-
Medical journals
- Career
Prediction of the pre-eclampsia in the first trimester – pilot study
Authors: M. Dugátová 1; J. Luha 2; E. Korňanová 1; M. Borovský 1
Authors‘ workplace: First Department of Gynecology and Obstetrics, Comenius University and University Hospital, Bratislava, Slovakia 1; Institute of Medical Biology, Genetics and Clinical Genetics, School of Medicine, Comenius University, Bratislava, Slovakia 2
Published in: Ceska Gynekol 2019; 84(4): 276-282
Category:
Overview
Aim of study: Aim of study was to find out the possibility of prediction of pre-eclampsia in the first trimester among patients with risk factors.
Type of study: Prospective study.
Name and seat of workplace: 1st Department of Gynecology and Obstetrics, Comenius University in Bratislava, the University Hospital of Bratislava.
Methods: Study included 77 women, who were examined in first trimester between 11+0 and 13+6 weeks of gestation from 1. 6. 2016 to 1. 6. 2017 in 1st Department of Gynecology and Obstetric in the Comenius University Hospital in the Bratislava and enlisted patients delivered until 31. 2. 2018. The study was approved by Hospital Ethics Committee and all patients signed consent form. We included patients, who ran at least one risk factor for developing pre-eclampsia, nulliparous, pregnancy after assisted reproduction technology, body mass index ≥ 25 in the beginning of pregnancy, age of patients and multiple gestation pregnancy. In addition, an ultrasound scans were performed in the first trimester. Doppler ultrasound pulsality index of uterine artery, pregnancy-associated plasma protein and mean arterial pressure were used for prediction of the pre-eclampsia. Sensitivity and specificity of test were calculated. We assessed pre-eclampsia according to diagnostic criteria of the American College Obstetricians and Gynecologists. In the last part, we compared perinatal and maternal outcomes in the pre-eclampsia group and in the control group.
Statistical analysis: Statistical analysis was realized by the IBM SPSS Statistics 25 Software. Risk factors were analysed by using Fisher exact test and Odds ratio. Mann-Whitney test and a one way analysis of variance were used for comparison the pre-eclampsia group and the control group.
Results: Patients, whose had got conceived after assisted reproduction technology, had significant higher probability of pre-eclampsia (Odds ratio = 7.7, p = 0.028). Patients with multiple gestation pregnancy had also significant higher risk of pre-eclampsia (Odds ratio = 16.5, p = 0.031). Mean arterial pressure was only significant as predictive test in 12th weeks of gestation. Adverse perinatal outcomes and higher rate of cesarean section were in the preeclampsia group.
Conclusion: Mean arterial pressure is easy to use and cost-effective predictor, but sensitivity was only 66.6% and specificity 49%.
Keywords:
pre-eclampsia – pregnancy-associated plasma protein (PAPP-A) – mean arterial pressure – risk factors – doppler ultrasound of uterine artery – pulse index
INTRODUCTION
Pre-eclampsia is a disease developed during pregnancy after 20th week of gestation and can be connected with increased maternal and fetal morbidity. Almost 3% of all pregnant women in Europe experience pre-eclampsia [6]. At least 42% of all maternal deaths annually, and 15% of all preterm deliveries are due to pre-eclampsia [15]. Nowadays, increased body mass index (BMI), history of pre-eclampsia and chronic hypertension are assumed as basic clinical risk factors. Some authors published, that BMI ≥ 25 kg/m2 and weight gain ≥ 0.5 kg during one week of pregnancy, are basic risk factors of preeclampsia [15].
According to the National Institute for Health and Care Excellence (NICE) guideline, women should be considered to be at high risk of developing pre-eclampsia, if they have any one of following major factors (history of hypertensive disease in previous pregnancy, chronic kidney disease, autoimmune disease, diabetes mellitus or chronic hypertension) or any of two moderate factors (first pregnancy at age ≥ 40 years, interpregnancy interval > 10 years, body mass index at first visit ≥ 35 kg/m2 or family history of pre-eclampsia) [19]. However, only 30 % of patients with these risk factors are diagnosed with pre-eclampsia [6]. Other risk factors can be nulliparous, multiple gestation pregnancy, assisted reproduction technology (ART) [13].
According to American College of Obstetricians and Gynecologists (ACOG) 2013 preeclampsia is primarily defined by the occurrence of new onset of hypertension, blood pressure over 140/90 mm Hg and plus new onset of proteinuria (300 mg/24 h). However these two criteria are considered the classic definition of pre-eclampsia, some women present with hypertension and multisystemic signs. In absence of proteinuria, pre-eclampsia is diagnosed as hypertension in association of thrombocytopenia (platelets count less than 100,000/microliter), impaired liver function (elevated liver transaminases twice to normal), the new development renal insufficiency (elevated serum creatinine greater than 1.1 mg/dl) pulmonary edema, or new onset cerebral of visual disturbance [1, 14]. Measurement of blood pressure and detection of urinary protein are essential for diagnosis of the pre-eclampsia, but pre-eclampsia can be predicted more precisely by combination of Doppler ultrasound of uterine artery (UtA), pregnancy-associated plasma protein (PAPP-A) and placental growth factor (PlGF) and vascular endothelial growth factor (VEGF). These methods are performed in the first trimester from 11+0 to 13+6 week of gestation [3, 6, 11, 12]. PAPP-A a macromolecular glycoprotein is mainly synthesized by placental trophoblast cells and is then secreted into blood. It is generally used as an index of the first trimester screening test for Down´s syndrome, but blood level of PAPP-A can reflect level of hypoxia of the placenta [4].
MATERIAL AND METHODS
The pilot study consisted of 77 pregnant women, who were examined between 11+0 and 13+6 weeks of gestation. The prospective study was realized in the I. Department of Gynecology and Obstetrics, Comenius University Hospital in Bratislava from 01/06/2016 till 01/06/2017 and patients enlisted delivered until 31/02/2018. The study was approved by Hospital Ethics Committee and all patients signed consent form. We included patients, who ran at least one risk factor for developing pre-eclampsia. Primipara, pregnancy after ART, BMI ≥ 25 in the beginning of pregnancy, age of patients and multiple gestation pregnancy were assumed as risk factors. In addition, an ultrasound scan was performed in the first trimester. All ultrasound scans were realized transabdominally with Voluson E6 GE Healthcare, Chicago, United States (USA).
First trimester screenings were carried out: the crown-rump length (CRL), nuchal translucency, and the doppler examination of uterine artery in accordance with the Fetal Medicine Foundation guidelines. These scans were performed by a doctor certified by the Fetal Medicine Foundation. Pulsed wave doppler was used to assess the UtA pulsality index (PI). Pulsed wave doppler was used with the gate set at 2 mm and an angle of insonation less than 30 degrees. After obtaining three similar consecutive waveforms the PI was measured. Concentration of PAPP-A protein was evaluated from the separated plasma. Minimal volume of separated plasma was 15 microliter. Plasma was cultivated with monoclonal commercial antibody. We gained data about PAPP-A from first trimester screening test. We supposed that low plasma level of PAPP-A could be connected with higher risk of the development of pre-eclampsia [15, 17].
Blood pressure was measured in the first trimester. Before our study, we assumed that measurement of systolic, diastolic pressure and mean arterial pressure (MAP) can be used as predictive test. The second and the third control were performed in 20th and 30th week of gestation. Last measurement of blood pressure was made during admittance of parturiens to hospital before delivery. We defined preeclampsia according to ACOG criteria 2013 and recorded the complications during pregnancy and the way of childbirth. If patients were not able to come to a check-up, they sent information about measurement of blood pressure and delivery from other hospitals. From all the patients, three had abortion (one was induced because of Down´s syndrome, two were spontaneous). Complete data were gained from 74 patients.
The study was funded by the Grant of Comenius University. We created two groups of patients. The first one was control group without pre-eclampsia and another one was with pre-eclampsia. We found out sensitivity and specificity of the predictive tests. In the end there were compared maternal and perinatal outcomes in these two groups and were made graphic representation in Microsoft Excel.
STATISTICAL ANALYSIS
Statistical analysis was carried out by the IBM SPSS Statistics 25 Software. At first, we have used Shapiro-Wilk test to find out, which data deviated from normal distribution and therefore non parametric test was used for analysis of them. Nulliparous, age ≥ 30, pregnancy after ART, multiple-gestation, BMI ≥ 25 were supposed as risk factors for the development of pre-eclampsia. Significance of signs were analyzed by using Fisher exact test. Odds ratio (OR) was used to determine probabilities of incidence of a sign. Non parametric Mann-Whitney test and a one way analysis of variance (ANOVA) were used to compare the pre-eclampsia group and the unaffected control group. P-value (P) less than 0.05 was considered statistically significant.
RESULTS
Pre-eclampsia occurred in 6 patients. Prevalence of pre-eclampsia was 8.1% in the herein presented prospective study. All examined patients were divided into two groups – a pre-eclampsia group and a control unaffected group. Basic statistical analysis is presented in table 1. Then we used χ2 test, 2×2 contingency tables and also odds and odds ratios were determined. Data are presented in the table 2. Patients, who conceived after ART, ran significantly higher risk of developing pre-eclampsia p = 0.028. Patients with multiple gestation pregnancy ran significantly higher risk of developing pre-eclampsia p = 0.031, too. OR of pre-eclampsia for patients, who conceived after ART was 7.7 (95% confidence interval – CI) and OR of pre-eclampsia for multiple gestation pregnancy was 16.5 (95% CI). Fisher exact test proved, that multiple gestation pregnancy is significant more often after using ART, p = 0.042.
1. Descriptive values in the control unaffected group and in the pre-eclampsia group 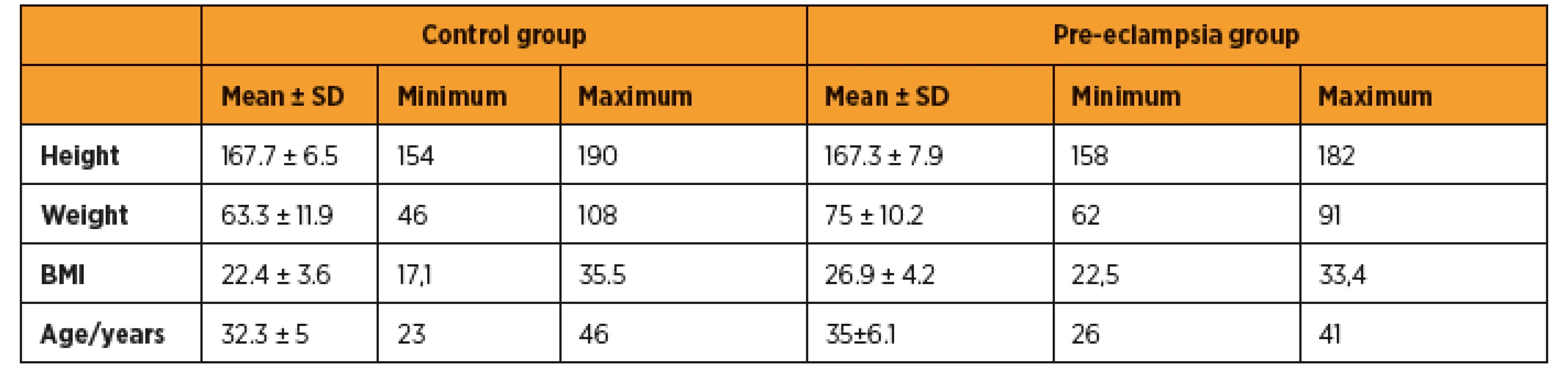
BMI – body mass index, SD – standard deviation 2. Risk factors for developing pre-eclampsia 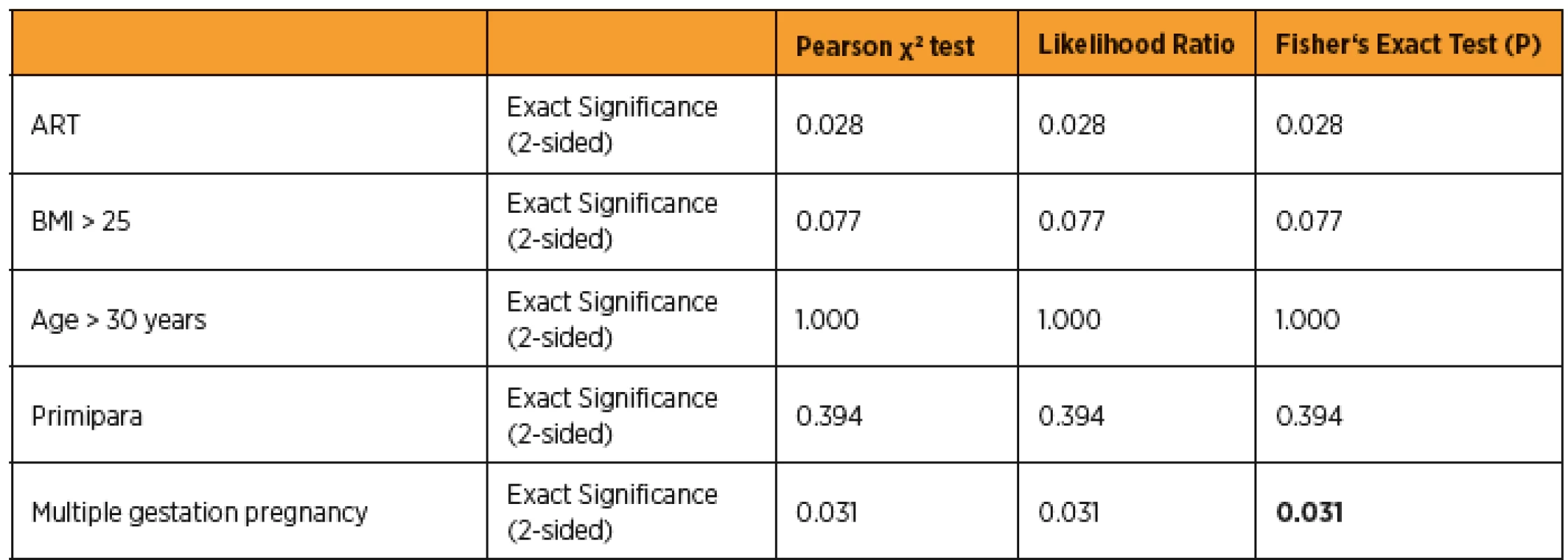
BMI – body mass index (BMI), ART – asissted reproduction technology, P-value less than 0.05 was considered as statistically significant In the next step, we aimed at the options of prediction of pre-eclampsia by using MAP, doppler UtA-PI and PAPP-A in the first trimester. We used Shapiro-Wilk test to find out normally distributed variables, than we applied ANOVA analysis for these data. Other abnormally distributed variables were analyzed by using non parametric Mann-Whitney test. We compared 95 percentile values of UtA PI and MAP (UtA-PI = 2.78, MAP = 101) and 5 percentile of PAPP-A MOM (PAPP-A MOM = 1.05) between pre-eclampsia and control group, then we calculated sensitivity and specificity of screening tests. Table 3 shows the results.
3. Predictive tests of pre-eclampsia 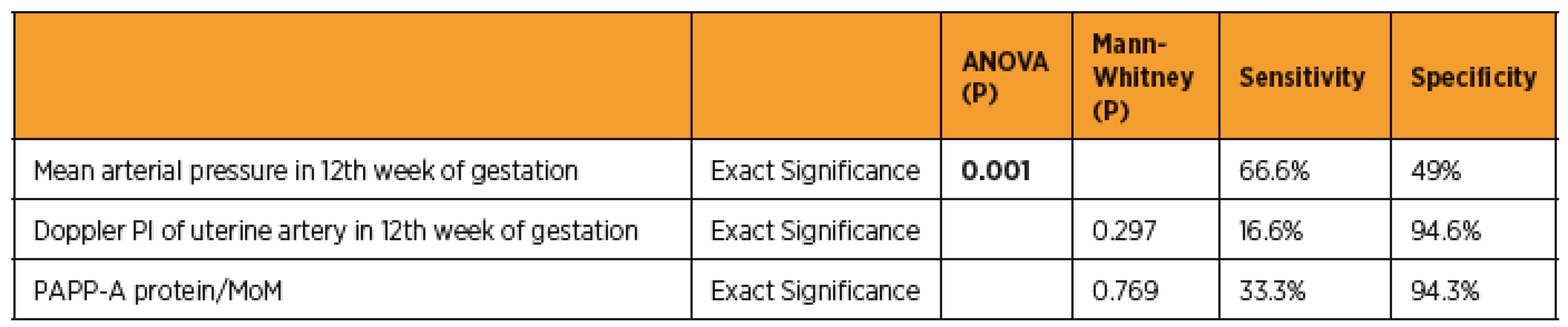
PI – pulsatility index, MoM – multiple of the median, PAPP-A – pregnancy-associated plasma protein, P-value less than 0.05 was considered as statistically significant In the last part, we analyzed perinatal outcomes and compared two groups (fig. 1). All patients with pre-eclampsia undertook caesarean section. 41 patients (60.3%) delivered vaginally and pregnancy in the rest 27 patients (37.9%) was terminated with caesarean section in the control group.
1. Comparison perinatal outcomes in the pre-eclampsia group and control group; the unit of weight is kilograms and height is centimeters 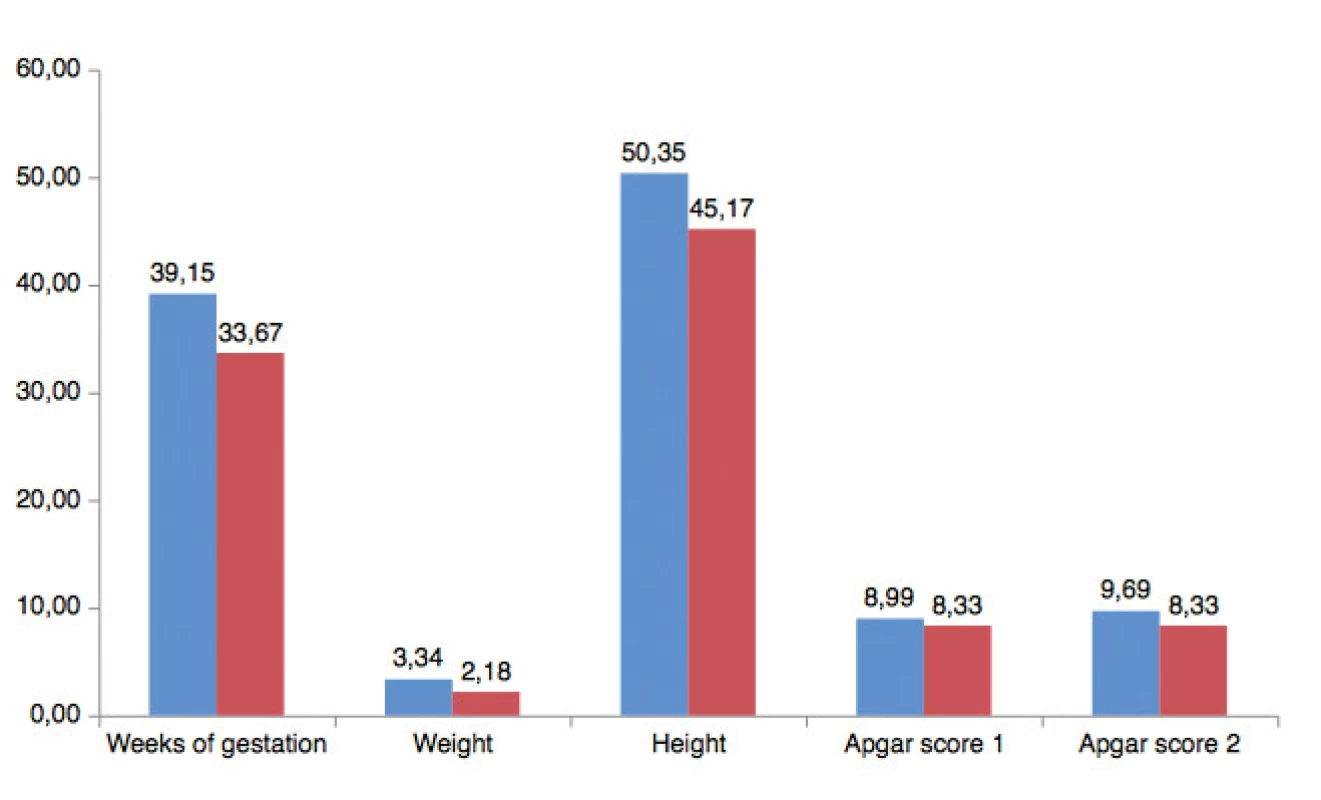
DISCUSSION
In the herein presented study, prevalence of pre-eclampsia was 8.10%. The prevailing age of patients was 35±6 in the pre-eclampsia group and 32.3 ± 5 years in the control group. There were also higher BMI, but lower weeks of gestation in the preeclampsia group compare to the control group. Patients with pre-eclampsia delivered on average in the 33rd week of gestation compared to 39th week of gestation of women in the control group. Newborns weighed 2176 ± 1025 in the pre-eclampsia group on average and the weight of infants were 3339 ±518 in the control group. In the Slovak republic, prevalence of preeclampsia was 2.8% between years 1997 and 2016 [5]. Subki et al. [16] carried out a retrospective study in Saudi Arabia and total 9493 deliveries were included. 224 women had hypertensive disorder during pregnancy (HDP), giving a prevalence of 2.4%. Of all the patients with HDP, the most prevalent hypertensive disorder was pre-eclampsia (54.9%). The mean age of patients with pre-eclampsia was 31.5 ± 6.9. Average BMI of women with pre-eclampsia was 27.9 ± 6.4. Patients delivered in 34.2 ± 3.2 week of gestation in average. Newborns weighed 2700.0 ± 800.0 in average.
Thomopoulos et al. [20] performed investigation of PubMed and the Cochrane Collaboration Library to identify if there is an increased risk of pregnancy hypertensive disorders following ART. They reviewed 1873 studies from PubMed and 182 studies from Cochrane database from the year 1978 to June 25, 2016. They found out, that IO was associated with an increased risk of pre-eclampsia. In vitro fertilization (IVF) treatment itself was significantly associated with an increased relative risk of pre-eclampsia. In another study, focused on ART and maternal morbidity, authors compared 2641 ART-derived pregnancies and 5282 spontaneous pregnancies as control. Pregnancies after ART were 1.49 times more likely to develop pre-eclampsia (95% CI 1.12–1.98) [23]. Significantly higher probability of pre-eclampsia at pregnancies following ART and multiple pregnancy gestation was also found out in herein presented study. Another risk factor has not been proved significant in our study. The results have shown, that the main risk factor is ART, because 3 patients (80%) with multiple gestation pregnancy conceived by using ART.
Many studies focused on serum parameters, which can predict pre-eclampsia or fetal growth restriction (FGR) in the first trimester. Table 4 shows comparison of the pre-eclampsia study. We have found out, MAP was significant as a screening test in 12th week of gestation. PAPP-A protein/MoM and UtA doppler weren´t significant as predictors of pre-eclampsia. The limitation of our study was small group with disease. Authors of some studies about pre-eclampsia suppose that low level of PAPP-A can be connected with this disease [2, 8, 9, 17, 22]. Some of them have proved it, others haven´t. Yu et al. [15], results are in the table 4. Murtoniemi et al. [9] published that UtA doppler and MAP is a significant and easy to examine predictor of pre-eclampsia. Livrinova et al. [7] focused on low level PAPP-A and adverse pregnancy outcome. These authors determined 0.4 MoM as a cut off level of PAPP. In this group, prevalence of hypertension or pre-eclampsia was 17.1%, compared to control group where prevalence of pre-eclampsia was 5.7%. The control group consisted of patients with PAPP over 0.4 MoM. Tserensambuu et al. [21] also stated, that PAPP-A, PlGF, UtA doppler, MAP are significant screening factors of pre-eclampsia in the first trimester. We found out that all patients with pre-eclampsia undertook cesarean section (100%). The rate of cesarean section was 37.9% in the control group.
4. Predictive test comparison in pre-eclampsia studies 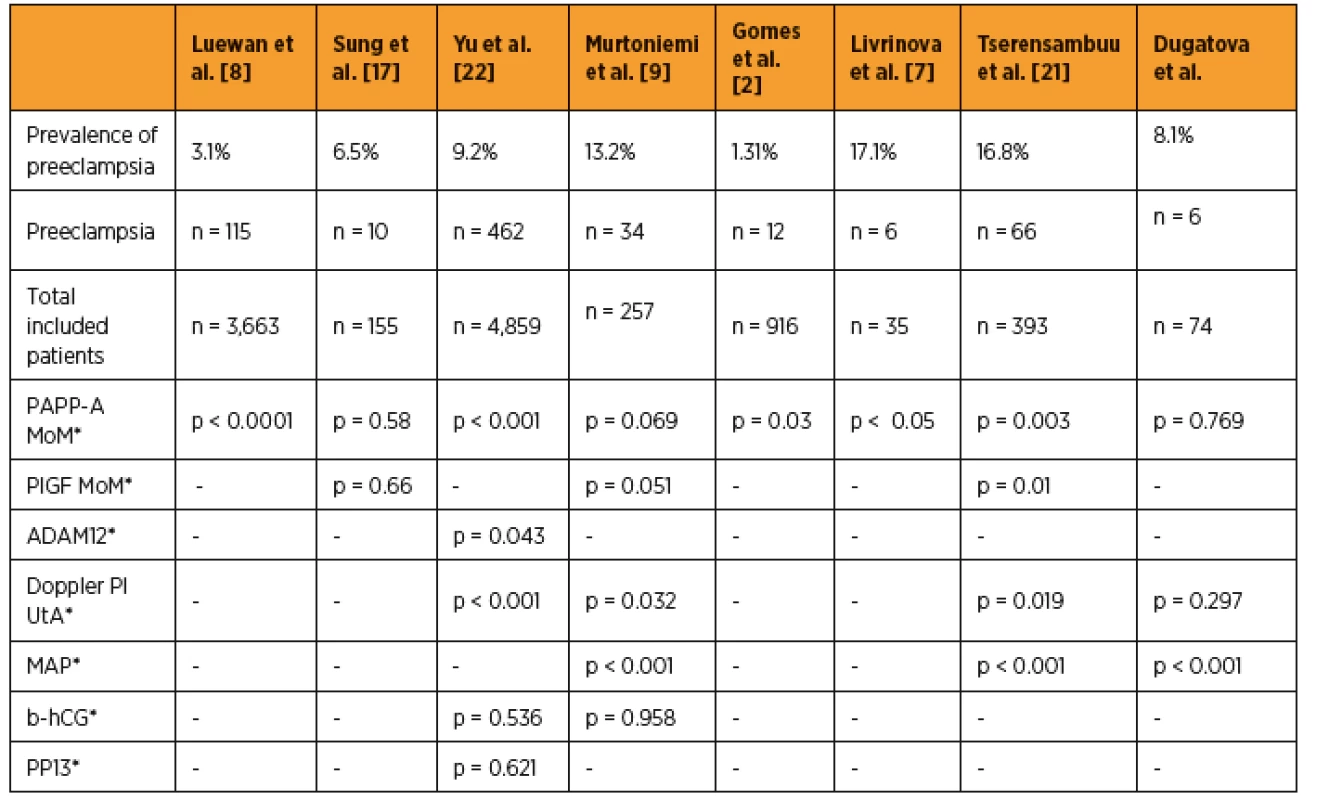
PAPP-A – pregnancy-associated plasma protein, MoM – multiple of the median, PlGF – placental growth factor, UtA – Doppler ultrasound of uterine artery, PI – pulsatility index, MAP – mean arterial pressure, ADAM12 – metalloprotease 12, PP13 – placenta protein 13, b-hCG – beta human chorionic gonadotropin, P-value less than 0.05 was considered as statistically significant The Italian study, focused on endothelial dysfunction and vascular stiffness in women with previous pregnancy complicated by pre-eclampsia, mentioned, that MAP, systolic and diastolic blood pressure was significantly higher in patients diagnosed with pre-eclampsia. All patients (100%) with early pre-eclampsia underwent cesarean section compared to 56.7% patients with late pre-eclampsia. Rate of cesarean section was 16.7% in the control group. Mean week of gestation at the time of delivery in the patients with early pre-eclampsia was 30 ± 6th compared to 37 ± 1th week of gestation in patients with late pre-eclampsia and 39 ± 1th week of gestation in the control group. Mean birth weight was 928 ± 539 at neonates delivered by patients with early pre-eclampsia, 2483 ± 561 at neonates delivered by patients with late pre-eclampsia and 3315 ± 485 in the control group [10]. In our study, we have also found out that neonates delivered by women with pre-eclampsia had a lower birth weight (control group 3340 g versus 2180 g in the pre-eclampsia group) and were delivered prematurally (control group mean 39 weeks of gestation versus mean 33 weeks of gestation in the pre-eclampsia group), too.
We have proved that pregnancy following ART and multiple gestation pregnancy are significant risk factors of pre-eclampsia. We have proved that MAP is useful and cost-effective predictors of pre-eclampsia. We have also found out that pre-eclampsia is connected with a higher rate of cesarean section. Correct measurement of blood pressure could lead to the reduction of pre-eclampsia and rate of cesarean section, too.
This work was supported by the Comenius university (grant/UK/152/2016).
MUDr. Monika Dugátová
1st Department of Gynecology and Obstetrics
Comenius University
Antolská 11
851 07 Bratislava
Slovakia
e-mail: mo.dugatova@gmail.com
Sources
1. American College of Obstetricians and Gynecologists. Hypertension Pregnancy – induced. Practice bulletin, 2013, 2, WQ 244.
2. Gomes, SM., Carlos-Alves, M., Trocado, V., et al. Prediction of adverse pregnancy outcomes by extreme values of first trimester screening markers. Obstet Med, 2017, 10(3), p. 132–137. doi: 10.1177/1753495X17704799.
3. Hlaváčik, M. Význam ultrazvukového vyšetrenia pri preeklampsii. Gynekol prax, 2017, 15(3), s. 148–151.
4. Jiang, M., Lash, EG., Zhao, X., et al. CircRNA-0004904, CircRNA-0001855, and PAPP-A: Potential novel biomarkers for the prediction of pre-eclampsia. Cell Physiol Biochem, 2018, 46, p. 2576–2586. doi: 10.1159/000489685.
5. Korbeľ, M., Nižňanská, Z., Havalda, A., et al. Preeklampsia – incidencia v Slovenskej republike v rokoch 1997–2016. Gynekol prax, 2017, 15(3), s.133–136.
6. Litwińska, E., Litwińska, M., Oszukowski, P., et al. Combined screening for early and late pre-eclampsia and intrauterine growth restriction by maternal history, uterine artery Doppler, mean arterial pressure and biochemical markers. Adv Clin Exp Med, 2017, 26(3), p. 439–448. doi: 10.17219/acem/62214.
7. Livrinova, V., Petrov, I., Samardziski, I., et. al. obstetric outcome in pregnant patients with low level of pregnancy-associated plasma protein a in first trimester. Open Access Macedonian J Med Sci, 2018, 6(6), p. 1028–1031. https://doi.org/10.3889/oamjms.2018.238.
8. Luewan, S., Teja-intr, M., Sirichotiyakul, S., et al. Low maternal serum pregnancy-associated plasma protein-A as a risk factor of pre-eclampsia. Singapore Med J, 2018, 59(1), p. 55–59. https://doi.org/10.11622/smedj.2017034.
9. Murtoniemi, K., Villa, MP., Matomäk, J., et al. Prediction of pre-eclampsia and its subtypes in high-risk cohort: hyperglycosylated human chorionic gonadotropin in multivariate models. BMC Pregnancy Childbirth, 2018, 18, p. 279. https://doi.org/10.1186/s12884-018-1908-9.
10. Orabona, R., Sciatti, E., Vizzardi, E., et al. Endothelial dysfunction and vascular stiffness in women with previous pregnancy complicated by early or late pre-eclampsia. Ultrasound Obstet Gynecol, 2017, 49, p. 116–123. doi:10.1002/uog.15893.
11. Oťapková, P., Záhumenský, J. Neskorá preeklampsia. Slov Gynek Pôrod, 2017, 24, s. 114–117.
12. Parra-Cordero, M., Rodrigo, R., Barja, P., et al. Prediction of early and late pre-eclampsia from maternal characteristics, uterine artery Doppler and markers of vasculogenesis during first trimester of pregnancy. Ultrasound Obstet Gynecol, 2013, 41, p. 538–544. doi:10.1002/uog.12264.
13. Pipkin, F., Phil, D. Risk factors for pre-eclampsia. N Engl J Med, 2001, 344, p. 925–926. doi: 10.1056/NEJM200103223441209.
14. Reismullerova, L., Holoman, K., Polackova-Borosova, M., et al. Polycystic ovary syndrome – a risk factor of pre-eclampsia after in vitro fertilisation. Bratisl Lek Listy, 2015, 116(5), s. 311–315. doi: 10.4149/BLL_2015_058.
15. Stepan, H., Hund, M., Gencay, M., et al. A comparison of the diagnostic utility of the sFlt-1/PlGF ratio versus PlGF alone for the detection of pre-eclampsia/HELLP syndrome. Hypertens Pregnancy, 2016, 35(3), p. 295–305. http://dx.doi.org/10.3109/10641955.2016.1141214.
16. Subki, HA., Algethami, RM., Baabdullah, MW., et al. Prevalence, risk factors, and fetal and maternal outcomes of hypertensive disorders of pregnancy: A retrospective study in Western Saudi Arabia. Oman Med J, 2018, 33(5), p. 409–415. doi 10.5001/omj.2018.75.
17. Sung, UK., Roh, AJ., Eoh, JK., et al. Maternal serum placental growth factor and pregnancy-associated plasma protein A measured in the first trimester as parameters of subsequent pre-eclampsia and small-for-gestational-age infants: A prospective observational study. Obstet Gynecol Sci, 2017, 60(2), p. 154–162. https://doi.org/10.5468/ogs.2017.60.2.154.
18. Tan, YM., Koutoulas, L., Wright, KHD., et al. Protocol for the prospective validation study: ‚Screening programme for pre-eclampsia‘ (SPREE). Ultrasound Obstet Gynecol, 2017, 50, p. 175–179. doi:10.1002/uog.17467.
19. Tan, YM., Wright, D., Syngelaki, A., et al. Comparison of diagnostic accuracy of early screening for pre-eclampsia by NICE guidelines and a method combining maternal factors and biomarkers: results of SPREE. Ultrasound Obstet Gynecol, 2018, 51, p. 743–750. doi: 10.1002/uog.19039. Epub 2018 Mar 14.
20. Thomopoulos, C., Salamalekis, G., Kintis, K., et al. Risk of hypertensive disorders in pregnancy following assisted reproductive technology: overview and meta-analysis. J Clin Hypertens (Greenwich), 2017, 19(2), p. 173–183. doi: 10.1111/jch.12945.
21. Tserensambuu, U., et al. The use of biochemical and biophysical markers in early screening for pre-eclampsia in Mongolia. Med Sci, 2018, 6, p. 57. doi: 10.3390/medsci6030057.
22 Yu, N., Cui, H., Chen, X., et al. First trimester maternal serum analytes and second trimester uterine artery Dopple r in the prediction of pre-eclampsia and fetal growth restriction. Taiwan J Obstet Gynecol, 2017, 56(3), p. 358–361. PMID: 28600048.
23. Zhu, L., Zhang, Y., Liu, Y., et al. Maternal and live-birth outcomes of pregnancies following assisted reproductive technology: A retrospective cohort study. Sci Reports, 2016, 6, p. 35141. doI: 10.1038/srep35141.
Labels
Paediatric gynaecology Gynaecology and obstetrics Reproduction medicine
Article was published inCzech Gynaecology

2019 Issue 4-
All articles in this issue
- Diagnosis of endometriosis 1st part – Overview of diagnostic approaches
- Diagnosis of endometriosis 2nd part – Ultrasound diagnosis of endometriosis (adenomyosis, endometriomas, adhesions) in the community
- Diagnosis of endometriosis 3rd part – Ultrasound diagnosis of deep endometriosis
- Distal vaginal agenesis
- Bladder rupture after a fall on the ground in a patient with total uterine prolapse
- Management of patient with breast carcinoma diagnosed during pregnancy
- Retroperitoneal bleeding caused by spontaneus rupture of renal angiomyolipoma following cesarean section treated with selective embolisation
- New serum tumor markers S100, TFF3 and AIF-1 and their possible use in oncogynecology
- Depression, anxiety in ovarian cancer patient
- Prediction of the pre-eclampsia in the first trimester – pilot study
- Czech Gynaecology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Diagnosis of endometriosis 3rd part – Ultrasound diagnosis of deep endometriosis
- Diagnosis of endometriosis 2nd part – Ultrasound diagnosis of endometriosis (adenomyosis, endometriomas, adhesions) in the community
- Diagnosis of endometriosis 1st part – Overview of diagnostic approaches
- Distal vaginal agenesis
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

