-
Medical journals
- Career
THE RELATIONSHIP BETWEEN OBSTRUCTIVE SLEEP APNEA AND CRANIOFACIAL ANOMALIES IN ADULT PATIENTS
Authors: N. Tkadlecová; P. Černochová
Authors‘ workplace: Stomatologická klinika Lékařské fakulty Masarykovy univerzity a Fakultní nemocnice u sv. Anny, Brno
Published in: Česká stomatologie / Praktické zubní lékařství, ročník 121, 2021, 4, s. 98-107
Category: Review Article
Overview
Introduction: Obstructive sleep apnoea (OSA) is considered to be a serious condition associated with daytime sleepiness which leads to cognitive impairment, hypertension, cardiovascular diseases and diabetes mellitus. OSA is a sleep disorder with multifactorial etiology characterised by recurrent episodes of partial or complete obstruction of upper airways during sleep, leading to a decrease in blood oxygen saturation level to 70%.
Presentation: The prevalence of OSA is high in adult patients in developed countries. Orthodontists often look after patients with OSA, whose craniofacial and dental anomalies, biotype of the facial skeleton and specific pathologies of the cervical spine are associated with obstruction of upper and lower airways. Craniofacial and dental anomalies usually involve skeletal class II., which is commonly combined with Angle class II. and increased overjet, mandibular posteriorotation, shorter mandibular length, increased mandibular plane angle in combination with anterior open bite, mandibular retrognathia, bimaxillary retrusion, narrow and high hard palate presenting as lateral crossbite. Other important signs include thicker and elongated soft palate, enlarged tongue and inferior position of the hyoid bone. Hypertrophy of the adenoid, palatine and lingual tonsils is also an important anatomic factor in etiology of OSA. Imaging methods are frequently used to diagnose these anomalies: orthopantomograms, lateral cephalograms, computed tomography and magnetic resonance imaging.
The aim of the article is to review craniofacial structures which contribute to developing OSA and to compare eight European studies proving the relationship between OSA and these anomalies.
Conclusion: Interdisciplinary cooperation among an orthodontist, otorhinolaryngologist, neurologist and internist is very important for appropriate treatment of OSA. The orthodontic examination should be performed in every patient with diagnosis OSA due to high frequency of the craniofacial abnormalities that are usually missed.
Keywords:
polysomnography – obstructive sleep apnoea – adult patients – lateral cephalograms – craniofacial anomalies – orthodontic therapy – obstructive sleep apnoea
INTRODUCTION
The respiratory system is a difficult biological system consisting of specific organs and structures used for gas exchange (O2 a CO2) between cells and blood vessels. The respiratory tract is morphologically divided into the upper airways (nose, nasal cavity, sinuses) and the lower airways (larynx, trachea, bronchi and lungs) because the pathways for air and for food cross in the pharynx [1]. Knowledge of anatomy of the pharynx is crucial for understanding the pathophysiology of obstructive sleep apnoea. The pharynx is a complex organ composed of three parts – the nasopharynx, mesopharynx and hypopharynx. The size of the pharyngeal space is affected by a relative growth and size of soft tissues surrounding the dentofacial skeleton [2, 3]. The main functions of the pharynx are breathing, swallowing and phonation. The pharyngeal muscles are key structures enabling these functions by changing a shape of its lumen. While the pharyngeal muscles and the muscles of the soft palate are relaxed when breathing [1, 4], the activity of the pharyngeal muscles falls as a consequence of a decrease in function of the hypoglossal nerve, which is responsible for the contraction and dilatation of the pharyngeal muscles [5]. Resistance in the respiratory airways rises as a result. Higher pressure in the upper airways causes a reflective contraction of the pharyngeal muscles which avoids their collapse and a development of sleep apnoea [4]. Craniofacial and dental anomalies, cervical spine pathologies, hypertrophy of the lymphatic tissues, disruption of neuromuscular activity of the upper airways and other factors can lead to the pharyngeal collapse and contribute to a development of sleep–related disorders [6]. There are many studies investigating the relationship between pathologies of the cranial and cervical skeletal structures and the development of obstructive sleep apnoea (OSA). The aim of this review is to compare eight European studies which analyse relations between OSA and craniofacial abnormalities diagnosed by lateral cephalogram, and to emphasize relevant anatomical structures of the head for studies on OSA.
DEFINITION AND TYPES OF SLEEP APNOEA
Apnoea is defined as a cessation of breathing for more than 10 seconds. Hypopnoea is defined as a 50% decrease in transport of gas through the alveolar capillary membrane to the blood for 10 seconds or more. It is also accompanied by a 3% decrease in blood oxygen saturation which leads to waking up [4, 7, 8]. The syndrome of obstructive sleep apnoea is considered to be one of the most frequent sleep-related breathing disorders [9]. The prevalence of OSA is high, 34% in men and 17% in women. However, the syndrome is not diagnosed very often [10]. OSA comprises 3 main types: obstructive, central and the mixed. Apnoea is considered to be obstructive in case of present breathing effort and at least 5 obstructive respiratory events per hour during sleep. Typical symptoms of OSA include daytime sleepiness, loud snoring and awakening due to gasping [4, 11]. The central sleep apnoea is characterised by an absence of breathing effort caused by a dysfunction of the central nervous system. In some cases, the central sleep apnoea is associated with Cheyne-Stokes respiration [12–14]. Mixed apnoea is defined as a combination of central and obstructive sleep apnoea. Central apnoea without any respiratory effort appears first. Respiratory effort evolves later, leading to the development of obstructive apnoea [4].
The severity of sleep apnoea can be defined using AHI (apnea – hypopnea index) which is a number of obstructive events (apnea/hypopnea) per hour of sleep [15]. Based on this index, apnoea can be classified as mild (AHI 5–15 apneas/hypopneas per hour of sleep), moderate (AHI 15–30) and severe (AHI > 30) [16].
Many risk factors for OSA have been identified. The most common risk factors are obesity, old age, diabetes mellitus, hypertension, smoking and alcohol. Other risk factors include craniofacial, cervical and dental abnormalities which play an important role in occurrence of OSA. These abnormalities are not associated with anatomic changes like inability to enter deep sleep, poor neuromuscular response or instability of ventilation control mechanisms [6, 17, 18]. On the other hand, OSA is a very important predictive factor for a development of cardiovascular diseases because it leads to hypertension and is also linked to atrial fibrillation, myocardial infarction and cardiac failure [19].
CRANIOFACIAL AND CERVICAL ANOMALIES AS RISK FACTORS FOR OSA
Craniofacial, cervical and dental anomalies are considered to be predisposing factors for OSA according to many studies. These abnormalities can be visible on lateral cephalometric radiographs, which are used as the main diagnostic tool especially thanks to its feasibility, low costs, reproducibility and minimal exposure to radiation [20, 21]. The cephalometric analysis can contribute to a diagnosis, reveal a patients predisposition to OSA and help orthodontists initiate the right treatment.
The following text lists and describes structures which are relevant to diagnosing obstructive sleep apnoea in Caucasian population.
POSITION OF MAXILLA AND MANDIBLE OF PATIENTS WITH OSA
An important factor for the development of OSA is jaw relations. Many studies have demonstrated that patients with OSA have decreased SNB angle, reduced length of mandible and growth posteriorotation [22, 23].
The position of jaws is determined by the ANB angle and WITS which classify patients into skeletal classes [24]. The syndrome of obstructive sleep apnoea is common among patients with skeletal class II, ANB > +5°. This angle is also a difference between the SNA angle (determines the sagittal position of the maxilla to the cranial base) and SNB angle (determines the sagittal position of the mandible to the cranial base) [24–26].
While reduced maxillary length (the distance between the anterior nasal spine and posterior nasal spine) has been demonstrated in many studies, the sagittal position of the maxilla to the cranial base does not vary [22, 27] (fig. 1).
1. Position of jaws related to the cranial base and pointing out the maxillary corpus
Point A: the most concave point of anterior maxilla;
Point B: the most concave point on mandibular symphysis;
N: nasion; S: the middle of sella turcica;
Spp: the most posterior point on spina nasalis posterior;
Spa: the most anterior point on spina nasalis anterior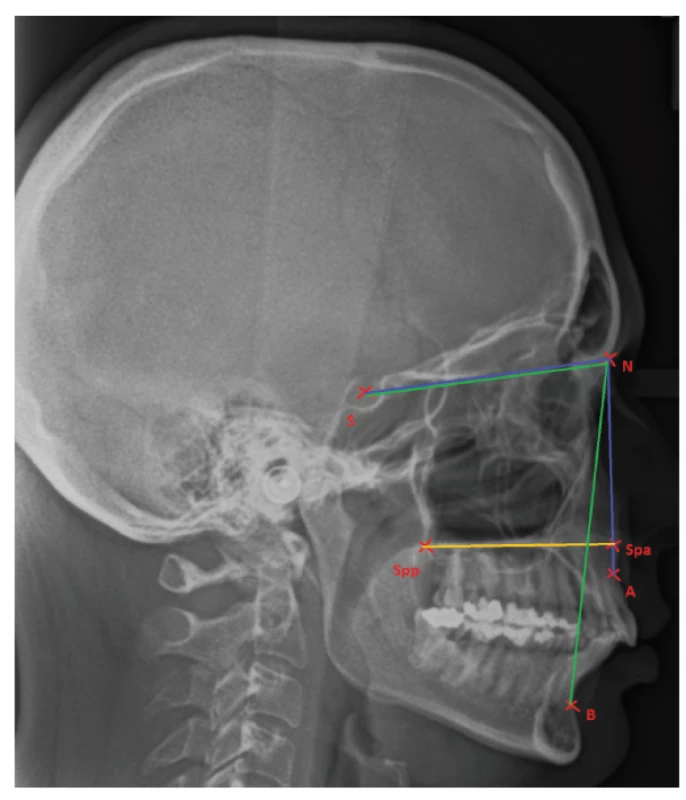
One of the important morphologic signs in patients with OSA is the high-vaulted hard palate which is usually present in combination with the narrow dental arch. This can result in a reduction of the oropharyngeal space, especially the space for the tongue [28].
Cranial base
Many studies have confirmed that patients with OSA have significantly shorter anterior cranial base, which is the line between the midpoint of the sella turcica (S) and the nasion (N) – the most anterior point of the frontonasal suture. Considerably shorter cranial base has also been noted, which is the line between the nasion (N) and the basion (Ba), the midpoint of the anterior margin of the foramen magnum [24, 29–31]. Additionally, patients with OSA have a reduced cranial base angle (S-N-Ba), manifesting as a bimaxillar retrusion and narrowed upper airways at the level of the oropharyngeal isthmus [22]. The bimaxillar retrusion is a statistically significant factor in Hispanic population in comparison with other ethnic groups [32]. According to Eva Hellsing, the upper airways patency is dependent on head posture when performing lateral cephalograms [33]. Solow et al. studied the position of the head and neck in patients with OSA and in healthy individuals. When interpreting lateral cephalographic radiographs, it has been proved that patients with OSA have an increased angle of cranio-cervical angulation (fig. 2), which is related to the hypertrophic adenoids causing obstruction of the upper airways. After adenoidectomy, the posture of the head and neck changed in these patients and a reduction in the angle of cranio-cervical angulation was observed in some cases [34].
2. Fig. 2 Shows the position of the head to the cervical column; the craniocervical angle (NSL-OPT); taken from [34] NSL: line from N (nasion) to S (sella);
OPT: inclination of the cervical column![Fig. 2 Shows the position of the head to the cervical column; the craniocervical angle (NSL-OPT); taken from [34]
NSL: line from N (nasion) to S (sella);<br> OPT: inclination of the cervical column](https://www.prolekare.cz/media/cache/resolve/media_object_image_small/media/image_pdf/44f32625e43a05ffa1e8e32234fd985a.png)
Facial height
Changes in anterior facial height and in its lower portion have been noted in patients with OSA. Both of these are longer in patients with OSA in comparison with non-OSA subjects [35–38] (fig. 3).
3. Lateral cephalogram expressing the term „anterior facial height“
N: nasion;
S: the middle of sella turcica;
Spp: the most posterior point on spina nasalis posterior;
Spa: the most anterior point on spina nasalis anterior;
Me: menton, the lowest point on mandibular symphysis;
Gn: gnathion, the lowest point of the midline of the lower jaw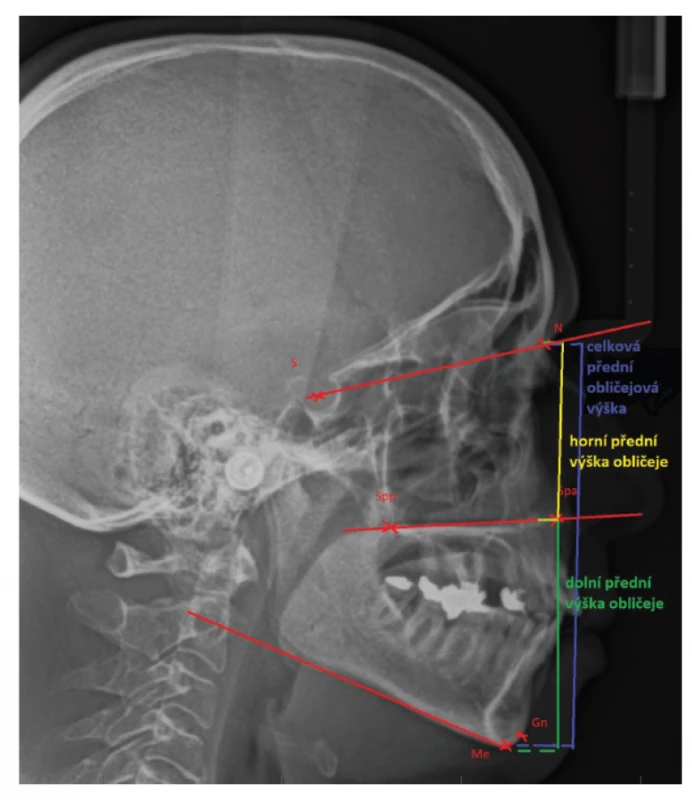
Size of the tongue and soft palate
Examination of the tongue and soft palate is a necessary part of a dental visit. Approximate size of these structures can be checked by a simple inspection. However, diagnosing macroglossia is much more challenging – indentations on the lateral side of the tongue are a typical sign [4]. Relative size of the tongue and soft palate can be evaluated using the Mallampati score which was originally used to assess a difficulty of an intubation. On examination, patient’s mouth is wide open and a doctor measures a distance between the soft palate and the root of the tongue. Patients with severe obstructive sleep apnoea are usually classified as IV. grade of Mallampati score – the soft palate and uvula are not visible [4, 39]. Lateral cephalogram, CT and MRI can be used for a more precise assessment of the size of the tongue and soft palate. Many publications have found the elongated and thick soft palate, along with an increased size of the tongue, are linked to OSA (fig.4) [22, 40–42]. Muscle fibres of the tongue attach to the hyoid bone, so its position is dependent on the location of the hyoid bone. Inferior position of the hyoid bone lowers the base of the tongue which results in narrowed upper airways [43, 44].
4. The length of soft palace – the distance between Spp (spina palatina posterior) and the point P (the tip of soft palate); the width of soft palate in the widest place;
the length of tongue – distance between the point Tt (the anterior tip of tongue) and the point Eb (base of epiglottis);
H – position of hyoid bone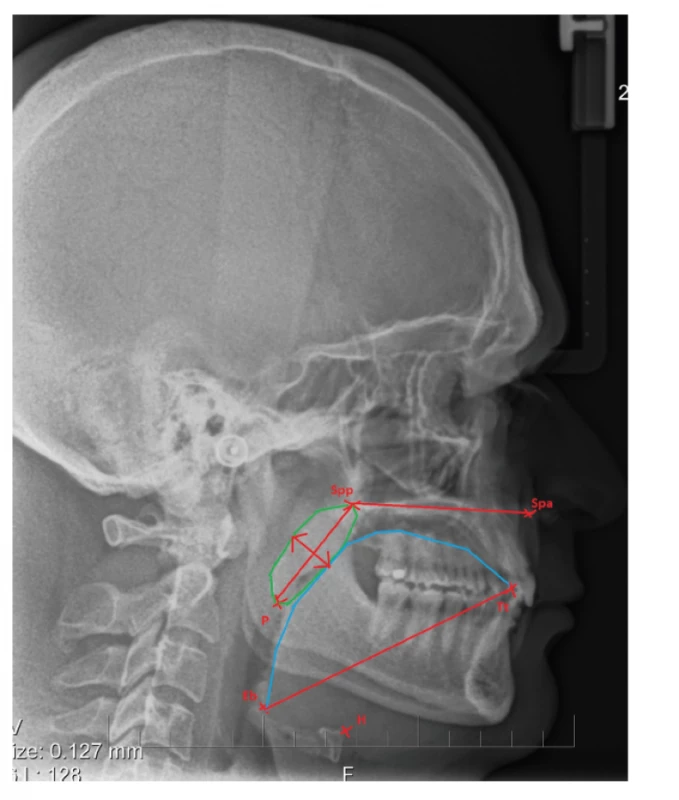
Position of the hyoid bone
The hyoid bone is located under the body of the tongue. Its shape resembles a horseshoe. The hyoid bone consists of two pairs of processes: the greater horns and the lesser horns, and of the body. The hyoid bone is palpable in very thin persons. It is connected to other anatomical structures by ligaments and muscles which are classified into two groups: the suprahyoid muscles and infrahyoid muscles. Activity of these muscles causes changes the position of the hyoid bone [45]. Inferiorly positioned hyoid bone was a common finding in many studies in comparison with non-OSA control group (fig. 4) [38, 41, 46–49]. The distance between the hyoid bone and the mandibular plane was measured in most cases. However, some authors measured the distance between the hyoid bone and the third or fourth cervical vertebra or the distance between the hyoid bone and the epiglottic vallecula [41, 42, 48]. As it has already been mentioned, the position of the hyoid bone is related to the position of the tongue and its base is located in the hypopharyx in case of the inferior position of the hyoid bone. This can be the main reason for failure of the treatment of patients with OSA when splints are used for a mandibular advancement [50, 51]. The position of the hyoid bone plays a key role in the upper airways patency. The inferior position of the hyoid bone and the base of the tongue can lead to an increase in a mandibular load because higher energy is required to elevate the tongue. As a result, patients with apnoea sleep with their mouth open which worsens their underlying obstructive sleep apnoea [52, 53].
Pharynx
The oropharynx is the most frequent site of obstruction of the upper airways and is subdivided into the retropalatal part and the retroglossal part [4]. Four groups of the pharyngeal muscles enable constriction and dilatation of the lumen of the upper airways. These muscles control the position of the soft palate, tongue, hyoid bone and posterolateral pharyngeal wall [54]. During cephalometric evaluation of the upper airways dimensions, McNamara analysis can be used. The dimensions of the oropharynx are measured in two places. The first one is the area between the soft palate and the most anterior point of the posterior pharyngeal wall. The second one is in the retrolingual area [55]. Many patiens with OSA have been found to have narrowed upper airways on a cephalogram by multiple studies [22. 29, 56]. Although it is a low cost method with a low exposure to radiation, it is impossible to precisely diagnose the narrowest portion due to the absence of 3D imaging. Nowadays, computed tomography is deemed to be a more reliable diagnostic tool to determine the narrowest segment of the airways and can also be used to compare the airways before and after maxillofacial surgery [57].
Dental anomalies
Patients with OSA are often diagnosed with dental anomalies. Most of them are caused by mouth breathing, presence of adenoid hypertrophy, bad habits and also a delay in starting the orthodontic treatment. Many studies have shown a correlation between OSA and narrow maxilla and high-vaulted hard palate with unilateral/bilateral lateral cross bite (fig. 5a, fig. 5b), frontal open bite and significant crowding in the upper and lower dentition [37, 58, 59]. Other frequently observed factors include protrusion of the central incisors and the increased overjet which are associated with skeletal class II and in many cases with Angle class II [60]. Kushida et al. created a morphometric model for prediction of OSA in adult patients. This model has proved to be very useful thanks to its reproducibility. The model is based on patient´s BMI index, neck circumference and specific parametres measured in the oral cavity, such as palatal height, width of the upper and lower jaws and the overjet [61].
5. a Lateral cross bite in patient with extracted upper first premolars and increased overjet 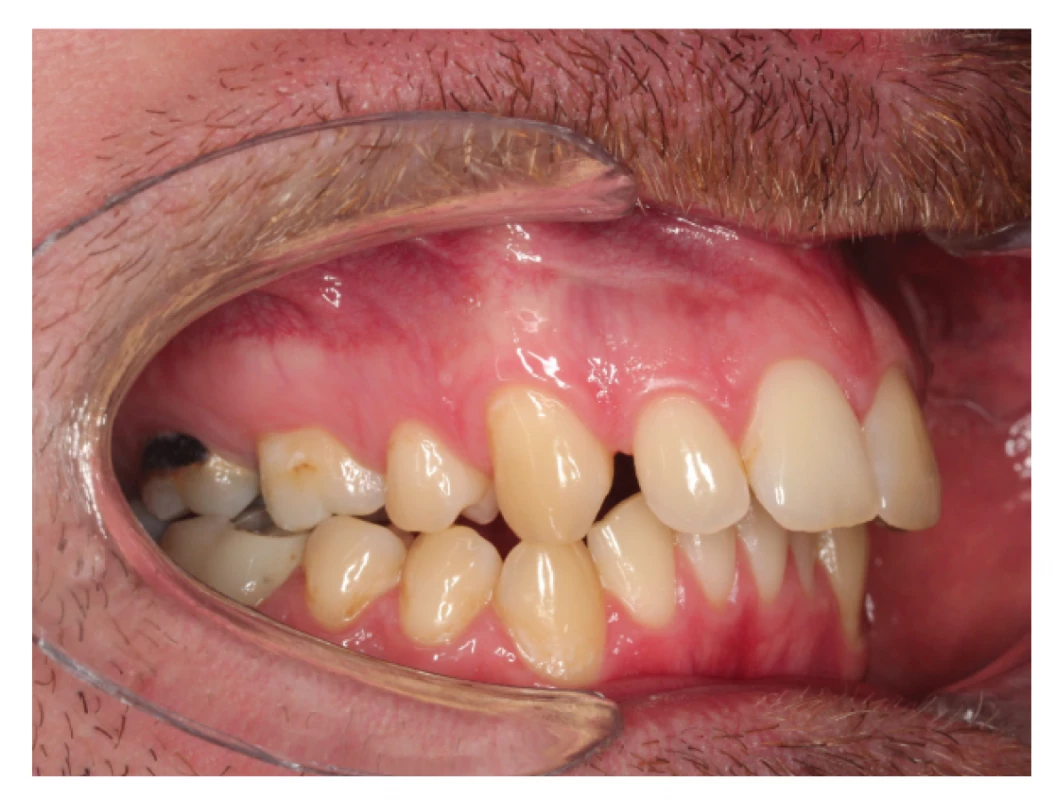
Starting treatment of orthodontic anomalies at a young age is essential with a positive effect on the jaw growth. Rapid maxillary expansion and mandibular advancement are effective treatment options for growing patients [58, 62]. Kaditis et al. suggested the algorithm for treatment of children with OSA. This approach involves observing the patient´s weight, treatment with nasal corticosteroids in case of tonsillar hypertrophy with the aim of its reduction or adenoidectomy, treatment with removable orthodontic appliances, continuous positive airway pressure, maxillofacial surgery or tracheostomy in case of previous treatment failure [63].
THE RELATIONSHIP BETWEEN CRANIOFACIAL ANOMALIES AND OSA IN EUROPE
European studies published between 1990 and 2020 were selected and compared. Both materials and methods were evaluated in the same way in all these studies. All of the studies focused on analysis of the craniofacial structures on lateral cephalometric radiographs of patients with OSA. The compared studies took place in European countries (England, France, Netherlands, Italy, Poland, Croatia and Turkey) and examined patients with OSA with different values of the AHI. The patients were divided into many different groups (men, women, age, BMI). The study population comprised of 16–99 patients (Tab. 1). The results of the patients with OSA were mostly compared with non-OSA subjects or with the normalized values [29, 42, 50, 64–68].
1. European studies determining correlation between OSA and craniofacial anomalies 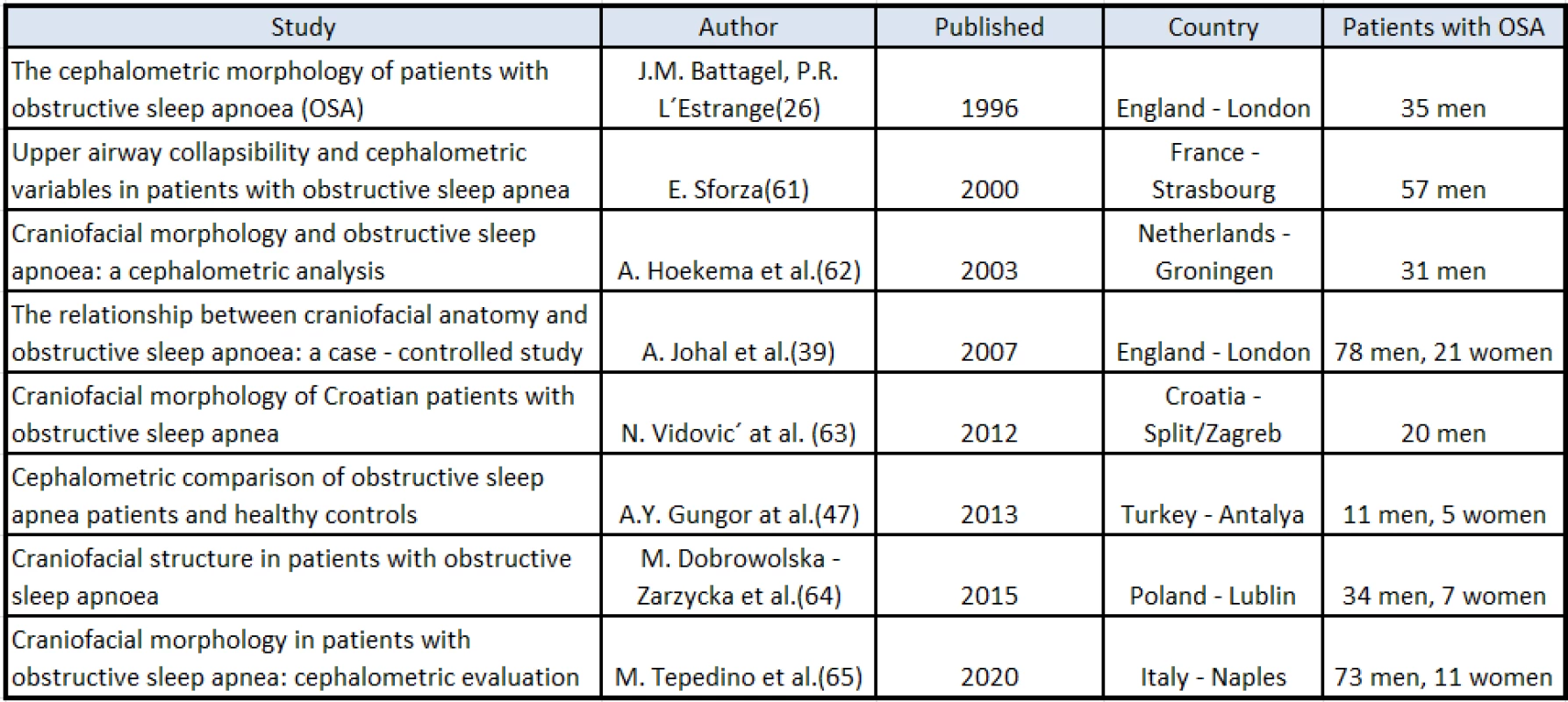
All the examined patients underwent polysomnography and severity of OSA was monitored by the RDI (respiratory disturbance index) from 5.0 to 97.0 or by the AHI from 4.0 to 80.0.
Battagel et al. from London conducted a comprehensive study. They analysed lateral cephalometric radiographs in a group of 35 men whose OSA had been diagnosed by polysomnography. The control group included 24 men without a past medical history of respiratory diseases, problems with snoring or daytime sleepiness. The age ranged between 26.0 and 73.5 years, the average age was 50.9 years. The control group composed of 31 men aged 25.9–50.5 years, with the average age of 41.8 years. The control group statistically differed from the experimental one in weight and lower BMI. The average BMI (24.5) in the control group was in normal range, whereas the average BMI of patients with OSA was 30.6, which falls within the obesity range [29].
In contrast, the team of orthodontists from Lublin in Poland studied a group of 41 patients with the AHI higher than 4, consisting of 8 women (17%) and 34 men (83%) aged between 38 and 74 years. 75% of the patients were at the age of 50–65 years and their AHI ranged from 9.1 to 80.6 (30.8 on average) episodes of apnoea per 1 hour of sleep. BMI was between 24 and 41 (the average of 30.9) and 22 patients (54%) were classified as obese in total [64].
Some studies included control groups of non – OSA subjects. These comprised of 20 to 37 men with lower BMI than patients in the experimental group who had been diagnosed with obstructive sleep apnoea.
The same methodology was used in all these studies for analysis of the craniofacial structures. The lateral cephalometric radiographs were performed in all the subjects thanks to its accuracy, good reproducibility, minimal exposure to radiation and low cost in comparison with modern imaging methods, such as computed tomography (CT) or magnetic resonance imaging (MRI). The observed craniofacial structures were described, measured and compared with standard values or values obtained by measurements in reference groups.
The cephalometric analysis of morphologic structures and anatomic diameters was in most studies (England, Italy, Croatia, Turkey) carried out using conventional points of the skeletal structures and soft tissues [29, 42, 50, 65, 68]. The authors marked 22 points in the area of the cervical vertebrae, oropharynx, epiglottis, soft palate and tongue, and circumscribed the intermaxillar space, soft palate and tongue consequently [29].
Various statistical methods for analysis of the measured parametres were used, especially analysis of variety (ANOVA) or Pearson’s correlation coefficient.
The scientists confirmed morphology of the orofacial structures and some surrounding cranial structures in patients with OSA significantly differ from the average values of healthy European population, especially the upper airways patency. The width of the upper airways was measured on lateral cephalometric radiographs in two sagittal planes (upper and lower) and significantly narrowed upper airways were found in 75.6% of patients with OSA [64]. The diameter of the upper pharyngeal space, according to McNamara´s diagnosis, is 10.4 mm on average. In a study conducted by Dobrowolska – Zarzycka et al., the diametre was lower. The same results were also confirmed by other authors [29, 66].
The association between the development of OSA and a relative enlargement of the tongue and a thickening of the soft palate was proved.
Many studies also found that the greater distance between the hyoid bone and the mandibular plane contributes to the development of OSA. The hyoid bone was located more inferiorly in comparison with the control group. This was confirmed by measuring the distance from the hyoid bone to the C3, its distance to the most posterior point of the mandibular symphysis and its distance to the Frankfurt plane [67]. The soft palate was longer and wider, and also its area was larger [42].
The studies showed that the most relevant predisposing factor for the development of OSA is the length of the mandible – its shortening, especially of the distance between the gonion and the menton or the distance between the gonion and the B point is in correlation with OSA. The length of the body of the mandible was shorter by 5.9 mm on average in patients with OSA than in the control group. Similarly, the distance was shorter between the gonion and the point B, on average, by 5.6 mm [29]. The size of the area of the intermaxillar space is also a predisposing factor for the development of OSA, which was shown in the study of Battagel et al. They discovered that a reduction in the space of the intermaxillar area is by 4.1 cm2 on average in patients with OSA [29]. In addition, the length of the intermaxillary space (the distance between the posterior pharyngeal wall and the tip of the lower incisors) was shorter by 5.7 mm than in the control group [29]. Other authors found out a similar correlation [42, 46]. Johal et al. showed shortening of this distance by 3.1 mm on average in patients with OSA compared to the control group [42]. All patients with OSA had measured size of the breathing area reduced by 66% compared with patiens in the control group.
The authors discovered many important distances and angles on the skull with the aim of showing their correlation with obstructive sleep apnoea. Vidovic et al. demonstrated a shorter distance from the point S to the point N (the anterior cranial base) [68]. Johal et al. and Hoekema et al. showed a statistically significant difference between the angles SNA and SNB [42, 67]. Finally, other significant factors which are associated with the development of OSA are: reduction of the middle facial height, protrusion of the upper central incisors or the distance between the hyoid bone and the C3. It is obvious that obstructive sleep apnoea depends on an interaction of many factors which cause relaxation of the pharyngeal muscle tone, their collapse and the development of apnoea or hypopnoea. The size and patency of the nasopharyngeal lumen depends on congenital and acquired anatomic abnormalities of the skeleton, caused by infections of the upper airways.
All the craniofacial factors mentioned above are worsened by a higher BMI.
CONCLUSION
Medline, Pubmed and Scopus databases were used as resourses. Including patients from European countries who had been diagnosed with obstructive sleep apnoea (OSA) and who had a lateral cephalometric radiograph performed was a major defining criterion.
To prove the impact of orthodontic anomalies on OSA, lateral cephalometric radiography is commonly used to identify various morphological skeletal abnormalities as well as anomalies of soft tissues of the face, oral cavity, and pharynx. Although computed tomography and magnetic resonance imaging are more accurate to diagnose OSA, a lateral cephalogram is diagnostically, economically, and medically sufficient.
Orthodontists are important for diagnosis of anatomical deviations, prediction, prevention, and treatment of OSA in early stages of the syndrome. Inhaled/exhaled ratio may be worsened by a pathological development of soft tissues and skeletal structures or a significantly raised BMI.
In conclusion, other studies have shown that orthodontists are able to precisely detect morphological anomalies of the airways, differentiate the disease and refer a patient to different specialties for further assessment. Prompt diagnosis and treatment may minimize detrimental effects of the syndrome.
MDDr. Nela Tkadlecová
Stomatologická klinika a ortodontické oddělení
Fakultní nemocnice u svaté Anny
Pekařská 53
625 00 Brno
e-mail: nela.tkadlecova@fnusa.cz
Sources
1. Páč L. Anatomie člověka II : splanchnologie, kardiovaskulární systém, žlázy s vnitřní sekrecí. 1. vyd. Brno, Czechia: Masarykova univerzita; 2010, 192.
2. Lopatienė K, Dabkutė A, Juškevičiūtė V. Vertical and sagittal morphology of the facial skeleton and the pharyngeal airway. Stomatologija. 2016; 18(1): 21–25.
3. Ghoneima A, AlBarakati S, Jiang F, Kula K, Wasfy T. Computational fluid dynamics analysis of the upper airway after rapid maxillary expansion: a case report. Prog Orthod. 2015; 16 : 10.
4. Šonka K. Apnoe a další poruchy dýchání ve spánku. 1. vyd. Praha: Grada Publishing; 2004.
5. Tangel DJ, Mezzanotte WS, White DP. Influence of sleep on tensor palatini EMG and upper airway resistance in normal men. J Appl Physiol (1985). 1991; 70(6): 2574–2581.
6. Khan A, Than KD, Chen KS, Wang AC, La Marca F, Park P. Sleep apnea and cervical spine pathology. Eur Spine J. 2014; 23(3): 641–647. doi: 10.1007/s00586-013-3046-4
7. Děje na alveolokapilární membráně a perfúze plic. Funkce buněk a lidského těla. Skripta. [Internet]. [cit. 10. 4. 2021]. Dostupné z: http://fblt.cz/skripta/vi-dychaci-soustava/3-deje-na-alveolokapilarni-membrane-a-perfuze-plic/
8. Moran M. Syndrom spánkové apnoe a pohybová aktivita ve spánku. Med pro Praxi. 2008; 5(11): 440–442. [cit. 19. 12. 2008]. Dostupné z: https://www.medicinapropraxi.cz/artkey/med-200811-0010.php
9. Svanholt P, Petri N, Wildschiødtz G, Sonnesen L, Kjaer I. Associations between craniofacial morphology, head posture, and cervical vertebral body fusions in men with sleep apnea. Am J Orthod Dentofac Orthop. 2009;135(6): 702.e1–9; discussion 702–703.
10. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013; 177(9): 1006–1014.
11. Banabilh SM. Orthodontic view in the diagnoses of obstructive sleep apnea. J Orthod Sci. 2017; 6(3): 81–85.
12. Thorpy MJ. Classification of sleep disorders. Neurotherapeutics. 2012; 9(4): 687–701. [cit. 14. 9. 2020]. Dostupné z: https://doi.org/10.1007/s13311-012-0145-6
13. Hall MJ, Xie A, Rutherford R, Ando S, Floras JS, Bradley TD. Cycle length of periodic breathing in patients with and without heart failure. Am J Respir Crit Care Med. 1996; 154(2 Pt 1): 376–381.
14. Naughton MT. Cheyne-Stokes respiration: friend or foe? Thorax. 2012; 67(4): 357–360.
15. Epstein LJ, Kristo D, Strollo PJ, Friedman N, Malhotra A, Patil SP, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009; 5(3): 263–276.
16. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002; 165(9): 1217–1239.
17. Abdissa D. Prevalence of obstructive sleep apnea risk and associated factors among patients with type 2 diabetes mellitus on follow up at Jimma Medical Center, Southwest Ethiopia. J Clin Transl Endocrinol. 2020; 21 : 100234.
18. Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013; 188(8): 996–1004.
19. Javaheri S, Barbe F, Campos-Rodriguez F,Dempsey JA, Khayat R, Javaheri S, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. 2017; 69(7): 841–858. [cit. 3. 12. 2020]. Dostupné z: http://www.sciencedirect.com/science/article/pii/S0735109717300098
20. Borges P de TM, Silva BB da, Moita Neto JM, Borges NE de S, Li LM. Cephalometric and anthropometric data of obstructive apnea in different age groups. Braz J Otorhinolaryngol. 2015; 81(1): 79–84.
21. Ryu HH, Kim CH, Cheon SM, Bae WY, Kim SH, Koo SK, et al. The usefulness of cephalometric measurement as a diagnostic tool for obstructive sleep apnea syndrome: a retrospective study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015; 119(1): 20–31.
22. Neelapu BC, Kharbanda OP, Sardana HK, Balachandran R, Sardana V, Kapoor P, et al. Craniofacial and upper airway morphology in adult obstructive sleep apnea patients: A systematic review and meta-analysis of cephalometric studies. Sleep Med Rev. 2017; 31 : 79–90.
23. Miles PG, Vig PS, Weyant RJ, Forrest TD, Rockette HE. Craniofacial structure and obstructive sleep apnea syndrome – a qualitative analysis and meta-analysis of the literature. Am J Orthod Dentofacial Orthop. 1996; 109(2): 163–172. [cit. 15. 12. 2020]. Dostupné z: http://www.sciencedirect.com/science/article/pii/S0889540696701774
24. Kamínek M. et al. Ortodoncie. Praha: Galén; 2020. [cit. 15. 12. 2020]. Dostupné z: http://www.medvik.cz/link/MED00204841
25. Indriksone I, Jakobsone G. The upper airway dimensions in different sagittal craniofacial patterns: a systematic review. Stomatologija. 2014; 16(3): 109–117.
26. Kim SJ, Kim YS, Park JH, Kim SW. Cephalometric predictors of therapeutic response to multilevel surgery in patients with obstructive sleep apnea. J Oral Maxillofac Surg. 2012; 70(6): 1404–1412.
27. Jacobson A. The ‘Wits’ appraisal of jaw disharmony. Am J Orthod. 1975; 67(2): 125–138.
28. Victor LD. Obstructive sleep apnea. Am Fam Physician. 1999; 60(8): 2279–2286.
29. Battagel JM, L’Estrange PR. The cephalometric morphology of patients with obstructive sleep apnoea (OSA). Eur J Orthod. 1996; 18(6): 557–569.
30. Ficke J, Varacallo M. Anatomy, head and neck, foramen magnum. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020. [cit. 16. 12. 2020]. Dostupné z: http://www.ncbi.nlm.nih.gov/books/NBK526041/
31. Aihara K, Oga T, Harada Y, Chihara Y, Handa T, Tanizawa K, et al. Analysis of anatomical and functional determinants of obstructive sleep apnea. Sleep Breath Schlaf Atm. 2012; 16(2): 473–481.
32. Liu Y, Lowe AA, Zeng X, Fu M, Fleetham JA. Cephalometric comparisons between Chinese and Caucasian patients with obstructive sleep apnea. Am J Orthod Dentofac Orthop. 2000; 117(4): 479–485.
33. Hellsing E. Changes in the pharyngeal airway in relation to extension of the head. Eur J Orthod. 1989; 11(4): 359–365.
34. Solow B, Ovesen J, Nielsen PW, Wildschiødtz G, Tallgren A. Head posture in obstructive sleep apnoea. Eur J Orthod. 1993; 15(2): 107–114.
35. Kikuchi M, Higurashi N, Miyazaki S, Itasaka Y. Facial patterns of obstructive sleep apnea patients using Ricketts’ method. Psychiatry Clin Neurosci. 2000; 54(3): 336–337. [cit. 16. 12. 2020]. Dostupné z: https://onlinelibrary.wiley.com/doi/abs/10.1046/j.1440-1819.2000.00703.x
36. Capistrano A, Cordeiro A, Capelozza Filho L, Almeida VC, Silva PI de CE, Martinez S, et al. Facial morphology and obstructive sleep apnea. Dent Press J Orthod. 2015; 20(6): 60–67.
37. Lowe AA, Santamaria JD, Fleetham JA, Price C. Facial morphology and obstructive sleep apnea. Am J Orthod Dentofacial Orthop. 1986; 90(6): 484–491. [cit. 16. 12. 2020]. Dostupné z: http://www.sciencedirect.com/science/article/pii/0889540686901083
38. Tangugsorn V, Skatvedt O, Krogstad O, Lyberg T. Obstructive sleep apnoea: a cephalometric study. Part I. Cervico-craniofacial skeletal morphology. Eur J Orthod. 1995; 17(1): 45–56.
39. Mallampati SR, Gatt SP, Gugino LD, Desai SP, Waraksa B, Freiberger D, et al. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J. 1985; 32(4): 429–434.
40. Welch KC, Foster GD, Ritter CT, Wadden TA, Arens R, Maislin G, et al. A novel volumetric magnetic resonance imaging paradigm to study upper airway anatomy. Sleep. 2002; 25(5): 532–542.
41. Stipa C, Cameli M, Sorrenti G, Ippolito DR, Pelligra I, Alessandri-Bonetti G. Relationship between cephalometric parameters and the apnoea-hypopnoea index in OSA patients: a retrospective cohort study. Eur J Orthod. 2020; 42(1): 101–106.
42. Johal A, Patel SI, Battagel JM. The relationship between craniofacial anatomy and obstructive sleep apnoea: a case-controlled study. J Sleep Res. 2007; 16(3): 319–326.
43. Yanase Y, Amano N, Satoda T, Masuda Y, Kawagishi S, Yoshino K. Neostriatal stimulation activates tongue-protruder muscle, but not tongue-retractor or facial muscles: an electrical and chemical microstimulation study in rats. Brain Res. 2001; 893(1–2): 282–286.
44. Lam B, Ooi CGC, Peh WCG, Lauder I,Tsang KWT, Lam WK, et al. Computed tomographic evaluation of the role of craniofacial and upper airway morphology in obstructive sleep apnea in Chinese. Respir Med. 2004; 98(4): 301–307.
45. Sineľnikov RD, Čihák R, Lemež L. Atlas anatomie člověka. Sv. 1. Nauka o kostech, kloubech, vazech a svalech. Praha: Avicenum; 1980. [cit. 20. 12. 2020]. Dostupné z:http://www.medvik.cz/link/MED00063898
46. Riha RL, Brander P, Vennelle M, Douglas NJ. A cephalometric comparison of patients with the sleep apnea/hypopnea syndrome and their siblings. Sleep. 2005; 28(3): 315–320.
47. Faber CE, Grymer L. Available techniques for objective assessment of upper airway narrowing in snoring and sleep apnea. Sleep Breath Schlaf Atm. 2003; 7(2): 77–86.
48. Sforza E, Bacon W, Weiss T, Thibault A, Petiau C, Krieger J. Upper airway collapsibility and cephalometric variables in patients with obstructive sleep apnea. Am J Respir Crit Care Med; 2000; 161 (2 Pt 1): 347–352. [cit. 20. 12. 2020]. Dostupné z: https://pubmed.ncbi.nlm.nih.gov/10673170/
49. Partinen M, Guilleminault C, Quera-Salva MA, Jamieson A. Obstructive sleep apnea and cephalometric roentgenograms. The role of anatomic upper airway abnormalities in the definition of abnormal breathing during sleep. Chest. 1988; 93(16): 1199–1205. [cit. 20. 12. 2020]. Dostupné z: https://pubmed.ncbi.nlm.nih.gov/3371099/
50. Gungor AY, Turkkahraman H, Yilmaz HH, Yariktas M. Cephalometric comparison of obstructive sleep apnea patients and healthy controls. Eur J Dent. 2013; 7(1): 48–54.
51. Battagel JM, Johal A, Kotecha B. A cephalometric comparison of subjects with snoring and obstructive sleep apnoea. Eur J Orthod. 2000; 22(4): 353–365.
52. Arya D, Tripathi A, Singh SV, Tripathi S, Nagar A, Mishra A. A pilot study to evaluate posttreatment cephalometric changes in subjects with OSA. J Prosthet Dent. 2010; 103(3): 170–177.[cit. 20. 12. 2020]. Dostupné z: http://www.sciencedirect.com/science/article/pii/S0022391310600248
53. Caballero P, Alvarez-Sala R, García-Río F, Prados C, Hernán MA, Villamor J, et al. CT in the evaluation of the upper airway in healthy subjects and in patients with obstructive sleep apnea syndrome. Chest. 1998; 113(1): 111–116.
54. Ayappa I, Rapoport DM. The upper airway in sleep: physiology of the pharynx. Sleep Med Rev. 2003; 7(1): 9–33. [cit. 20. 12. 2020]. Dostupné z: http://www.sciencedirect.com/science/article/pii/S1087079202902388
55. McNamara JA. A method of cephalometric evaluation. Am J Orthod. 1984; 86(6): 449–469. [cit. 20. 12. 2020]. Dostupné z: http://www.sciencedirect.com/science/article/pii/S000294168490352X
56. Sprenger R, Martins LAC, Dos Santos JCB,de Menezes CC, Venezian GC, Degan VV. A retrospective cephalometric study on upper airway spaces in different facial types. Prog Orthod. 2017; 18(1): 25.
57. Veys B, Pottel L, Mollemans W, Abeloos J, Swennen G, Neyt N. Three-dimensional volumetric changes in the upper airway after maxillomandibular advancement in obstructive sleep apnoea patients and the impact on quality of life. Int J Oral Maxillofac Surg. 2017; 46(12): 1525–1532. [cit. 20. 12. 2020]. Dostupné z: http://www.sciencedirect.com/science/article/pii/S0901502717315308
58. Katyal V, Pamula Y, Daynes CN, Martin J, Dreyer CW, Kennedy D, et al. Craniofacial and upper airway morphology in pediatric sleep-disordered breathing and changes in quality of life with rapid maxillary expansion. Am J Orthod Dentofacial Orthop. 2013; 144(6): 860–871. [cit. 22. 12. 2020]. Dostupné z: http://www.sciencedirect.com/science/article/pii/S0889540613008299
59. Huynh NT, Morton PD, Rompré PH, Papadakis A, Remise C. Associations between sleep-disordered breathing symptoms and facial and dental morphometry, assessed with screening examinations. Am J Orthod Dentofac Orthop. 2011; 140(6): 762–770.
60. Zonato AI, Martinho FL, Bittencourt LR,de Oliveira Camponês Brasil O, Gregório LC, Tufik S. Head and neck physical examination: comparison between nonapneic and obstructive sleep apnea patients. Laryngoscope. 2005; 115(6): 1030–1034.
61. Kushida CA, Efron B, Guilleminault C. A predictive morphometric model for the obstructive sleep apnea syndrome. Ann Intern Med. 1997; 127(8 Pt 1): 581–587.
62. Villa MP, Rizzoli A, Miano S, Malagola C. Efficacy of rapid maxillary expansion in children with obstructive sleep apnea syndrome: 36 months of follow-up. Sleep Breath. 2011; 15(2): 179–184. [cit. 22. 12. 2020]. Dostupné z: https://doi.org/10.1007/s11325-011-0505-1
63. Kaditis A, Kheirandish-Gozal L, Gozal D. Algorithm for the diagnosis and treatment of pediatric OSA: A proposal of two pediatric sleep centers. Sleep Med. 2012; 13(3): 217–227. [cit. 22. 12. 2020]. Dostupné z: http://www.sciencedirect.com/science/article/pii/S1389945711003844
64. Dobrowolska-Zarzycka M, Dunin-Wilczyńska I, Szymańska J. Craniofacial structure in patients with obstructive sleep apnoea. Folia Morphol. 2016; 75(3): 311–315.
65. Tepedino M, Illuzzi G, Laurenziello M, Perillo L, Taurino AM, Cassano M, et al. Craniofacial morphology in patients with obstructive sleep apnea: cephalometric evaluation. Braz J Otorhinolaryngol. 2020; 18.
66. Sforza E, Bacon W, Weiss T, Thibault A, Petiau C, Krieger J. Upper airway collapsibility and cephalometric variables in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2000; 161(2 Pt 1): 347–352.
67. Hoekema A, Hovinga B, Stegenga B, De Bont LGM. Craniofacial morphology and obstructive sleep apnoea: a cephalometric analysis. J Oral Rehabil. 2003; 30(7): 690–696.
68. Vidović N, Mestrović S, Dogas Z, Buković D, Brakus I, Brakus RB, et al. Craniofacial morphology of Croatian patients with obstructive sleep apnea. Coll Antropol. 2013; 37(1): 271–279.
Labels
Maxillofacial surgery Orthodontics Dental medicine
Article was published inCzech Dental Journal

2021 Issue 4
Most read in this issue- BIOCERAMIC-BASED ROOT CANAL SEALERS
- THE RELATIONSHIP BETWEEN OBSTRUCTIVE SLEEP APNEA AND CRANIOFACIAL ANOMALIES IN ADULT PATIENTS
- APPLICATION OF TRIBOLOGICAL METHODS FOR PREDICTION OF WEAR OF DENTAL FILLING MATERIALS
- EDITORIAL
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career