-
Medical journals
- Career
Study of influence of 1,3-oxazole-4-yl-phosphonic acid derivative on nitric oxide system indicators in rats with arterial hypertension
Authors: Iryna Nizhenkovska Kateryna Sedko Olena Kuznetsova
Authors‘ workplace: Department of Pharmaceutical
Published in: Čes. slov. Farm., 2018; 67, 154-159
Category: Original article
Overview
Arterial hypertension is a leader among the most common chronic cardiovascular diseases which covers 30–45% of world population with a dynamics of further growth. 1,3-oxazole-4-yl-phosphonic acid derivative (abbreviated as oxazole derivative) is a novel original compound with vasodilating activity. The purpose of this work was to study the NO-mediated mechanism of vasodilating effect of this derivative according to the activity of eNOS, iNOS and NO2- through their content in rats on a resistant arterial hypertension model. The oxazole derivative intraperitoneally administered at a dose of 25 mg/kg prevented development of arterial hypertension by promotion of recovery of eNOS, iNOS and NO2- activity in the studied aorta, heart, and blood serum to the normal level recorded in intact animals. It can be concluded that the mechanism of antihypertensive action of the oxazole derivative is mediated by the NO system.
Key words:
arterial hypertension • nitric oxide • 1,3-oxazole-4-yl-phosphonic acid derivative
Introduction
Arterial hypertension (AH) is a leader among the most common chronic cardiovascular diseases and covers 30–45% of world population with a dynamics of further growth1). One of causes for exacerbation of this problem is a significant number of patients with uncontrolled blood pressure (BP) level, which is partly due to incorrect regimens of treatment of this pathology and the presence of resistant forms of arterial hypertension2). In this regard, a development of new antihypertensive drugs and a search for biologically active compounds meeting the basic requirements for an ideal hypotensive drug remain relevant3).
1,3-Oxazole-4-yl-phosphonic acid derivative (abbreviated name – oxazole derivative) is a novel original compound with vasodilating activity, the antihypertensive effect of which has been confirmed in previous experimental in vitro4) and in vivo5) studies. In addition, relatively low toxicity of this compound has been established at different routes of administration6). However, the mechanism of the vasodilating effect of oxazole derivative has not been investigated and is still unknown.
Currently, nitric oxide (NO) and its metabolites as markers of arterial hypertension and their role in the formation of endothelial dysfunction are being actively studied7). Under physiological conditions, NO functions as a powerful vasodilator when regulating the vascular tone. The main target of nitric oxide in the vascular system is gem-soluble guanylate cyclase. By activating guanylate cyclase, NO increases formation of cGMP in smooth muscle cells, which is the main intracellular messenger in the cardiovascular system, and causes vascular relaxation8). In its turn, synthesis of NO in the body is influenced by the enzymes of nitric oxide synthase NOS in the presence of NADPH, FAD, FMN, tetrahydrobiopterin, calmodulin, and Ca2+ ions9). It is known that in smooth muscles of rats with arterial hypertension a decrease in endothelial NO-synthase (eNOS) activity and an increase in inducible (cytokine-dependent) NO-synthase (iNOS) activity is observed10).
Previously, we studied isolated aorta segments of rats with regard to the influence of the L-NAME NO-synthase blocker on the vasodilator activity of 1,3-oxazole-4-yl-phosphonic acid derivative, but preservation of the efficient effect of oxazole derivative against the background of NO-synthase blockade gave rise to assuming that the influence of these two chemical compounds on vessels is realized through different targets11). Therefore, the purpose of this paper was to study the NO-mediated mechanisms of the vasodilating effect of oxazole derivative according to the activity of eNOS, iNOS and NO2- content as one of the products of NO metabolism in rats on a resistant arterial hypertension model.
Experimental part
The studies were conducted on 36 white viripotent Wistar rats of (181.8 ± 3.54) g average weight (No. of approval from the animal care committee of Bogomolets National Medical University: 56/2018). All manipulations with animals were performed according to the European Convention on the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (Strasbourg, 1986).
The resistant arterial hypertension was simulated by salt load – salt drinking (1% solution of sodium chloride) with free access to it during 21 days12). The rats recorded with a BP increase by 10% or more were considered to be animals with the formed AH. Animals not recorded with a BP increase 21 days after beginning of salt load were not included into groups’ allocation and were withdrawn from the trial.
All rats were kept under identical laboratory conditions on the ordinary diet in a vivarium, under “day/night” light regime at 20–22 С and had free access to drinking water. The mean blood pressure and heart rate in unanesthetized rats were registered by a sphygmomanometric method using a specialized cuff with a pulse sensor mounted on the rat’s tail and analysis of periodicity of blood flow oscillations at a Ugo Basile unit (Italy, 2005). The experimental rats were preheated to 28–32 °C for 10–15 minutes, and indicated temperature was maintained throughout the experiment to ensure blood circulation in the tail in the required volume and stabilization of the blood flow.
The rats were divided into 3 groups of 12 individuals in each by random sample technique: intact, blank and basic. The intact group included rats without salt loading and without other manipulations (drug administration, etc.) with free access to drinking water. The blank group included rats with the model of stable arterial hypertension being formed during 21 days by way of salt load and starting from the 14th day after the start of salt load was administered with a mixture of Tween-80 and water for injection in a ratio of 1 : 10 as a solvent by the scheme equivalent to that, which was administered to the animals of the basic group. The basic group included rats with an 8% and above elevated blood pressure level at the 14th day after the start of salt load. Starting from the 14th day, animals of this group were receiving oxazole derivative at a dose of 25 mg/kg (ED50)5) intraperitoneally, once per day, daily, within 7 days. This group of animals was formed to determine the effect of 1,3-oxazole-4-yl-phosphonic acid derivative with regard to the prevention of the development of hypertension or decreasing degree of hypertension.
For analysis of the studied biochemical parameters, blood serum, the heart and the aorta were taken at the 22nd day after AH simulation commencement.
Frozen tissues were directly homogenized manually in a homogenization buffer in volumes three times the weight of the tissues. The homogenization buffer containing 320 mM sucrose, 50 mM Tris base, 0.1 mM EDTA, 1 mM dithiothreitol, 10 μg/ml leupeptin, 10 μg/ml soybean trypsin inhibitor, and 2 μg/ml aprotinin was adjusted to pH 7.0 with HCl before addition of 10 mg/ml of phenylmethylsulfonyl fluoride (PMSF). The crude homogenates were then centrifuged at 14.000 g for 40 min at 4 °C. The supernatants were analyzed for determination of NOS activity.
Determination of total NO-synthase activity
For the purpose of determining the total activity of NO-synthase (eNOS and iNOS), a combination of classical method and its modern modification adapted for spectrophotometric measurement of one of the reaction products – L-citrulline was employed13, 14). Sample aliquots containing 100 μg of protein were being incubated in a total volume of 100 μl of substrate mixture of the following composition (mmol/l): KH2PO4 – 50, MgCl2 – 1, CaCl2 – 2, NADPH – 1, L-arginine – 2, pH 7.0 within 60 min at 37 °C. The reaction was stopped by addition of 0.3 ml of 2NHClO4. Controls were the samples containing a complete substrate mixture and pre-denatured 2NHClO4 protein. The mixture was centrifuged at 3500 rpm within 10 minutes, and L-citrulline content was determined in a supernatant protein-free mixture by a highly specific spectrophotometric method in color reaction with antipyrine.
Determination of inducible and endothelial NO-synthase activity
The method of determination differs from the previous one by that for determining the iNOS activity in incubation mixture, and 2 mmol of EDTA was added instead of CaCl2.
Calculation of endothelial nitric oxide synthase (eNOS) activity
eNOS activity was calculated by subtracting the iNOS activity from the total NOS activity. Enzymes activity was expressed in picomoles of newly formed L-citrulline per 1 min calculated using 1 mg of total protein in sample.
Determination of citrulline content
Citrulline was determined by a highly sensitive colorimetric method15). Non-protein sample aliquots were mixed with 2 ml of reagent (1 ml of 59 mmol diacetylmonoxime, 1 ml of 32 mmol antipyrine and 55 μmol of iron (II) sulphate in 6NH2SO4), boiled within 15 min in a water bath; after cooling, the magnitude of extinction was determined at 465 nm. The amount of citrulline was determined using a calibration chart.
Determination of nitrite ions content
Nitrite ions content was determined by the Griess method with modifications16). 1.8 ml of distilled water, 200 μl of 1% solution of sulphanilic acid in a 5% phosphoric acid solution was added to 200 μl of homogenate. Then it was left for 7 minutes in a dark place at room temperature. Then, 200 μl of 1% solution of alpha-naphthylethylenediamine with 5% orthophosphoric acid was added, stirred, and left in a dark place at room temperature for 10–15 minutes. Optical density of samples was measured at 539 nm. NO2- scontent was determined according to a calibration graph which was constructed using different concentrations of NaNO2 and recalculated on 1 mg of total protein.
Determination of protein concentration
Concentration of protein was determined by the Lowry method17). The method is based on the formation of colored products of aromatic amino acids with Folin’s reagent in conjunction with biuret reaction to peptide bonds.
Statistical calculations were carried out using BioStat 2009 v 5.8.4. (Manufacturer – Analyst Soft). Depending on the normality of the distribution of the compared series, the research groups were compared with the blank and intact groups with the help of Student’s criterion for independent samples and with the initial value using the paired Student’s criterion. The significance level for all criteria was 0.05.
Results
eNOS, iNOS activity and NO2- content in the rat aorta, heart and blood serum is represented in Tables 1, 2, 3. In the blank group of rats with persistent arterial hypertension, an expected decrease in eNOS activity was observed at 56.9% (p < 0.05) in the aorta, 44.9% (p < 0.05) in the heart, and 62.9% (p < 0.05) in blood serum relative to the intact group of animals. At the same time, under pathological conditions caused by AH formation, iNOS activity increased almost three-fold (p < 0.05) in the aorta, by 2.3 times (p < 0.05) in the heart, and two-fold (p < 0.05) in blood serum. NO2- content increased to a greater extent in blood serum and the heart by 48.4% (p < 0.05) and 42.7% (p < 0.05), respectively, than in the aorta – by 34.5% (p < 0.05) in comparison with intact animals.
1. Endothelial NO-syntase activity in the aorta, heart, and blood serum of rats, nmol × min–1 × mg–1 of protein (M ± m, n = 12) 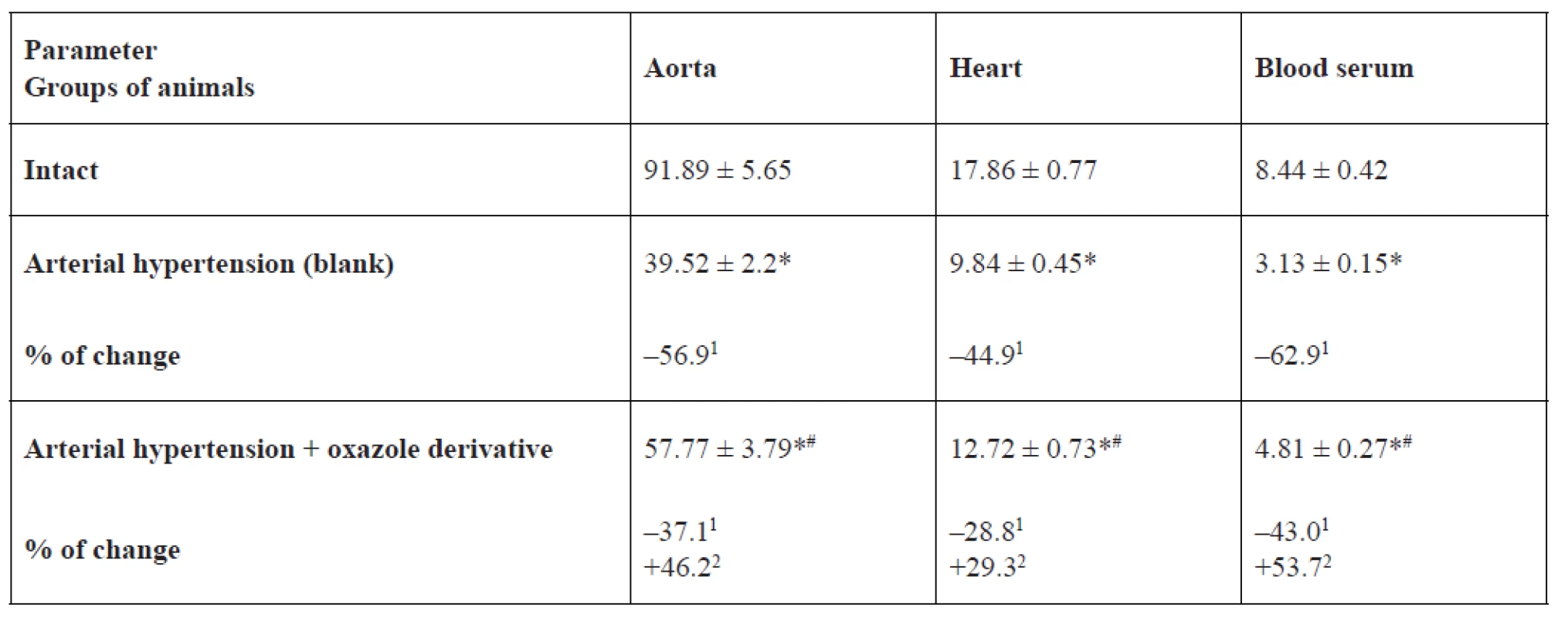
*p < 0.05 relative to the intact group, #p < 0.05 relative to the blank group
1% of changes relative to the intact group, 2% of changes relative to the blank group2. Inducible NO-syntase activity in the aorta, heart, and blood serum of rats, nmol × min–1 × mg–1 of protein (M ± m, n = 12) 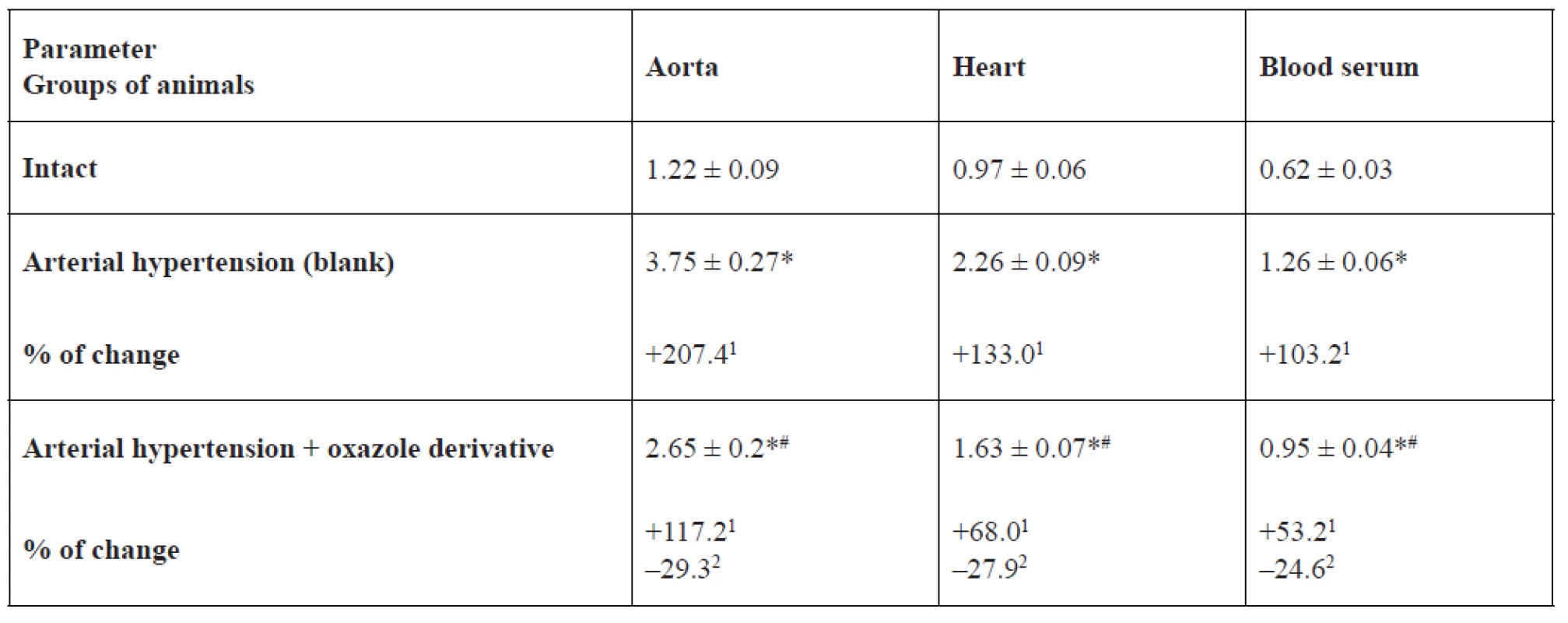
*p < 0.05 relative to the intact group, #p < 0.05 relative to the blank group
1% of changes relative to the intact group, 2% of changes relative to the blank group3. Content of nitrite ions in the aorta, heart and blood serum of rats, nmol × mg–1 of protein (M ± m, n = 12) 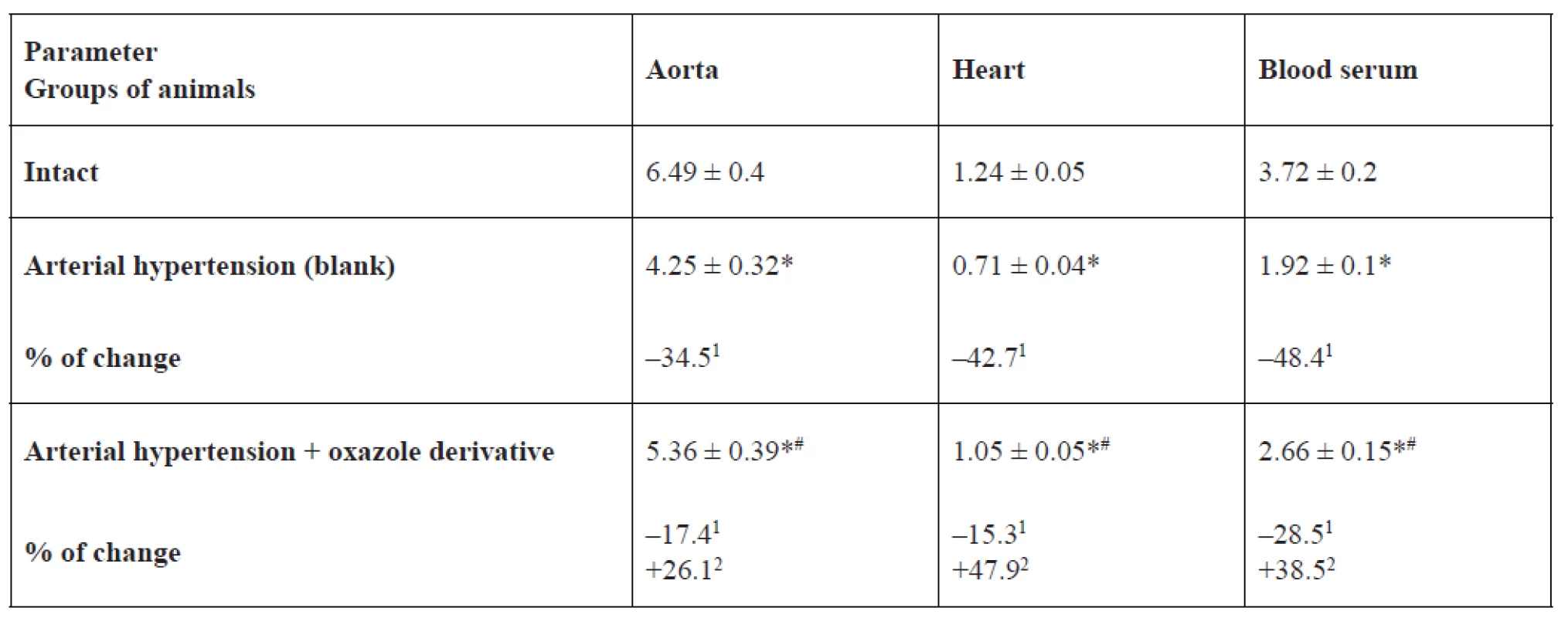
*p < 0.05 relative to the intact group, #p < 0.05 relative to the blank group
1% of changes relative to the intact group, 2% of changes relative to the blank groupIn the basic group of rats, which were receiving 1,3-oxazole-4-yl-phosphonic acid peritoneally at a dose of 25 mg/kg in order to prevent hypertension development or reduce hypertension degree, its ability to normalize the studied parameters was detected. For example, eNOS activity was higher by 53.7% (p < 0.05) in blood serum, by 46.1% (p < 0.05) in the aorta, and by 29.3% (p = 0.066) in the heart than in rats with arterial hypertension. The effect of oxazole derivative also covered the recovery of AH-related enhanced iNOS activity. The above indicator was lower by 29.3% (p < 0.05) in the aorta, by 27.9% (p < 0.05) in the heart, by 24.6% (p < 0.05) in blood serum versus the corresponding values in the group of rats with arterial hypertension. Vasodilating activity of the test compound is also confirmed by an increase in NO2 content in blood serum of rats by 1.4 times (p < 0.05) versus the group of rats with arterial hypertension.
Discussion
A decrease in endothelial NO-synthase activity which was detected in the aorta, heart and blood serum in an experimental model of hypertension may be associated with a decrease in the expression of this enzyme gene18). At the same time, we have shown that at hypertension, the activity of inducible NO-synthase, which is low under normal conditions, increases in the tissues under study.
The changes in the nitric oxide system during hypertension established in this experiment probably relate to a decreased ability of endothelial cells to release relaxation factors together with unchanged or increased formation of vasoconstrictive agents. Production of cyclooxygenase-dependent prostaglandins and free radicals is increased leading to a decrease in NO activity19).
At a moderate level of synthesis, active forms of oxygen act as specific signalling molecules and are involved in the regulation of immune processes, operation of the circulatory, endocrine and other body systems. The signalling function of active forms of oxygen is realized due to their activating influence on the transcription factors through the complex redox-sensitive signalling systems of cells.
In pathological conditions, the system of generation of nitric oxide becomes an auxiliary factor in the development of oxidative stress in a cell. It is known that with high levels of superoxide radicals and nitric oxide, peroxynitrite is formed which is a strong oxidizing agent that can cause significant damage to molecules. In such a way, the developed peroxynitrite may lead to even more damage of cells of the cardiovascular system. Thus, the increase in the activity of inducible NO-synthase we detected in the cardiovascular system tissues was caused by activation of the enzyme by pro-inflammatory cytokines, such as alpha-tumour necrosis factor (TNF-α), and others. In this case, NO molecules formed under the action of inducible NO-synthase were converted into peroxynitrite when affected by free radicals, which resulted in intensification of free radical processes in the cardiovascular system organs (increased lipid peroxidation, formation of products of oxidative modification of proteins, DNA damage)20). The oxazole derivative normalizes the level of iNOS in blood serum and cardiovascular tissues and may be a blocker of one of the stages of the above mechanism.
In addition, nitric oxide belongs to the factors influencing the processes of inhibition of growth. Endothelial dysfunction with NO deficiency, increased expression of growth factors, local vasoactive substances, proteins and proteinases of the matrix may cause vascular re-modelling and damage of vessel’s structure. As a result, at hypertension, vessels have a thickened middle membrane, reduction of the lumen and an increase in extracellular matrix. Increasing mass of smooth muscle cells raises the level of vasoconstriction in response to neurohormones, leads to an increase in peripheral vascular resistance and thus contributes to further development of arterial hypertension21).
The recovery of NO-synthase system detected in our studies may relate to the effect of 1,3-oxazole-4-yl-phosphonic acid derivative on the level of bradykinin, which is a potent stimulant for release of endothelium-dependent relaxation factors (nitric oxide, endothelium-dependent hyperpolarization factor, prostacyclin)22). In particular, the endothelial hyperpolarization factor is an unstable metabolite of arachidonic acid, the release of which depends on the concentration of intracellular calcium and calmodulin23). Prostacyclin is formed in endothelial cells and activates adenylate cyclase of smooth muscle cells. This leads to increasing formation of cAMP, which leads to a relaxing effect that increases vasodilation caused by nitric oxide24).
Another likely mechanism of action of the studied oxazole derivative on the endothelial function may be blockade of the formation of angiotensin II, which is considered as an inducer of oxidative stress. Mechanisms of action of angiotensin II on the production of ROS (superoxide radicals) are related to stimulation of NADPH/NADH oxidases. Thus, a decrease in angiotensin II leads to a decrease in the intensity of oxidative processes, the products of which reduce the NO level. Decreasing of angiotensin II formation may occur through the inhibition of angiotensin-converting enzyme, or under the conditions of AT1 receptors blockade25).
Moreover, the effect of the test compound on nitric oxide system we detected may relate to the decrease in the level of calcium in cells cytosol. Such effect may be observed as a result of the decrease in transmembrane calcium delivery in smooth muscle cells of vessels and due to the NO cGMP-mediated mechanism in endothelial cells. In particular, the vasodilating effect of other oxazole derivatives is manifested by inhibition of СК2 protein kinase and a decreased intracellular calcium level26).
The effect of the studied drug may also be due to its antioxidant action. Thus, an increase in the superoxide dismutase activity leads to a decrease in NO destruction in vessels wall or influence on the expression of genes by endothelial NO-synthase, contributing to the increase of its level in the studied organs in an experimental hypertension model27).
Conflicts of interest: none.
Received Juny 8, 2018 / Accepted August 6, 2018
I. Nizhenkovska • Kateryna Sedko (Matskevych) (✉) • O. Kuznetsova
Department of Pharmaceutical
Biological and Toxicological Chemistry of Bogomolets National Medical University, Ukraine
Vyborgska, 49, flat 26, 03067 Kyiv, Ukraine
e-mail: katerynasedko@ukr.net
Sources
1. Mancia G., Fagard R., Narkiewicz K., Redón J., Zanchetti A., et al. Task Force Members. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013; 31, 1281–1357.
2. Chow C. K., Teo K. K., Rangarajan S., Islam S., Gupta R., et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA 2013; 310, 959–968.
3. Popov V. V., Bulanova N. A., Ivanov G. G. Sovremennie mysheni antygypertenzyvnoj terapyi. Dannie klynycheskyh issledovanyj. Chast 1. Aktualnie voprosi klynycheskoj farmakology 2012; 8(1), 88–94.
4. Nizhenkovska I. V., Romanenko A. V., Sedko K. V., Grusha M. M., Brovarets V. S., Golovchenko A. V. Studying of vasoaktive properties of diethyl esther of 5-alkylamine-2-{N-[N-benzoyl-(4-methylbenzal) glycyl] aminomethyl}-1,3-oxasole-4-ylphosphonic acid on rat’s isolated aorta. Pharmacology and drug toxicology 2015; 6, 76–83.
5. Nizhenkovska I. V., Zaichenko H. V., Sedko K. V., Golovchenko A. V., Golovchenko O. I. Research of the influence of derivative of 1,3-oxazole-4-il-phosphonic acid on arterial pressure and heart rate of rabbits. Recipe 2018; 21(1), 75–83.
6. Nizhenkovska I. V, Sedko K. V, Golovchenko O. V., Golovchenko O. I. The study of acute toxicity of 1, 3-oxazole-4-il-phosphonic acid derivative in intraperitoneal administration. Visnik farmacii 2018; 1(93), 43–48.
7. Kolesnyk M. Ju. Narušenyja v systeme oksyda azota kardyomyocytov u kris so spontannoj hypertenzyej na fone eksperymentalʹnoj hyperhlykemyy i ateroskleroza. Tavryčeskyj medyko-byolohyčeskyj vestnyk 2012; 15(59), 154–157.
8. Mojbenko O. O., Sahač V. F., Tkačenko M. M., Korkuško O. V. Fundamentalʹni mexanizmy diji oksydu azotu na sercevo-sudynnu systemu jak osnovy patohenetyčnoho likuvannja jiji zaxvorjuvanʹ. Fiziol. Žurn. 2004; 50(1), 11–30.
9. Sybirna N. O., Ljuta M. Ja., Klymyšyn N. I. Molekuljarni mexanizmy deponuvannja oksydu azotu v erytrocytax. Studia Biologica 2010; 4(1), 143–160.
10. Sullivan J., Pardieck J., Hyndmann K., et al. Renal NOS activity, expression, and localization in male and female spontaneously hypertensive rats. AJP Journal 2010; 51, 31–32.
11. Nizhenkovska I. V., Romanenko A. V., Sedko K. V., Grusha M. M. Vplyv blokady NO-syntazy na vazodylatacijnyj efekt dietylovoho esteru 5-alkilamino-2-{N-[N-benzojil-(4-metylbenzyliden) hlicyl] aminometyl}-1,3-oksazol-4-il-fosfonovoji kysloty na sehmentax izolʹovanoji aorty ščuriv. Pharmaceutical review 2016; 4, 52–56.
12. Badyal D. K., Lata H., Dadhich A. P. Animal models of hypertension and effect of drugs. Indian J. Pharmacol. 2003; 35, 349–362.
13. Salter M. Widespread tissue distribution, species and changes in activity of Ca2+ dependent and Ca2+ independent nitric oxide syntases. FEBS Lett. 1991; 291(1), 145–149.
14. Chin S. Y., Pandey K. N., Shi S. J., Kobori H. Increased activity and expression of Ca2+ dependent NOS in renal cortex of ANG II-infused hypertensive rats. Amer J Physiol. 1999; 277(5), 797–804.
15. Boyde J. R., Rahmotullah M. Optimization of conditions for the colorimetric determination of citrulline, using diacetil monoxime. Anal Biochem. 1980; 107, 424–431.
16. Green L. C. Analysis of nitrate, nitrite and [15N] nitrate in biological fluids. Anal. Biochem. 1982; 126(1), 131–138.
17. Lowry O. H. Protein measurement with Folin phenol reagent. J. Biol. Chem. 1951; 193(1), 265–275.
18. Dosenko V. E., Zagoriy V. Yu., Haytovich N. V. Allelic polymorphism of endothelial NOsynthase gene and its functional manifestations. Acta Biochem. Pol. 2006; 53(2), 299–302.
19. Kovalenko O. Je., Lytvyn O. V. Osoblyvosti cerebralʹnoho krovopostačannja jak osnova dlja topičnoji diahnostyky ta patohenetyčnoji terapiji. Liky Ukrajiny 2012; 10(166), 26–31.
20. Nahorna O. O., Beleničev I. F., Horčakova N. O., Mazur I. A., Čekman I. S. Vplyv anhiolinu na pokaznyky systemy oksydu azotu v miokardi ščuriv z eksperymentalʹnoju xroničnoju sercevoju nedostatnistju. Visnyk problem biolohiji ta medycyny 2016; 4(134), 96–99.
21. Sočka N. V. Dysfunkcija endoteliju u ditej z arterialʹnoju hipertenzijeju. Naukovo-praktyčnyj žurnal dlja pediatriv ta likariv zahalʹnoji praktyky – simejnoji medycyny 2013; 3(21), 57–64.
22. Holte H. R., Bjornstad-Ostensen A., Berg T. The role of endogenous bradykinin in blood pressure homeostasis in spontaneously hypertensive rats. Br J Pharmacol. 1996; 118(8), 1925–1930.
23. Garland C. J., Dora K. A. EDH: endothelium-dependent hyperpolarization and microvascular signaling. Acta Physiologica 2017; 219(1), 152–161.
24. Lupynskaja Z. A. Endotelyj sosudov – osnovnoj rehuljator mestnoho krovotoka. Vestnyk KRSU 2003; 7, 35–41.
25. Kovaleva O. N., Aščeulova T. V., Herasymčuk N. N., Safarhalyna-Kornylova N. A. Rolʹ oksydatyvnoho stressa v stanovlenyy y prohressyrovanyy hypertonyčeskoj bolezny. Naučnыe vedomosty Belhorodskoho hosudarstvennoho unyversyteta 2015; 4(21), 5–10.
26. Jakovenko I. N., Šablykin O. V., Kozačenko O. P., Brovarecʹ V.S. Vazodylatujuči efekty N-(2-aryl-4-tiokarbamojil-1,3-oksa-zol-5-il)-β-alaniniv – specyfičnyx inhibitoriv protejinkinazy SK2. Žurnal orhaničnoji ta farmacevtyčnoji ximiji 2012; 10(39), 55–58.
27. Iskra R. Ja., Vlizlo V. V. Superoksyddysmutazna, katalazna ta NO-syntazna aktyvnistʹ u tkanynax ščuriv za vplyvu cytratu xromu. Nauk. zap. Ternop. Nac. ped.. un-tu. Ser. 2012; 3(52), 107–113.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2018 Issue 4-
All articles in this issue
- Biological role of copper as an essential trace element in the human organism
- Study of influence of 1,3-oxazole-4-yl-phosphonic acid derivative on nitric oxide system indicators in rats with arterial hypertension
- Gas chromatography-mass spectrometry study of the root and herb of Smallanthus sonchifolius
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Biological role of copper as an essential trace element in the human organism
- Gas chromatography-mass spectrometry study of the root and herb of Smallanthus sonchifolius
- Study of influence of 1,3-oxazole-4-yl-phosphonic acid derivative on nitric oxide system indicators in rats with arterial hypertension
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

