-
Medical journals
- Career
Study of possible sex features of ramipril and candesartan treatment under experimental arterial hypertension in rats
Authors: Natalia Tsubanova; Alona Kovpak; Igor Zamorskyi
Published in: Čes. slov. Farm., 2022; 71, 116-120
Category: Original Article
doi: https://doi.org/https://doi.org/10.5817/CSF2022-3-116Overview
The article presents the results of a preclinical study of ramipril and candesartan in an experimental group of hypertensive rats of different sexes. Antihypertensive therapy was performed for 21 days. The drugs were administered daily in moderate therapeutic doses calculated for rats using the coefficient of species sensitivity. It was found that the course of experimental hypertension has gender differences, and in males, according to blood pressure, the level of NO metabolites is more pronounced. The use of ramipril from the group of ACE inhibitors and candesartan from the ARBs group in experimental hypertension in rats has gender differences. Ramipril is likely to be more effective in normalizing blood pressure and endothelial function in males than females. The use of candesartan did not show significant gender differences, but there was a tendency for females to be slightly more effective than males. Established gender differences in hypertension pharmacotherapy should be considered to optimize treatment.
Keywords:
hypertension – gender differences – ramipril – candesartan
Introduction
Today, there are more and more scientific studies on the gender features of the pathogenesis and pharmacotherapy of cardiovascular system diseases (CVD), including hypertension1, 2). Research in recent decades has shown that sex steroid hormones and their receptors are, at least in part, determinants of sex differences in cardiovascular outcomes. In particular, it is well known that hormone receptors, including estrogen, progesterone, and androgen receptors, are expressed in the vascular system; and the female sex hormone estrogen has a cardioprotective effect3, 4).
Epidemiological studies show that women with a preserved menstrual cycle are more protected from CVD than men of the same age. In women, cardiovascular disease appears ten years later than in men, which coincides with the period of estrogen loss in postmenopausal women. The hormone estrogen acts through two classic estrogen receptors (ER): ERα and ERβ. In addition, recent studies show that G-proteinbound ER may mediate some of the rapid action of estrogen in the vascular network5). These receptors mediate the action of steroid hormones through both genomic and rapid non-genomic influences. A study by Hester et al. found that women are more susceptible to the development of pulmonary arterial hypertension (PAH). Female patients with PAH show better right ventricular function and increased survival than their male counterparts. A phenomenon is called the “estrogen paradox” 6).
Gender differences also indicate arterial stiffness, which progressively increases with age and is an independent predictor of cardiovascular risk. Evidence suggests gender differences in the timing of age-related arterial stiffness and the associated risk of cardiovascular disease, which increases disproportionately in postmenopausal women. According to DuPont et al., the relationship between arterial rigidity and mortality in women is almost twice as high as in men7). Clinical manifestations of arterial stiffness suggest the presence of various sex-specific mechanisms, and there is evidence that with age in men and women, collagen content decreases. Still, the decrease in elastin in blood vessels is characteristic mainly of men. The relationship between arterial rigidity and mortality in women is almost twice as high as in men. Clinical manifestations of arterial stiffness suggest the presence of various sex-specific mechanisms, and there is evidence that with age in men and women, collagen content decreases. Still, the decrease in elastin in blood vessels is characteristic mainly of men8, 9).
Current strategies for treating hypertension (AH) are based on evidence-based medicine. A large number of clinical studies recommend prescribing drugs (LP) from the group of angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) from which antihypertensive therapy is started. ACE inhibitor ramipril and candesartan from the ARB group are widely used in medical practice10, 11).
This experiment aimed to study possible gender characteristics of the effectiveness of ramipril and candesartan in experimental hypertension in rats.
Experimental part
The study was performed on laboratory rats of both sexes with spontaneous arterial hypertension (spontaneously hypertensive rats, SHR), weighing 240–285 g (laboratory animal nursery “Biomodelservice”, Kyiv). Animals received ramipril (the 5 mg tablets manufactured by Merkle GmbH, Germany) and candesartan (the 4 mg tablets manufactured by Ranbaxy, India). The tablets were crushed into a fine powder and given to the rats along with the diet. The studied drugs were administered as monotherapy. They were applied in the minimum therapeutic doses for humans, which were listed for rats using the coefficient of Rybolovlev12). The doses were 0.3 mg/kg and 0.2 mg/ kg for ramipril and candesartan, respectively.
To measure blood pressure (BP) in rats, a noninvasive blood pressure measurement system (Blood Pressure Analysis System sTM BP-2000 Series II, Visitech Systems, USA) with automatic temperature control in an immobilization tunnel consisting of a computer with installed software and measuring instruments.
Studies of biochemical parameters were performed according to standard methods. Eighty rats were used in this study. The animals were kept in standard vivarium conditions. The research was conducted following the National General Ethical Principles of Animal Experiments (Ukraine, 2001), which comply with the provisions of the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (Strasbourg, 1986)13). The Commission on Bioethics did not find any moral and ethical norms violations during the research work.
The obtained experimental data were processed statistically using Student’s t-test, using a statistical analysis program (Version 6. AnalystSoft Inc., StatPlus14).
Results and discussion
The primary indicator of the effectiveness of AH is the normalization of blood pressure. SHR rats received ramipril or candesartan monotherapy at moderate therapeutic doses. Blood pressure was studied on the 7th and 21st days of the experiment (Table 1).
1. Study of gender differences in the effect of ramipril and candesartan on blood pressure in SHR rats 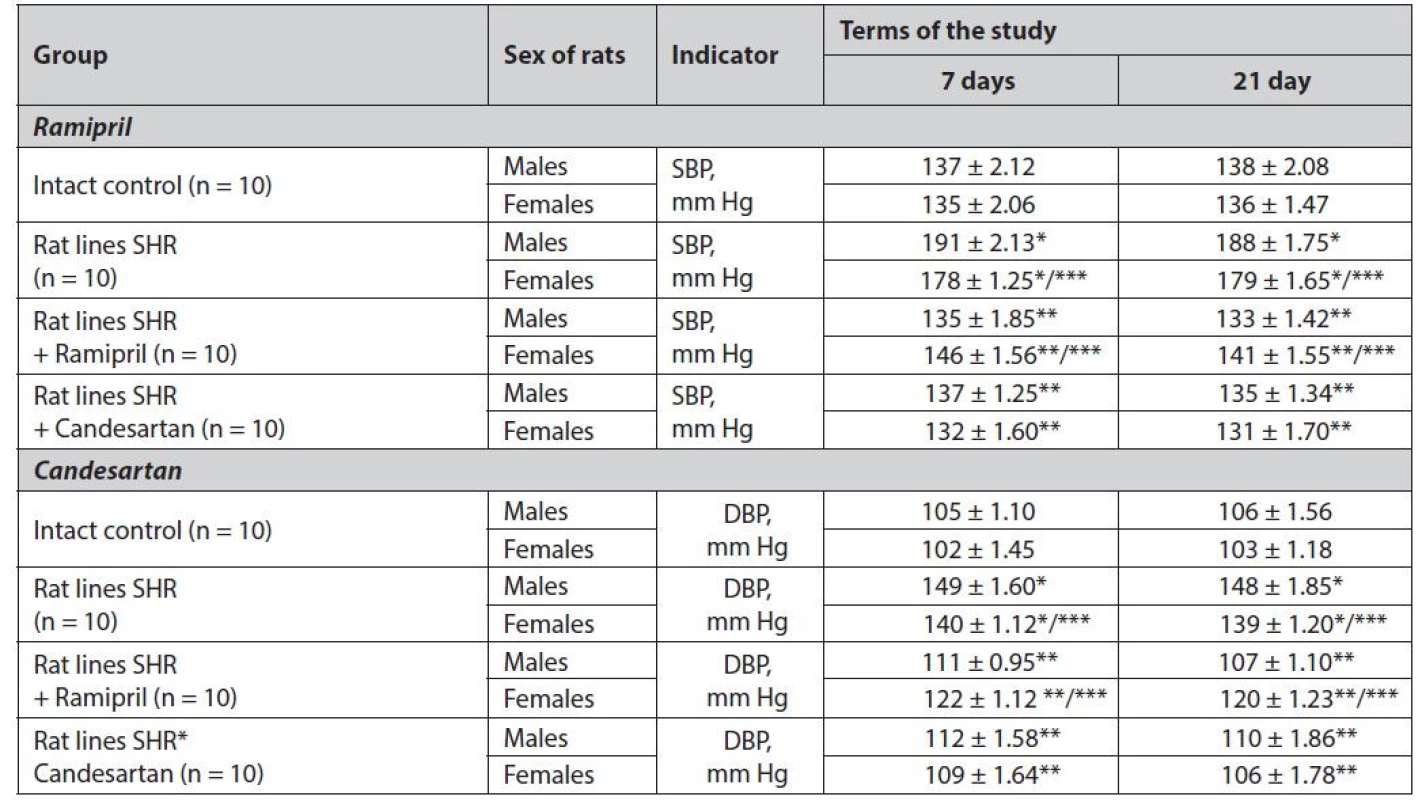
SBP – systolic blood pressure, DBP – diastolic blood pressure
*statistically significant difference with the group of intact control p ≤ 0.001
**statistically significant difference with the group of hypertensive rats p ≤ 0.001
***statistically significant difference between males and females of one group p ≤ 0.01According to the study, it can be concluded that males of reproductive age are more sensitive to the formation of hypertension than females of reproductive age, as verified by a probable increase in SBP and DBP in male rats by 5–7% (p ≤ 0.01) relative to female rats.
Treatment with ramipril also had statistically significant gender differences. In male rats, ramipril was more effective in reducing SBP and DBP on days 7 and 21 of treatment. In female rats, ramipril was probably 6–11% less effective (p ≤ 0.01) than males. It should be noted that, although gender differences were found in the normalization of blood pressure for ramipril, in female rats, ramipril was still effective against the group of control pathology (p ≤ 0.001).
When candesartan was administered, there was no statistically significant difference in the SBP and DBP normalization in hypertensive rats. Nevertheless, a slight trend toward higher efficacy in females was found.
The data obtained by us coincide with the clinical observation of Skybytsky et al., which showed that ACE inhibitor – zofenopril is more effective in men, while ARBs – valsartan better normalizes blood pressure in women15).
The lower efficiency of ACE inhibitors in females can be explained by the fact that women, in contrast to men, have less synthesis and activity of ACE inhibitors. Also, there is evidence that in women, PAS is activated mainly by the ACE-independent pathway, with the participation of chymases, cathepsin G, the enzyme CAGE, and others. Tissue bioactive substances lead to the synthesis of angiotensin II16).
Regarding the duration of antihypertensive therapy (Table 1), stable normalization of blood pressure can be achieved after seven days of administration of ramipril or candesartan.
The second evaluation point was determining the gender differences in the ramipril or candesartan effects on coagulation and fibrinolysis in rats with hypertension (Table 2).
2. Study of gender differences in the effect of ramipril and candesartan on coagulation and fibrinolysis rates in SHR rats 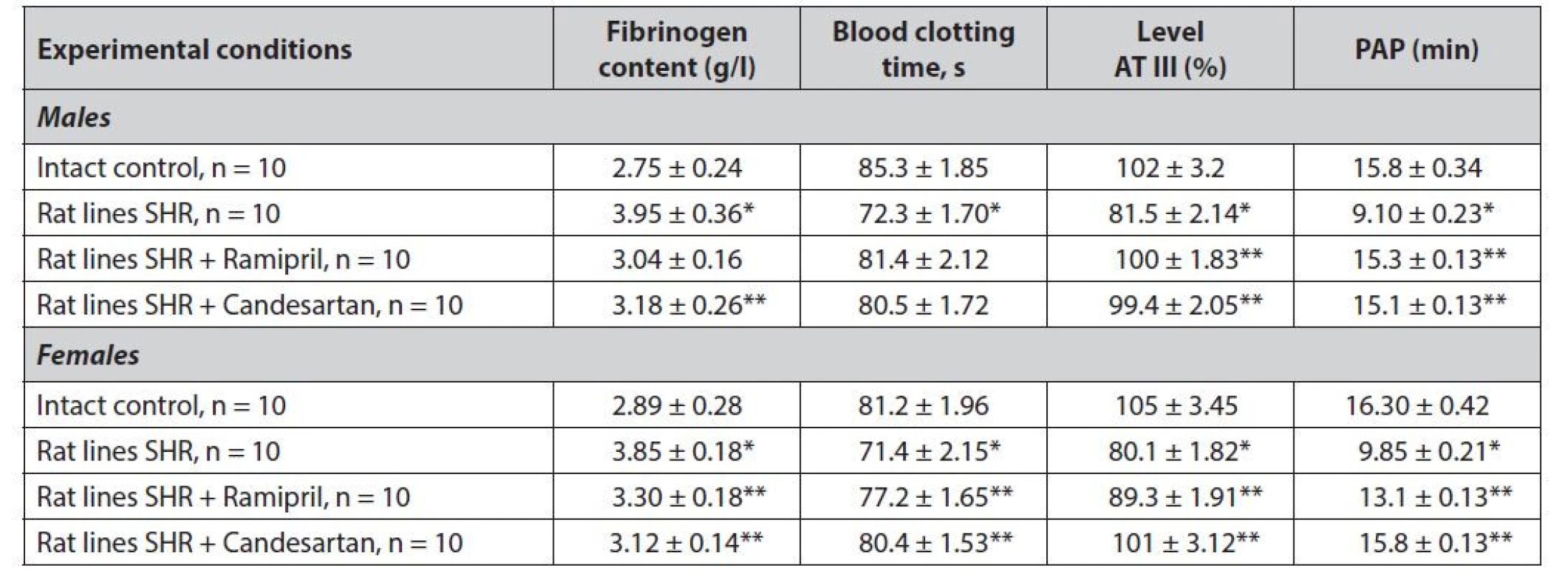
AT III – antithrombin III, PAP – potential plasminogen activity
* statistically significant difference with the group of intact control p ≤ 0.001
** statistically significant difference with the group of hypertensive rats p ≤ 0.001
*** statistically significant difference between males and females of one group p ≤ 0.01Based on the obtained data, it can be noted that hypertensive rats were characterized by a significant shift in balance (blood clotting – fibrinolysis, in the direction of thrombosis). For both male and female rats of the SHR series, a probable increase in fibrinogen content of 1.4 times (p ≤ 0.001) in males, 1.3 times (p ≤ 0.001) in females, a significant increase in blood clotting time in males increased by 1.2 times (p ≤ 0.001) in females 1.1 times (p ≤ 0.001) were found. With inversion of the blood coagulation system in hypertensive rats of both sexes, a strong decrease in fibrinolytic activity was registered.1.7 times (p ≤ 0.001), in females 1.6 times (p ≤ 0.001).
When ramipril or candesartan monotherapy was used for 21 days, these parameters were normalized to physiological levels. It should be noted that the ARB candesartan had an equally effective effect on both males and females. In contrast, ramipril exhibited a steady trend towards a more effective normalization of the “coagulation-fibrinolysis” system in males. The pharmacological effect of ramipril on females was less pronounced, although the above indicators did not show a statistically significant difference with males.
The leading link in endothelial dysfunction in hypertension is synthesizing an important endothelial factor, nitric oxide (NO), and related enzymatic and non-enzymatic systems. It led to a study of the level of NO metabolites in females and males of hypertensive rats treated with ramipril or candesartan. The results are shown in Table 3.
3. Study of gender differences in the effect of ramipril and candesartan on the level of NO metabolites in SHR rats 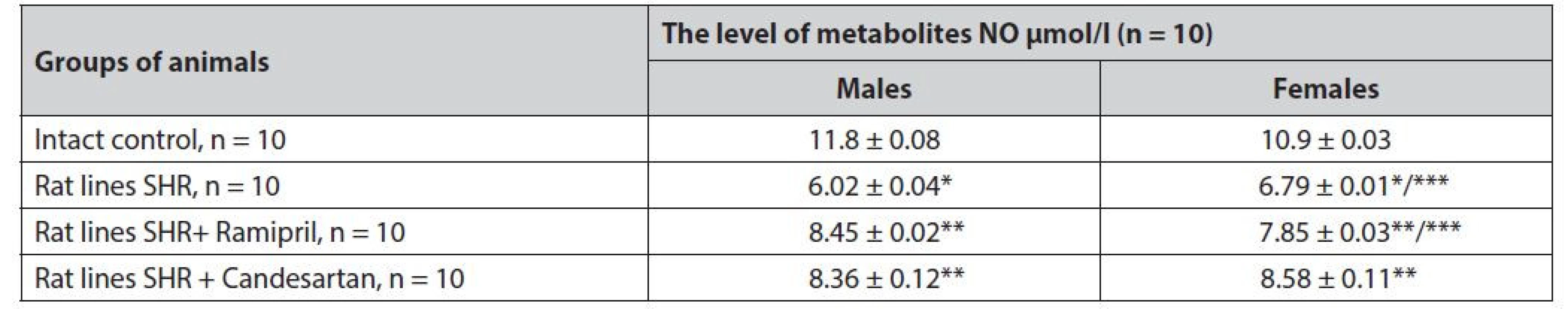
* statistically significant difference with the group of intact control p ≤ 0.001
** statistically significant difference with the group of hypertensive rats p ≤ 0.001
*** statistically significant difference between males and females of one group p ≤ 0.01Gender differences were reported for the group of hypertensive rats. The pathology became more pronounced in males, with more significant endothelial dysfunction, verified by a probably lower (12%, p ≤ 0.01) level of NO metabolites compared to females of the same group.
Treatment of the average therapeutic dose of ramipril more effectively restored the above in males. For females treated with ramipril, the normalization of NO metabolites was 1.1 times lower (p ≤ 0.01).
Candesartan had no statistically significant differences in the recovery of endothelial function in males and females, although a tendency to be more effective in females was reported.
Conclusions
Experimental hypertension in males, almost by indicators: systolic blood pressure, diastolic blood pressure, and the level of NO metabolites, becomes more pronounced than in female rats. Under the same conditions, males are more sensitive to the formation of persistent hypertension than females. Using ramipril from ACE inhibitors and candesartan from ARBs in experimental hypertension in rats has gender differences. Therapeutic, daily administration of ramipril is likely to be more effective in normalizing blood pressure and endothelial function in males than in females. The higher efficacy of ramipril in males has also been reported in the recovery of the coagulationfibrinolysis system. The use of candesartan did not show significant gender differences, but there was a tendency to be somewhat more effective in females than in males. The formation and pharmacotherapy of hypertension have considerable gender differences that need to be considered to optimize treatment. ACE inhibitor ramipril is more effective in male rats, while candesartan from the ARBs group has no statistically significant gender differences.
Conflict of interest: none.
Received February 18, 2022 / Accepted April 27, 2022
N. Tsubanova
National University of Pharmacy, Kharkiv, Ukraine
Alona Kovpak, I. Zamorskyi
Bukovinian State Medical University Teatralnaya 2, 58002 Chernivtsi, Ukraine
e-mail: alona.kovpak@gmail.com
Sources
1. Regitz-Zagrosek V., Kararigas G. Mechanistic pathways of sex differences in cardiovascular disease. Physiol. Rev. 2017; 97(1), 1–37. https://doi.org/10.1152/physrev. 00021.2015
2. Marques A. P., Szwarcwald C. L., Pires D. C., Rodrigues J. M., Almeida W. D. S., Romero D. Factors associated with arterial hypertension: a systematic review. Cien Saude Colet. 2020; 25(6), 2271–2282. Portuguese. doi: https://doi.org/10.1590/1413-81232020256.26972018
3. Eric D. A. Sex hormones across the menstrual cycle in pulmonary arterial hypertension: adding a new layer of complexity. Ann. Am. Thorac. Soc. 2021; 18(2), 209–211. https://doi.org/10.1513/AnnalsATS.202011-1379ED
4. Tang C., Luo Y., Li S., Huang B., Xu S., Li L. Characteristics of inflammation process in monocrotaline-induced pulmonary arterial hypertension in rats. Biomed. Pharmacother. 2021; 133, 111081. https://doi.org/10.1016/j. biopha.2020.111081
5. Zimmerman M. A., Budish R. A., Kashyap S., Lindsey S. H. GPER-novel membrane oestrogen receptor. Clin. Sci. 2016; 130(12), 1005–1016. https://doi.org/10.1042/ CS20160114
6. Hester J., Ventetuolo C., Lahm T. Sex, gender, and sex hormones in pulmonary hypertension and right ventricular failure. Compr. Physiol. 2020; 10(1), 125–170. https:// doi.org/10.1002/cphy.c190011
7. DuPont J. J., Kenney R. M., Patel A. R., Jaffe I. Z. Sex differences in mechanisms of arterial stiffness. Br. J. Pharmacol. 2019;176(21), 4208–4225. https://doi.org/10.1111 /bph.14624
8. Ge X., Zhu T., Zhang X., Liu Y., Wang Y., Zhang W. Gender differences in pulmonary arterial hypertension patients with BMPR2 mutation: a meta-analysis. Respir. Res. 2020; 21(1), 44. https://doi.org/10.1186/s12931-020 - 1309-2
9. Agarwala A., Michos E. D., Samad Z., Ballantyne C. M., Virani S. S. The use of sex-specific factors in the assessment of women’s cardiovascular risk. Circulation 2020; 141(7), 592–599. https://doi.org/10.1161/CIRCULATIONAHA. 119.043429
10. Ojji D., Mayosi B., Francis V., Badri M., et al. for the CREOLE Study Investigators. Comparison of dual therapies for lowering blood pressure in black Africans. N. Engl. J. Med. 2019; 380, 2429–2439. https://doi.org/10.1056/NEJMoa1901113
11. Costa E. C., Hay J. L., Kehler D. S., Boreskie K. F. Effects of high-intensity interval training versus moderate-intensity continuous training on blood pressure in adults with pre-to established hypertension: a systematic review and meta-analysis of randomized trials. Sports Med. 2018; 48, 2127–2142.
12. Stefanova O. V. Preclinical drug studies. Guideline. Kyiv: Avicenna 2001.
13. Reznikov O. G. General ethical principles of animal experiments. Endocrinology 2003; 8(1), 142–145.
14. AnalystSoft Inc., StatPlus – program pro statistickou analýzu. Verze 6. Režim elektronického přístupu, www. analystsoft.com
15. Skibitsky V. V, Gorodetskaya E. V., Fendrikova A. V., Kudryashov E. A. Gender features of combined antihypertensive therapy in patients with hypertension and coronary heart disease. Cardiovasc. Ther. Prevent. 2014; 13 (5), 14–21.
16. Denton K. M., Hilliard L. M., Tare М. Sex-related differences in hypertension: seek and ye shall find. Hypertension 2013; 62, 674–677.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2022 Issue 3-
All articles in this issue
- Metronomic therapy in the treatment of cancer
- RNDr. PhMr. Ernest Alt – in memoriam
- Za prof. Ing. Petrom Kovácsom, DrSc. – in memoriam
- Pulsed electric field energy calculation to damage red galangal (Alpinia purpurata, K. Scumm) rhizome slices and its essential oil yield and quality with hydrodistillation
- Study of possible sex features of ramipril and candesartan treatment under experimental arterial hypertension in rats
- The effect of Aronia melanocarpa extract on the phospholipid composition of the rat myocardium during stress
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Metronomic therapy in the treatment of cancer
- The effect of Aronia melanocarpa extract on the phospholipid composition of the rat myocardium during stress
- Study of possible sex features of ramipril and candesartan treatment under experimental arterial hypertension in rats
- Pulsed electric field energy calculation to damage red galangal (Alpinia purpurata, K. Scumm) rhizome slices and its essential oil yield and quality with hydrodistillation
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

