-
Medical journals
- Career
Prevalence of Mycoplasma hominis and Ureaplasma urealyticum in women undergoing an initial infertility evaluation
Authors: R. Sleha 1,2; V. Boštíková 1; Radek Hampl 3; M. Salavec 4; P. Halada 5; Martin Štěpán 5; Š. Novotná 3; R. Kukla 2; E. Slehová 2; M. Kacerovský 5; P. Boštík 6
Authors‘ workplace: University of Defence, Faculty of Military Health Sciences, Department of Epidemiology, Hradec Kralove, Czech Republic 1; University of Pardubice, Faculty of Chemical-Technology, Department of Biology and Biochemistry, Pardubice, Czech Republic 2; The Centre of Assisted Reproduction SANUS, Pardubice, Czech Republic 3; Department of Dermatovenereology, Faculty of Medicine, Charles University, Hradec Kralove, Czech Republic 4; University Hospital, Department of Obstetrics and Gyneacology, Hradec Kralove, Czech Republic 5; University of Defence, Faculty of Military Health Sciences, The Centre of Advanced Studies, Hradec Kralove, Czech Republic 6
Published in: Epidemiol. Mikrobiol. Imunol. 65, 2016, č. 4, s. 232-237
Category: Original Papers
Overview
Aims:
Mycoplasma hominis and Ureaplasma urealyticum are potentially pathogenic bacterial species that are frequently isolated from the urogenital tract of women. These pathogens could be responsible for various genitourinary diseases and have been associated with adverse pregnancy outcomes and female fertility problems. The aim of this study was to analyse the presence of M. hominis and U. urealyticum in the cervical canal of uterus of women with and without fertility problems.Methods:
Endocervical swabs obtained from women with reproductive problems and fertile women were tested by both cultivation and polymerase chain reaction. The antimicrobial susceptibility to the azithromycin, ciprofloxacin, doxycycline and erythromycine of the isolated strains of M. hominis and U. urealyticum was also tested by the microdilution broth method.Results:
A total of 111 women with fertile problems were examined. U. urealyticum was detected in samples from 44 (39.6%) women. M. hominis was detected in significantly fewer samples, i.e. only from 9 (8.1%) samples. From these, 6 (5.4%) women were positive for both microorganisms. The fertile group consisted from 23 women. The presence of U. urealyticum was detected in 8 (34.7%) of them. M. hominis was detected only in the mixture with U. urealyticum in 3 (13.0%) cases. The most effective antibiotic against both species in our study was doxycycline.Conclusion:
The results show slightly higher incidence of M. hominis and U. urealyticum in the genitourinary tract of women with fertility problems compare with control group. The potential negative effect of these species on the reproduction ability of women was not observed.KEYWORDS:
mycoplasma – ureaplasma – women – infertility – assisted reproductionINTRODUCTION
Mycoplasma (M.) hominis and Ureaplasma (U.) urealyticum are members of unique class of microorganisms that are considered as opportunistic pathogens of humans. These microorganisms are commonly found within the genitourinary tract of healthy women, but the both species have been associated with an increased risk of developing certain pathogenic conditions, including acute urethritis, bacterial vaginosis, pelvic inflammatory disease and pyelonephritis [1–3]. In addition, numerous previous reports proposed that microbial invasion of M. hominis and U. urealyticum into the amniotic cavity were associated with adverse pregnancy outcomes. Inflammatory reactions within genitourinary tract of pregnant women represent common pathways that could lead to complicated pregnancy and initiate a premature spontaneous delivery [4–7].
Genital mycoplasmas and ureaplasmas could be transmitted from an infected genital tract of females to the fetus or neonate. Intrauterine infection caused by M. hominis or U. urealyticum can result in chorioamnionitis, dissemination to fetal organs or congenital pneumonia [8, 9]. The mycoplasma species were also isolated from the cerebrospinal fluid or from the brain of a several infants [10, 11]. There is considerable evidence for the role of M. hominis in post-partum and post-abortal fever [12].
Inflammation in women genital tract caused by these species could be also involved in female infertility. During past decade was observed the high frequency of U. urealyticum in women with fertility problems [13]. Reduced pregnancy rates after in vitro fertilisation were detected in women with cervical colonisation with ureaplasmas in comparison to women with successful embryo transfer. For women undergoing in-vitro fertilisation is still unclear whether or not test for these microorganisms or to prescribe antibiotics to colonised women [14]. However, the effect of the presence mycoplasmas in the genitourinary tract on the unexplained infertility remains still controversial.
The laboratory diagnosis of the M. hominis and U. urealyticum was originally based on the direct methods. The culture techniques are still valuable and sensitive, but very time-consuming. The current generation of diagnostic test is more often based on the nucleic acid amplification tests, such as polymerase chain reaction (PCR) and its modifications [15, 16, 17]. These approaches have been described as more rapid, specific and sensitive than traditionally used culture methods.
Mycoplasma or ureaplasma infections require the therapeutic use of antimicrobials. Mycoplasmas are naturally resistant to antibiotic compounds that are directed to inhibition of cell wall synthesis, such as penicillins and cephalosporins [18]. Tetracyclines, macrolides or quinolones are the major antibiotics used in the antimicrobial therapy of genital mycoplasmas [19]. However, their therapeutic efficacy may be unpredictable due to increasing resistance of these microorganisms [20].
In the present study, the prevalence of M. hominis and U. urealyticum were investigated in the two groups considered: fertile women and asymptomatic patients with fertility problems. We analysed the effect of these pathogens on the rate of conception in assisted reproduction. The antimicrobial susceptibilities of a large number of clinical isolates of M. hominis and U. urealyticum to different antibiotics were also determined.
MATERIALS AND METHODS
Study population and sample collection
Endocervical swabs and clinical data of investigated and control groups of women were collected. The study group consisted from 111 women with fertility problems, who were treated in the Centre of Assisted Reproduction in Pardubice (Czech Republic). The investigated women have not become pregnant after at least 1 year of having sex without using control methods. The fertile (control) groups were 18 female patients with pregnancy in the past, without clinical symptoms of urogenital infections from the University Hospital Hradec Kralove (Czech Republic) and 5 pregnant women in the 1st trimester of pregnancy. The patients and control groups did not receive antibiotics at least two weeks before the study. The swabs were transported to the laboratory in the mycoplasma transport medium (PPLO broth, BD, USA).
The study protocol was approved by the local ethics committees of Centre Assisted Reproduction SANUS and University Hospital Hradec Kralove.
Bacterial Strains
The reference strains of M. hominis (CIP 103715) and U. urealyticum (CIP 103755) were purchased from the Collection of Institute Pasteur, Paris, France. These microorganisms were used as positive controls.
Culture media and specimen processing
M. hominis and U. urealyticum strains were cultivated in PPLO broth. The following composition is given for the preparation of one litre of medium: 21 g BD Difco™ PPLO broth (Becton, Dickinson and Company, USA), 200 mL horse serum (LabMediaServis, Czech Republic), 100 mL freshly-prepared yeast extract, 1 g ampicillin (Biotika Bohemia, Czech Republic), 2 mL 1% phenol red (Sigma-Aldrich, United Kingdom) and 700 mL of purified water. The culture medium contains 0.8 g thallium acetate (Sigma-Aldrich) and 5 g L-arginine for M. hominis (Merck, Germany) and 1 mg amphotericin B (Sigma-Aldrich), 256 mg lincomycine (Sandoz, Slovenia) and 1 mL 40% urea (Himedia, Italy) for U. urealyticum. PPLO agar was prepared from 35 g of BD Difco™ PPLO agar (Becton, Dickinson and Company, USA) and supplemented the same way as the PPLO broth.
Clinical samples were inoculated into the both PPLO broth medium for M. hominis and U. urealyticum. Samples were incubated under 5% of CO2 at 37 °C and monitored daily for up to five days. A positive growth of each microorganism was considered when a colour change due to arginine or urea hydrolysis resulting in concomitant alkalization of the broth medium was observed and confirmed with the growth of typical colonies after subculture on PPLO agar.
All of the specimens and clinical isolates were stored at -80 °C until DNA extraction and antimicrobial susceptibility testing were performed.
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing was performed in PPLO broth without inhibitors (ampicillin, amphotericin B, lincomycine and thallium acetate) by microdilution method. Reference standard powders of ciprofloxacin, doxycycline and erythromycin were purchased from Sigma-Aldrich (United Kingdom), while azithromycin was obtained from Teva Pharmaceuticals (Czech Republic). All tested agents were dissolved in a sterile deionized water or ethanol as directed by the manufacturer and prepared stock solution by diluting in PPLO broth.
Serial 10-fold dilutions of each isolate were prepared in wells containing PPLO broth without antibiotics to determine the number of color-changing units (CCU). Each tested strain was run in duplicate on a sterile 96-well round-bottom plate composed of the 4 antimicrobials in concentration range 32–0.06 μg/mL, 1 negative control (no antimicrobials and no culture). Subsequently, each well was than inoculated of 104 to 105 CCU/mL of the standardized inoculum of tested strains and incubated under 5 per cent of CO2 at 37 °C.
1. Details of PCR primers used in this study 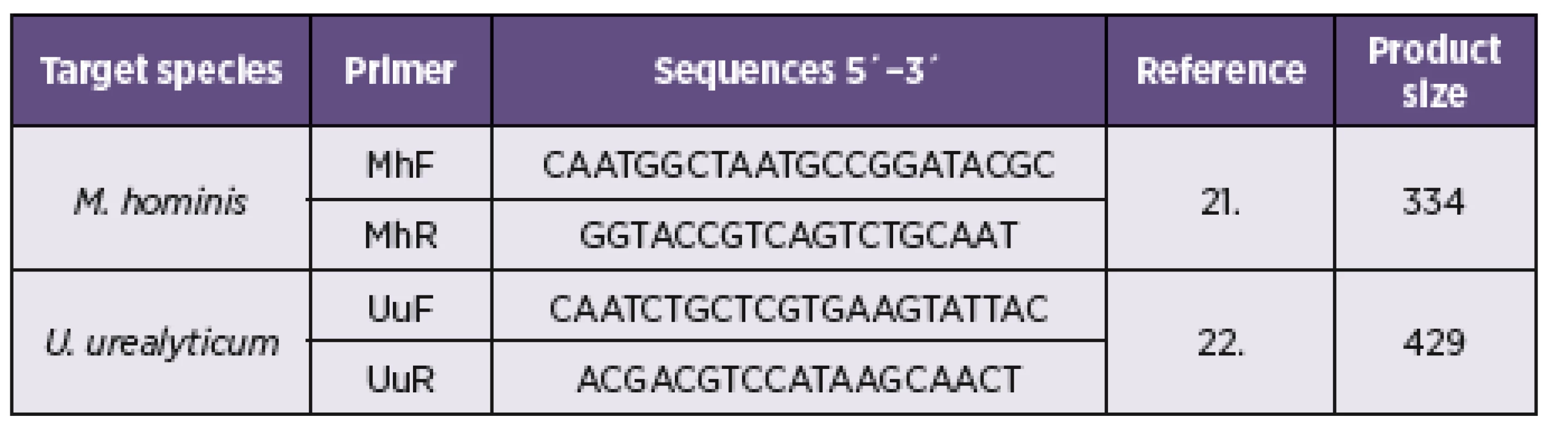
DNA extraction and PCR assay
Samples were vortexed and 300 µl transferred to a QIAamp DNA Mini Kit column (Qiagen, USA). For the DNA extraction, the manufacturer’s protocol was followed. The final elution volume of purified DNA was 200 µl. Isolated DNA samples were stored at -20 °C until the PCR assay performing.
The PCR was carried out in a final volume of 25 μL reaction mixture with 10x PCR-buffer (100 mM Tris-HCl, pH 8.3; 50 mM KCl), 2 mM MgCl2, 0.2 mM of each deoxyribonucleotide triphosphate, 0.5 μM of each oligonucleotide, 1.25 U ExTaq polymerase (Takara Bio Inc., Shiga, Japan), and 2 µl PCR templates (added last). Purified mycoplasmal DNA was used as a positive control for amplification. The published primer sequences targeted the highly conserved region of the 16S rDNA gene of M. hominis and amplified a unique 334 bp region [21]. U. urealyticum primers were obtained from the urease gene sequence (Table 1), a 429-bp fragment of the gene was amplified with this primer set [22]. The amplification was performed in a TP Profesional cycler (Biometra, Germany). The cycling conditions to amplify consisted of an initial denaturation at 94 °C for 4 min, 30 cycles of melting at 94 °C for 1 min, annealing at 62 °C for 1 min, and elongation at 72 °C for 1 min and a final extension at 72 °C for 1 min fragment analyzed by gel electrophoresis on 1.3% (w/v) agarose gels and visualised after staining with ethidium bromide using a UV transilluminator (Vilber Lourmat, France). Negative and positive controls were used in each experiment run.
RESULTS
During the study period June 2011 and March 2014 a total of 111 women with fertile problems and 23 women from control groups were investigated. The mean age for the study group was 34.3 ± 3.9 (ranged 24–47) years and 33.7 ± 5.4 (ranged 23–44) years for the control cohort. The causal factors of the infertility of women from the investigated group are presented in the Table 2.
2. The causal factor of the infertility of women from the investigated group (n = 111) and rate of presence of genital mycoplasmas 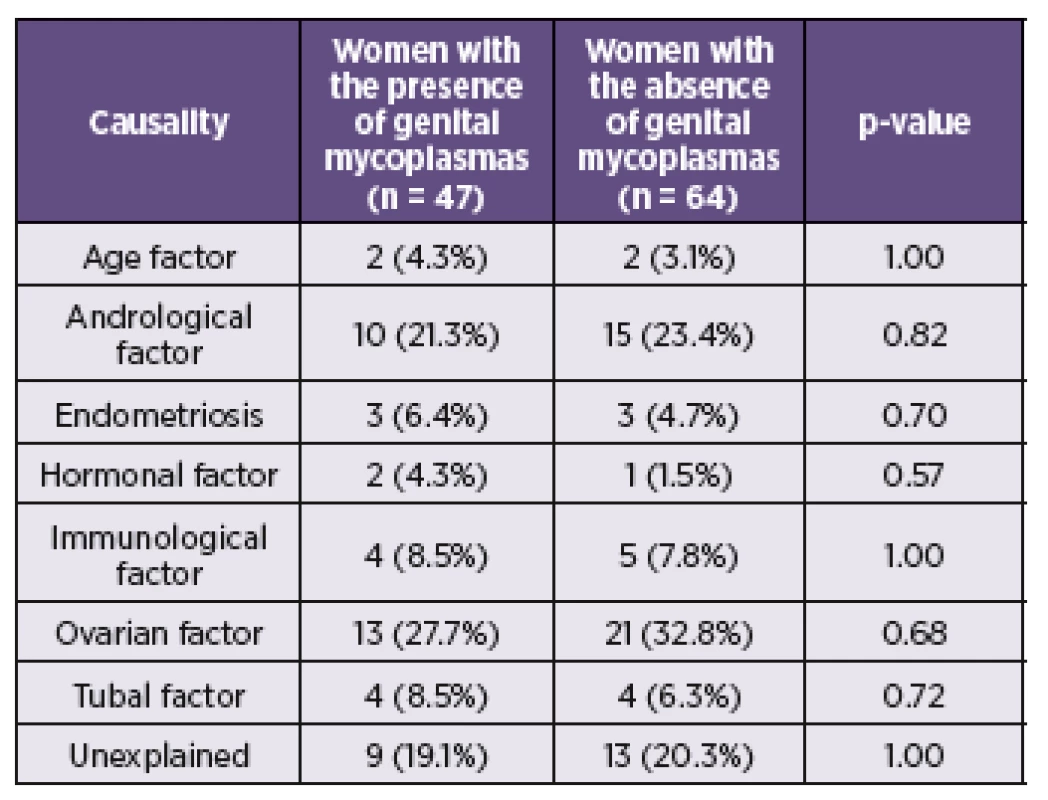
Legend: Categorical variables were compared using Fisher’s exact test. Statistically significant results are marked in bold. Variables are presented as number (%). Of the 111 subjects from the investigated group, 47 (42.3%) were by culture and PCR positive for M. hominis and/or U. urealyticum. Of these, 3 (2.7%) patients were positive only for M. hominis and 38 (34.2%) for U. urealyticum. In 6 (5.4%) cases were detected both species (Table 3). Among 23 fertile women from the control cohort were 8 (34.7%) positive for the presence of M. hominis and/or U. urealyticum. M. hominis has been found only in the mixture with U. urealyticum in 3 (13.0%) cases. U. urealyticum alone was detected in 5 (21.7%) samples (see Table 3). The presence of M. hominis and U. urealyticum in the relation with the causal factor of infertility is presented in the Table 2. In this study, the presence of the genital mycoplasmas was not associated with the higher rates of incidence for some causal factor of infertility.
3. Presence of U. urealyticum and M. hominis in the cervical swabs from fertile and infertile women 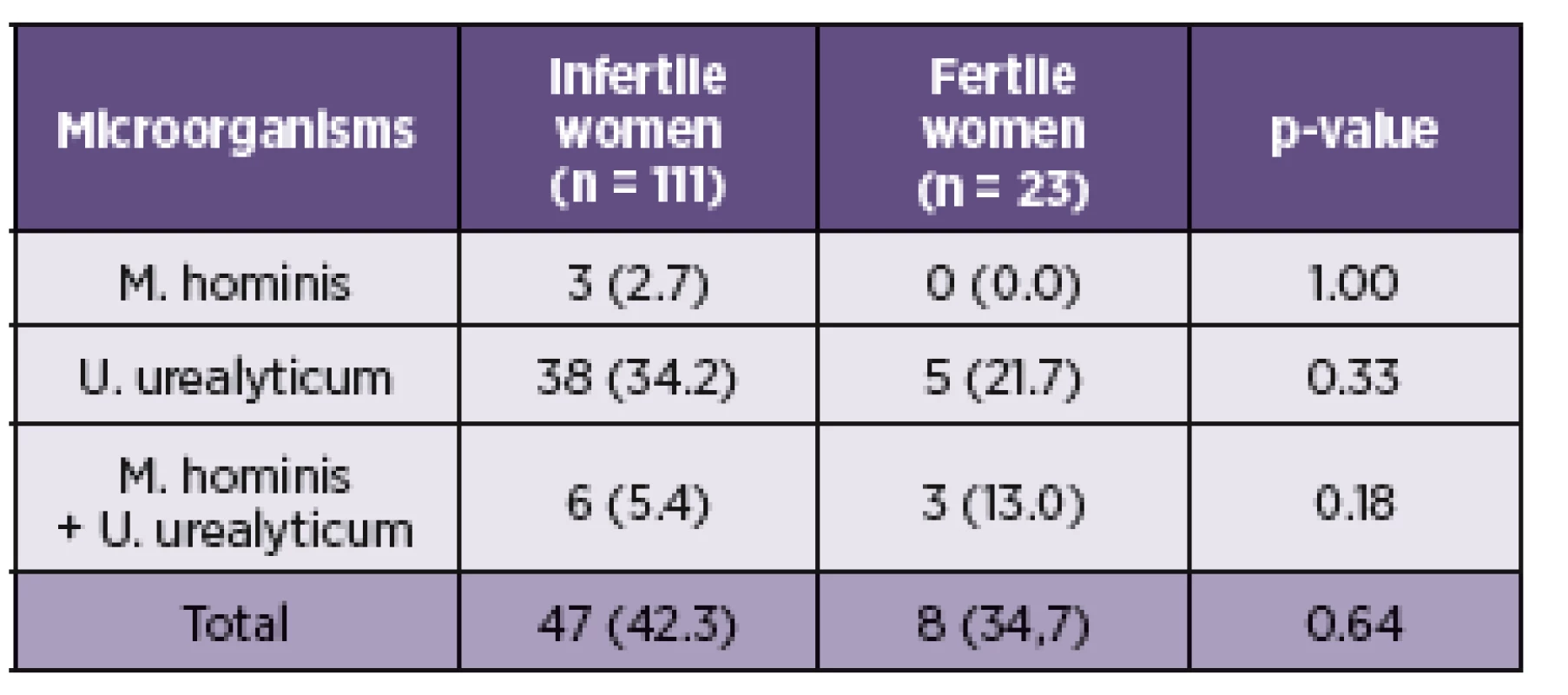
Legend: Categorical variables were compared using Fisher’s exact test. Statistically significant results are marked in bold. Variables are presented as number (%). The association between the presence of mycoplasmas and rates of conception in the cohort of infertile women was also monitored. Results for a total of 109 patients were obtained from the clinic of assisted reproduction (Table 4). No significant differences in the rate of conception between the group of pregnant and non-pregnant women from the assisted reproduction were observed.
4. Presence of <i>M. hominis</i> and <i>U. urealyticum</i> and the rate of conception in infertile women from the clinic of assisted reproduction 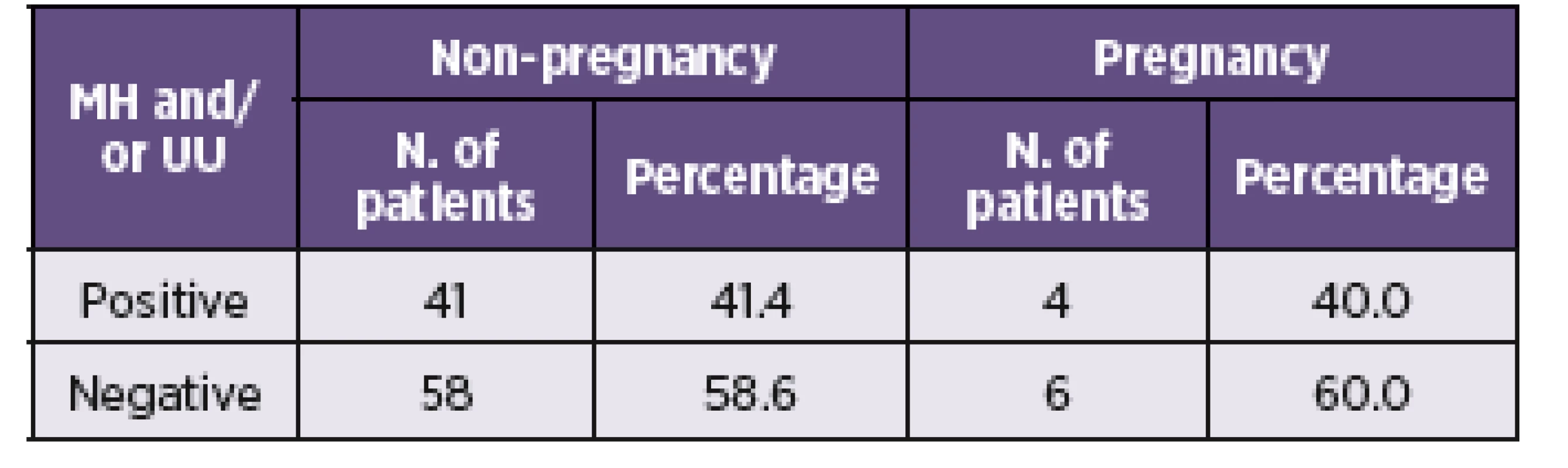
The minimal concentrations at which 50% (MIC50) and 90% (MIC90) of isolated strains were inhibited are shown in Table 5. Doxycycline was the most effective antibiotics against both tested species with MIC90 0.125 μg/mL for M. hominis and 0.5 μg/mL for U. urealyticum. In the present study, erythromycin and azithromycin susceptibility testing of M. hominis clinical isolates using microdilution method demonstrated a high frequency (100%) of resistance. The value of MIC90 of azithromycin and erythromycin for U. urealyticum were 4 and 8 μg/mL respectively. Ciprofloxacin inhibited growth of the M. hominis strains with MIC90 2 μg/mL and 16 μg/mL for U. urealyticum.
5. Antimicrobial susceptibility of clinical isolates of <i>M. hominis</i> and <i>U. urealyticum</i> from women with fertile problems 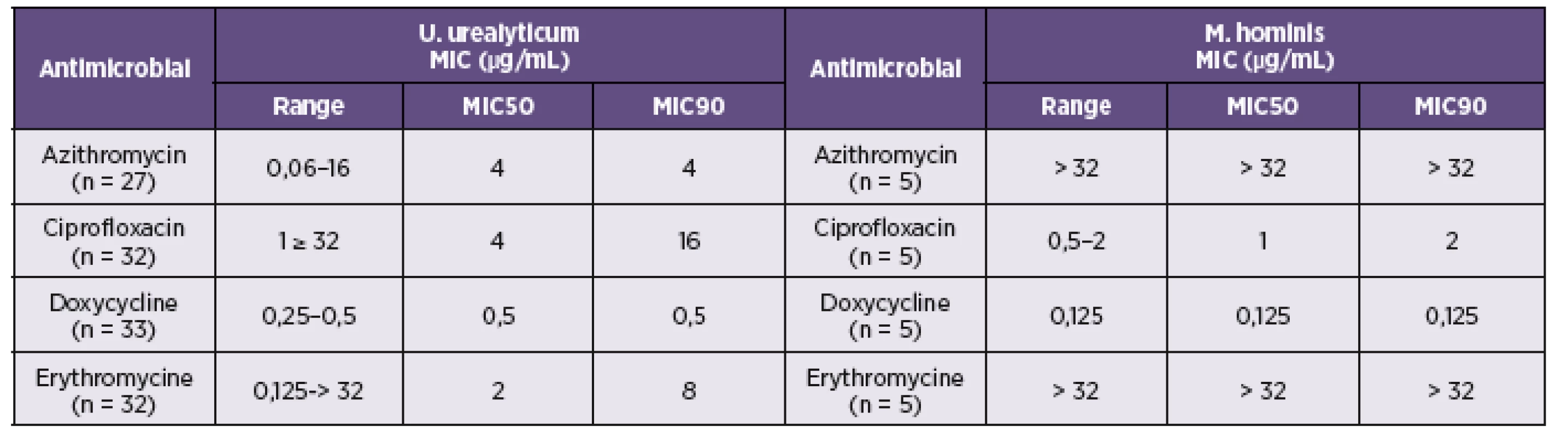
DISCUSSION
The presence of pathogenic microorganisms in the genitourinary tract of women could represent significant aetiologic factor that affects the female reproduction ability. M. hominis and U. urealyticum are common inhabitants in the cervices of many sexually active women and are associated with several urogenital infections (bacterial vaginosis, cervicitis, pelvic inflammatory disease). The inapparent colonisation by mycoplasmas or ureaplasmas could induce pro-inflammatory immune response in the endometrium that may an adverse effect on outcome of pregnancy [1, 3, 4]. However the pathological role of these species in the aetiology of the female fertility problems is still unknown.
To our best knowledge, this is the first report on the prevalence of M. hominis and U. urealyticum in the genitourinary tract of women from the assisted reproduction in the Czech Republic. In our study, the high prevalence of M. hominis and U. urealyticum (42.3%) was detected in the investigated group of infertile women in comparison with control subjects (34.7%). The frequency of the U. urealyticum was higher than that of M. hominis in both groups of women. These findings are consistent with other studies. The study between infertile and fertile couples showed significantly higher presence of these species in couples with fertility problems [23]. The similar prevalence of both microorganisms as in this study between women with fertility problems was obtained by Zdrodowska et al. (2006), who reveal the genital carriage of M. hominis and U. urealyticum in 1 (2.3%) and 16 (37.2%) cases respectively [24]. In contrast, another study detected no difference among fertile and infertile groups with respect of prevalence of M. hominis and U. urealyticum [25]. In the similar study, Witkin et al. (1995) detected significant lower colonisation rate of both species in cervices of women undergoing in vitro fertilisation, compared with our results. According to authors, U. urealyticum is present in the cervices of many culture-negative women. Its presence, however, does not influence IVF outcome subsequent to embryo transfer in women treated with tetracycline after oocyte retrieval [26]. In our study no significant differences in the rate of conception in relation to the presence of investigated species were observed. However to confirmation these results is to necessary monitoring in the greater number of patients.
The antimicrobial susceptibility testing of isolated strains was also performed. It is necessary to monitor the local drug situation to provide reasonable information for clinics. Doxycycline was the most effective antimicrobials against all of tested isolated strains of M. hominis and U. urealyticum in this study. These results confirmed that doxycycline could be still the drug of first choice for the treatment of the urogenital infections caused by mycoplasmas or ureaplasmas. However, local antimicrobial susceptibility testing is recommended due to high rate of mycoplasma resistance against tetracyclines. Tetracycline resistance is associated with the presence of transposon-borne tetM determinant [27]. The prevalence of tetM gene between clinical isolates of M. hominis is between 17-30% [28, 29]. The resistance to tetracyclines was described also for Ureaplasma ssp. isolates [30]. The high in-vitro activity was determined for ciprofloxacin which can be used as an alternative for the tetracyclines. But in recent years, high-level resistance to various fluoroquinolones of M. hominis strains were reported [31, 32]. Similar results for doxycycline and ciprofloxacin have been reported from Krausse et al. (2010) [31]. All of the tested strains of M. hominis were resistant against azithromycin and erythromycin. M. hominis is generally resistant to 14 - and 15-membered macrolides. This resistance has been mainly associated with a G2057A transition in domain V of 23S rRNA [33]. The lower inhibitory effect of erythromycin against clinical isolates of U. urealyticum was also recently reported [20].
CONCLUSION
Results of this work show higher cervical colonisation of M. hominis and U. urealyticum in infertile women compared with fertile group. However, in the present study the rate of conception in assisted reproduction treatment was not significantly affected by the presence of investigated microorganisms. Thus, the potential negative effect of both bacteria on the reproduction ability of women remains controversial. But, this colonisation may be predictive factor of prenatal and perinatal complications. Our results also indicate that doxycycline and ciprofloxacin are effective drugs against both microorganisms when empirical therapy is required.
No conflict of interest to declare.
Do redakce došlo 8. 10. 2015.
Adresa pro korespondenci:
Mgr. Radek Sleha, Ph.D.
Fakulta vojenského zdravotnictví, Katedra epidemiologie,
Hradec Králové
Třebešská 1575
500 01 Hradec Králové
e-mail: radek.sleha@unob.cz
Sources
1. Keane FE, Thomas BJ, Gilroy CB, et al. The association of Mycoplasma hominis, Ureaplasma urealyticum and Mycoplasma genitalium with bacterial vaginosis: observations on heterosexual women and their male partners. Int J STD AIDS, 2000; 11(6): 356–360.
2. Horner P, Thomas B, Gilroy CB, et al. Role of Mycoplasma genita-lium and Ureaplasma urealyticum in acute and chronic nongonococcal urethritis. Clin Infect Dis, 2001; 32(7): 995–1003.
3. Taylor-Robinson D, Jensen JS, Svenstrup H, et al. Difficulties experienced in defining the microbial cause of pelvic inflammatory disease. Int J STD AIDS, 2012; 23(1): 18–24.
4. Witt A, Berger A, Gruber CJ, et al. Increased intrauterine frequency of Ureaplasma urealyticum in women with preterm labor and preterm premature rupture of the membranes and subsequent cesarean delivery. Am J Obstet Gynecol, 2005; 193(5): 1663–1669.
5. Kacerovsky M, Baudys L. Preterm premature rupture of membranes and Ureaplasma urealyticum. Ceska gynekol, 2008; 73(3): 154–159.
6. Larsen B, Hwang J. Mycoplasma, ureaplasma and adverse pregnancy outcomes: A fresh look. Inf Dis Obstet Gynecol, 2010; ID 521921.
7. Kwak DW, Hwang HS, Kwon JY, et al. Co-infection with vaginal Ureaplasma urealyticum and Mycoplasma hominis increases adverse pregnancy outcomes in patients with preterm labor or preterm premature rupture of membranes. J Mat Fet NeoMed, 2014; 27(4): 333–337.
8. Egawa T, Morioka I, Morisawa T, et al. Ureaplasma urealyticum and Mycoplasma hominis presence in umbilical cord is associated with pathogenesis of funisitis. Kobe J Med Sci, 2007; 53(5): 241–249.
9. Jobe AH. Effects of Chorioamnionitis on the Fetal Lung. Clin Perinatol, 2012; 39(3): 441–457.
10. Gwee A, Chinnappan M, Starr M, et al. Ureaplasma meningitis and subdural collections in a neonate. Pediatr Infect Dis J, 2013; 32(9): 1043–1044.
11. Rao R, Ghanayem N, Kaufman B, et al. Mycoplasma hominis and Ureaplasma species brain abscess in a neonate. Pediatr Infect Dis J, 2002; 21(11): 1083–1085.
12. Koshiba H, Koshiba A, Daimon Y, et al. Hematoma and abscess formation caused by Mycoplasma hominis following cesarean section. Int J Womens Health, 2011; 3 : 15–18.
13. Michou V, Constantoulakis P, Makarounis K, et al. Molecular investigation of menstrual tissue for the presence of Chlamydia trachomatis, Ureaplasma urealyticum and Mycoplasma hominis collected by women with a history of infertility. J Obstet Gynaecol Res, 2014; 40(1): 237–242.
14. Witkin SS, Kligman I, Grifo JA, et al. Ureaplasma urealyticum and Mycoplasma hominis detected by the polymerase chain reaction in the cervices of women undergoing in vitro fertilisation: prevalence and consequences. J Assist Reprod Gen, 1995; 12(9): 610–614.
15. Redelinghuys MJ, Ehlers MM, Dreyer AW, et al. Antimicrobial susceptibility patterns of Ureaplasma species and Mycoplasma hominis in pregnant women. BMC Infect Dis, 2014; 14 : 171.
16. Naher HS, Said IH. Culturing and PCR Methods for detection of Mycoplasma hominis and Ureaplasma urealyticum in women with genitourinary tract infections. Int Res J Medical Sci, 2013; 1(3): 25–29.
17. Pascual A, Jaton K, Ninet B, et al. New diagnostic Real-Time PCR for specific detection of Mycoplasma hominis DNA. Int J Microbiol, 2010; pii:317512.
18. Bayraktar MR, Ozerol IH, Gucluer N, et al. Prevalence and antibiotic susceptibility of Mycoplasma hominis and Ureaplasma urealyticum in pregnant women. J Infect Dis, 2009; 14 : 90–95.
19. Waites KB, Duffy LB, Bébéar CM, et al. Standardized methods and quality control limits for agar and broth microdilution susceptibility testing of Mycoplasma pneumoniae, Mycoplasma hominis, and Ureaplasma urealyticum. J Clin Microbiol, 2012; 50(11): 3542–3547.
20. Beeton ML, Chalker VJ, Maxwell NC, et al. Concurrent titration and determination of antibiotic resistance in ureaplasma species with identification of novel point mutations in genes associated with resistance. Antimicrob Agents Chemother, 2009; 53(5): 2020–2027.
21. Blanchard A, Yanez K, Dybvig HL, et al. Evaluation of intraspecies genetic variation within the 16S rRNA gene of Mycoplasma hominis and detection by polymerase chain reaction. J Clin Microbiol, 1993; 31 : 1358–1361.
22. Blanchard A, Hentschel J, Duffy L, et al. Detection of Ureaplasma urealyticum by polymerase chain reaction in the urogenital tract of adults, in amniotic fluid, and in the respiratory tract of newborns. Clin Infect Dis, 1993;17(Suppl. 1): S148–153.
23. Lee JS, Kim KT, Lee HS, et al. Concordance of Ureaplasma urealy-ticum and Mycoplasma hominis in infertile couples: Impact on semen parameters. Urology, 2013; 81(6): 1219–1224.
24. Zdrodowska-Stefanow B, Klosowska WM, Ostaszewska-Puchalska I, et al. Ureaplasma urealyticum and Mycoplasma hominis infection in women with urogenital diseases. Adv Med Sci, 2006; 51 : 250–253.
25. Casari E, Ferrario A, Morenghi E, et al. Gardnerella, Trichomonas vaginalis, Candida, Chlamydia trachomatis, Mycoplasma hominis and Ureaplasma urealyticum in the genital discharge of symptomatic fertile and asymptomatic infertile women. New Microbiol, 2010; 33(1): 69–76.
26. Witkin SS, Kligman I, Grifo JA, et al. Ureaplasma urealyticum and Mycoplasma hominis detected by the polymerase chain reaction in the cervices of women undergoing in vitro fertilization: prevalence and consequences. J Assist Reprod Genet, 1995; 12(9): 610–614.
27. Roberts MC, Koutsky LA, Holmes KK, et al. Tetracycline-resistant Mycoplasma hominis strains contain streptococcal tetM sequences. Antimicrob Agents Chemother, 1985; 28(1): 141–143.
28. Cummings MC, McCormack WM. Increase in resistance of Mycoplasma hominis to tetracyclines. Antimicrob Agents Chemother, 1990; 34 : 2297–2299.
29. Dégrange S, Renaudin H, Charron A, et al. Tetracycline resistance in Ureaplasma spp. and Mycoplasma hominis: Prevalence in Bordeaux, France, from 1999 to 2002 and description of two tet(M)-positive isolates of M. hominis susceptible to tetracyclines. Antimicrob Agents Chemother, 2008; 52(2): 742–744.
30. Krausse R, Schubert S. In-vitro activities of tetracyclines, macrolides, fluoroquinolones and clindamycin against Mycoplasma hominis and Ureaplasma ssp. isolated in Germany over 20 years. Clin Microbiol Infect, 2010; 16 : 1649–1655.
31. Gruson D, Pereyre S, Renaudin H, et al. In vitro development of resistance to six and four fluoroquinolones in Mycoplasma pneumoniae and Mycoplasma hominis, respectively. Antimicrob Agents Chemother, 2005; 49(3): 1190–1193.
32. Pereyre S, Renaudin H, Charron A, et al. Emergence of a 23S rRNA mutation in Mycoplasma hominis associated with a loss of the intrinsic resistance to erythromycin and azithromycin. J Antimicrob Chemother, 2006; 57(4): 753–756.
Labels
Hygiene and epidemiology Medical virology Clinical microbiology
Article was published inEpidemiology, Microbiology, Immunology

2016 Issue 4-
All articles in this issue
- The incidence, mortality and risk factors of prostate cancer
- Epidemiological significance of the metabolic syndrome
- Differential diagnosis of the viral etiology of suspected mumps in a population with high vaccine coverage
- Factors modifying cord blood IgE levels – a pilot study
- Recurrent meningitis and inherited complement deficiency
- IGRA methods in the routine operation – QuantiFERON®-TB Gold or T-SPOT.TB?
- Prevalence of Mycoplasma hominis and Ureaplasma urealyticum in women undergoing an initial infertility evaluation
- An updated approach to viral hepatitis E
- Epidemiology, Microbiology, Immunology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Prevalence of Mycoplasma hominis and Ureaplasma urealyticum in women undergoing an initial infertility evaluation
- IGRA methods in the routine operation – QuantiFERON®-TB Gold or T-SPOT.TB?
- Epidemiological significance of the metabolic syndrome
- Differential diagnosis of the viral etiology of suspected mumps in a population with high vaccine coverage
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

