hepatitis E. Vox Sang, 2000;79(2):72–74.
74. WHO. Hepatitis E Vaccine: Composition, Safety, Immunogenicity and Efficacy A document prepared for Strategic Advisory Group of Experts on Immunization (SAGE) by the Hepatitis E Vaccine Working Group [online]. 2014 [cit. 2019-03-03]. Dostupné na www: < https://www.who.int/immunization/sage/meetings/2014/october/2_HepEvaccsafety_immunogenicity_efficacy_final_1Oct2014.pdf?ua=1,>
75. Sanyal AJ, Boyer TD, Terrault N, et al. Zakim and Boyer’s hepatology: a textbook of liver disease. 7th Ed. Philadelphia: Elsevier. 2018.
76. WHO. Hepatitis E vaccine: WHO position paper, May 2015. Wkly Epidemiol Rec, 2015;90(18):185–200.
77. Pérez-Gracia MT, Suay B, Mateos-Lindemann ML. Hepatitis E: an emerging disease. Infect Genet Evol, 2014;22 : 40–59.
Labels
Hygiene and epidemiology Medical virology Clinical microbiology
Article was published inEpidemiology, Microbiology, Immunology

2019 Issue 4
Most read in this issue- West Nile fever – autochthonous human cases in South Moravia in 2018 from the epidemiological perspective
- Epidemiology of hepatitis E virus infection
- Tuberculosis in elderly in the Czech Republic
- Estimating the impact of overweight and obesity on cancer risk in the Czech and Slovak populations
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

