-
Medical journals
- Career
OCT ANGIOGRAPHY AND DOPPLER SONOGRAPHY IN NORMAL-TENSION GLAUCOMA
Authors: J. Lešták 1; M. Fůs 1; A. Benda 1; K. Marešová 2
Authors‘ workplace: Oční klinika JL Fakulty biomedicínského inženýrství ČVUT v Praze 1; Oční klinika Lékařské fakulty Univerzity Palackého a Fakultní nemocnice Olomouc 2
Published in: Čes. a slov. Oftal., 76, 2020, No. 3, p. 120-123
Category: Original Article
doi: https://doi.org/10.31348/2020/20Overview
Aims: To investigate the dependence of blood vessel density and velocity in ophthalmic artery and arteria centralis retinae of the same eye in patients with normotensive glaucoma.
Methods: The sample consisted of 20 patients with normotensive glaucoma (NTG). There were 17 women (mean age 56.1) and 3 men (mean age 60 years). Inclusion criteria for study: visual acuity 1.0 with correction up to ±3 dioptres, approximately equal changes in the visual field, whereby it was incipient NTG and diagnosis was confirmed by electrophysiological examination, without further ocular or neurological disease. Parameters of vessel density (VD) were evaluated by Avanti RTVue XR (Optovue). Perfusion parameters such as peak systolic velocity (PSV), end diastolic velocity (EDV) and resistive index (RI) were evaluated for ophthalmic artery (AO) and arteria centralis retinae (ACR) using Doppler sonography (Affinity 70G Philips, probe 5–12 MHz). Visual field (VF) was evaluated by automated perimeter (Medmont M700) using fast threshold glaucoma strategy test. The sum of sensitivity levels in apostilb (asb) were evaluated in range 0–22 degrees of visual field. Resulting values of VF were compared with VD and perfusion parameters in AO and ACR at the same eye.
Results: Pearson’s correlation coefficient was used to evaluate the dependence. Data shows, that changes in visual fields are mainly caused by peripapillary VD of small and all vessels, and vessels throughout measured image area also. Correlation of small vessels throughout measured image area was weak (r = 0.23). Moderate negative correlation was found for PSV in AO and peripapillary small VD (r = -0.46), all peripapillary VD (r = -0.49), VD in whole area (r = -0.45), then between EDV in AO and VD in whole area (r = -0.42). Other correlations between VD and perfusion parameter were insignificant.
Conclusions: Study confirms, that changes of visual field in NTG patients are mainly caused by VD rather than perfusion parameters, especially in AO. Perfusion parameters in ACR are not significantly correlated with changes of VF in NTG patients.
Keywords:
visual field – Doppler sonography – OCT angiography – vessel density – normotension glaucoma
INTRODUCTION
In normal-tension glaucoma (NTG), damage takes place to the nerve fibres of the ganglion cells on the level of the optic nerve papilla and its anterior section [1]. It is known from the literature that in NTG the arteria ophthalmica (AO) and arteria centralis retinae (ACR) are altered [2,3,4]. As a result, it was the aim of this study to determine whether a dependency exists between VD and flow parameters in the ACR and AO.
MATERIAL AND METHODS
The observed cohort consisted of 20 patients with NTG, comprising 17 women with an average age of 56.1 years (43-79 years) and 3 men with an average age of 60 years (51-66 years). Among the inclusion criteria for the study were the following: visual acuity of 1.0, if applicable with correction of less than ± 3 dioptres, approximately the same changes in the visual field in all patients, diagnosis of incipient NTG confirmed by electrophysiological examination, no other ocular or neurological pathology. Vessel density (VD) was determined with the aid of Avanti RTVue and XR (Optovue). Whole image (WI) and peripapillary (PP) values of VD were analysed. In both cases, both all blood vessels (VDa) and small blood vessels (VDs) were analysed. The perfusion parameters were obtained using Doppler sonography on an instrument Affinity 70G (Philips, probe 5–12 MHz). Peak systolic velocity (PSV), end diastolic velocity (EDV), resistance index (RI) in the arteria ophthalmica (AO) and arteria centralis retinae (ACR) were measured. The visual field was evaluated according to a glaucoma test with a fast threshold strategy (Medmont M700). The sum of sensitivity levels in apostilbs (asb) was evaluated within the range of 0–22 degrees of the visual field. The results of sensitivity levels in the visual field were compared with VD and the measured flow parameters of the same eye. A Pearson’s correlation coefficient was used for assessment of the dependency between selected parameters.
RESULTS
The average measured values are shown in tables 1 and 2. On the basis of these values it is possible to determine that changes in visual fields are contributed to especially by peripapillary VD (VDa and VDs), as well as all vessels of VD from the entire area of measurement with the stated tool (WIVDa). Small vessels from the area of WI (WIVDs) have no significant influence (r = 0.23). In the case of dependency between VD and flow parameters in the ACR and CO, a medium indirect dependency was determined between PPVDa and PSVAO (r = -0.49), PPVDs and PSVAO (r = -0.46) and WIVDa and PSVAO (r = -0.45). A similar relationship was recorded also between EDVAO and PPVDa (r = -0.41), EDVAO and PPVDs (r = -0.41) and EDVAO and WIVDa (r = -0.42). Other correlations between VD and flow parameters were insignificant. Our results demonstrated that changes in the visual field are contributed to mainly by the vascular component of VD, specifically more PPVDa, PPVDs and WIVDa than WIVDs. Only a weak correlation was recorded between the perfusion parameters and visual field (PSVAO = -0.23; PSVACR = 0.26; EDVAO = -0.27; EDVACR = 0.22).
1. Resulting values of Pearson’s correlation coefficient. Very weak correlation (r = 0.00–0.19), weak correlation (r = 0.20–0.39), medium correlation (r = 0.40–0.59), strong correlation (r = 0.60–0.79), very strong correlation (r = 0.80–1.00). The mean measured values and their standard deviation are presented in the last two rows of the table. 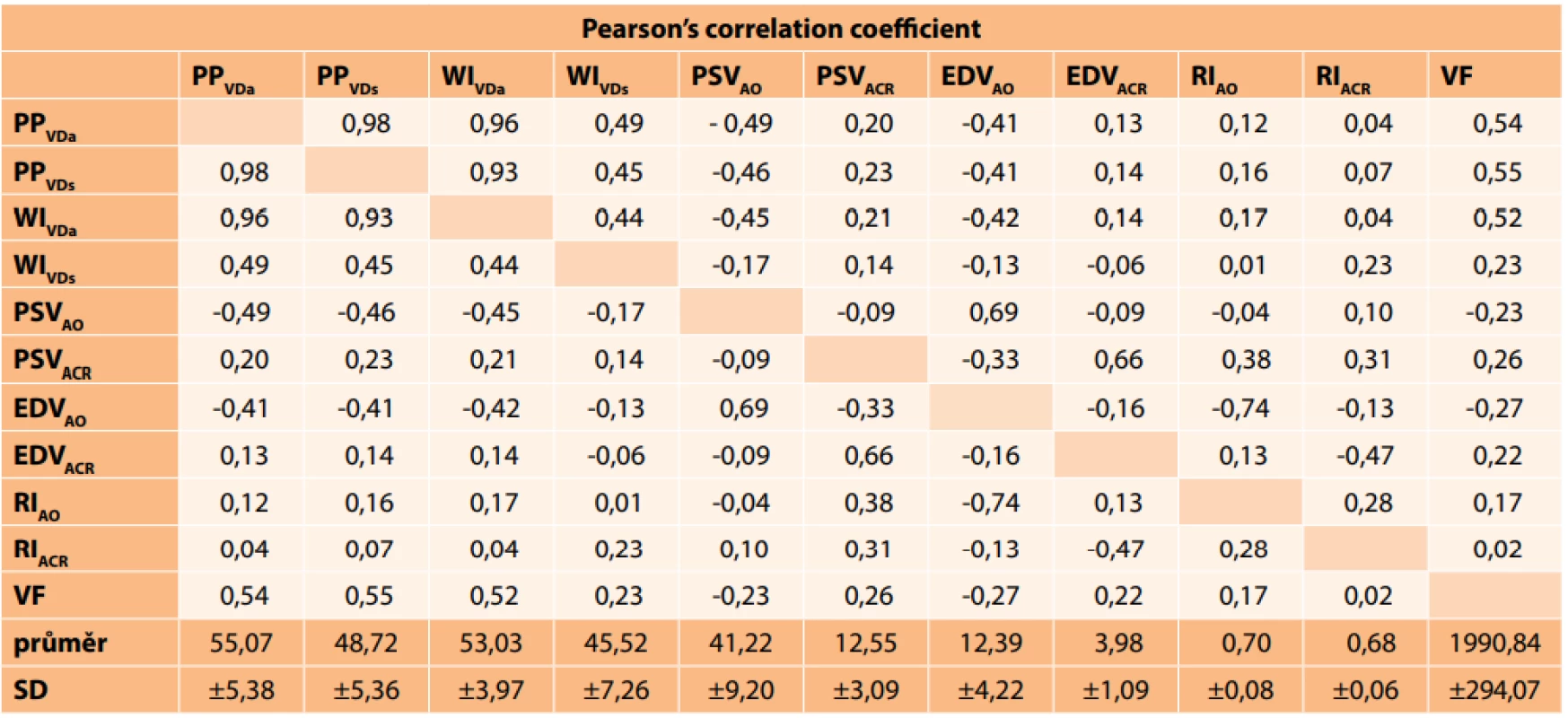
PPVDa (peripapillary vessel density of all vessels), PPVDs (peripapillary vessel density of small vessels), WIVDa (vessel density cév celého obrazu), WIVDs (vessel density of vessels of whole image), PSV (peak systolic velocity), EDV (end diastolic velocity), RI (resistance index), ACR (arteria centralis retinae), AO (arteria oftalmica), VF (visual field), SD (standard deviation). 2. Mean measured values of velocity and resistance index in ACR and AO. *Physiological values were taken from the study by Plange et al. [2]. ![Mean measured values of velocity and resistance index in ACR and AO. *Physiological values were taken from the study by
Plange et al. [2].](https://www.prolekare.cz/media/cache/resolve/media_object_image_small/media/image_pdf/1cf4f9907d9ebf1d6e4eb50460406135.png)
ACR (arteria centralis retinae), AO (arteria ophthalmica), PSV (peak systolic velocity), EDV (end diastolic velocity), RI (resistance index), cm/s (centimetres per second), SD (standard deviation), S (significant), NS (not significant) DISCUSSION
Haemodynamic changes leading to ischaemia may be one of the important manifestations in NTG. As a result, the parameters measured with the aid of Doppler sonography may also serve as a potential diagnostic tool in NTG [3,4].
Six years ago, Mi et al. wrote in a summary study that to date no comprehensive technique exists for evaluating ocular blood flow. Different measurements provide different details on vascular parameters, and it is necessary to interpret them differently. Precision and multi-functionality are therefore trends of prospective application of current approaches to the assessment of ocular blood flow (OBF) [5].
The most popular method in clinical conditions is colour Doppler imaging (CDI). This is used for evaluating the velocity of blood flow through the retrobulbar blood vessels with the aid of the resistance index as a parameter. A higher value of the resistance index represents greater vascular resistance, which indicates a disorder of perfusion. Examination by CDI is an older method in comparison with OCT angiography, and has been used in ophthalmology since the end of the 20th century. In our study we did not attempt to compare the measured perfusion values with a group of healthy subjects. As an example, we present a comparison with the results of other authors [2]. Vascular dysregulation leads to unstable ocular blood flow, which may result in ischaemia and damage to the optic nerve [6,7].
OCT angiography is a relatively new, non-invasive and reproducible method. The initial results of studies have demonstrated a high diagnostic potential in glaucoma, and in the future they could contribute to the management of this pathology [8]. Through a comparison of OCT angiography with the method of laser flow measurement in patients with NTG, the authors determined a higher diagnostic value using OCT angiography than flow measurement [9]. For the study of glaucoma, examination of perfusion parameters of superficial retinal microcirculation has greater validity in the peripapillary region than in the macular region [10].
Our examination in NTG demonstrated that VD plays a large role in changes in the visual field. We demonstrated a lower dependency in the case of WIVDs. Changes in the density of blood vessels in the peripapillary region in NTG have also been recorded by other authors [7,9,11,12,13,14,15,16].
Tepelus et al. determined that eyes with NTG also had lower choriocapillary perfusion density than a control group [14].
We can concur with this conclusion also on the basis of our own examination. We believe that in NTG a very important role is played by the loss of capillaries mainly in the region of the optic nerve papillary and its anterior section. Changes of flow parameters determined by Doppler ultrasonography confirm this assumption. We are not yet able to state by which process this loss of capillaries takes place. An explanation may be offered by the study by Cheng et al., who determined higher viscosity of blood in patients with NTG, which may be linked to impaired deformability of erythrocytes, in connection with a change of their rigidity. Higher viscoelasticity and viscosity of blood in patients with NTG, at a low flow speed, was caused by increased aggregability of erythrocytes. Impaired deformability of erythrocytes in NTG patients is also susceptible to the development of abnormalities of distal microcirculation. Increased blood viscosity and low effectiveness of blood oxygen transport may lead to hypoperfusion of the optic nerve in patients with NTG [17]. For the stipulated quantity of blood to flow through the given region, its speed must be increased upon a disorder of the bloodstream. We determined this also in our own cohort (PSV). All the above matters may then contribute to the pathogenesis of NTG.
CONCLUSION
Our results demonstrated that changes in the visual field are contributed mainly by the vascular component of VD, specifically more PPVDa, PPVDs and WIVDa than WIVDs. We recorded an indirect medium correlation between VD and PSVAO. The other correlations between VD and flow parameters were insignificant. VD contributes more to changes in the visual field than perfusion parameters in the AO. Perfusion in the ACR does not play a significant role in changes in the visual field in NTG.
Sources
1. Lešták J, Nutterová E, Pitrová Š. High tension versus normal tension glaucoma. A comparison of structural and functional examinations. J Clinic Exp Ophthalmol, 2012; S5 : 006. doi:10.4172/2155-9570.S5-006.
2. Plange N, Remky A, Arend O. Colour Doppler imaging and fluorescein filling defects of the optic disc in normal tension glaucoma. Br J Ophthalmol. 2003 Jun;87(6):731–736.
3. Xu S, Huang S, Lin Z, Liu W, Zhong Y. Color Doppler Imaging Analysis of Ocular Blood Flow Velocities in Normal Tension Glaucoma Patients: A Meta-Analysis. J Ophthalmol, 2015 : 919610. doi: 10.1155/2015/919610. Epub 2015 Oct 29.
4. Kuerten D, Fuest M, Bienert M, Walter P, Plange N. Ocular hemodynamics in Acute Nonarteritic Anterior Ischemic Optic Neuropathy Compared with normal tension glaucoma. J Glaucoma. 2019 Apr;28(4):334–340.
5. Mi XS, Yuan TF, So KF. The current research status of normal tension glaucoma. Clin Interv Aging. 2014 Sep;16(9):1563-1571.
6. Flammer J, Mozaffarieh M. What is the present pathogenetic concept of glaucomatous optic neuropathy? Surv Ophthalmol. 2007 Nov;52 : 162–173.
7. Ehrlich R, Harris A, Siesky BA. et al. Repeatability of retrobulbar blood flow velocity measured using color Doppler imaging in the Indianapolis Glaucoma Progression Study. J Glaucoma. 2011 Dec;20(9):540–547.
8. Alnawaiseh M, Lahme L, Eter N, Mardin C. Optical coherence tomography angiography: Value for glaucoma diagnostics. Ophthalmologe. 2019 Jul;116(7):602–609.
9. Takeyama A, Ishida K, Anraku A, Ishida M, Tomita G. Comparison of Optical Coherence Tomography Angiography and Laser Speckle Flowgraphy for the Diagnosis of Normal-Tension Glaucoma. J Ophthalmol. 2018 Jan;31. doi: 10.1155/2018/1751857.
10. Richter GM, Chang R, Situ B. et al. Diagnostic Performance of Macular Versus Peripapillary Vessel Parameters by Optical Coherence Tomography Angiography for Glaucoma. Transl Vis Sci Technol. 2018 Dec 6;7(6):21. doi: 10.1167/tvst.7.6.21. eCollection 2018 Nov.
11. Bojikian KD, Chen CL, Wen JC. et al. Optic Disc Perfusion in Primary Open Angle and Normal Tension Glaucoma Eyes Using Optical Coherence Tomography-Based Microangiography. PLoS One. 2016 May 5;11(5):e0154691. doi: 10.1371/journal.pone.0154691. eCollection 2016.
12. Scripsema NK, Garcia PM, Bavier RD. et al. Optical Coherence Tomography Angiography Analysis of Perfused Peripapillary Capillaries in Primary Open-Angle Glaucoma and Normal-Tension Glaucoma. Invest Ophthalmol Vis Sci. 2016 Jul 1;57(9):OCT611-OCT620. doi: 10.1167/iovs.15-18945.
13. Igarashi R, Ochiai S, Sakaue Y. et al. Optical coherence tomography angiography of the peripapillary capillaries in primary open-angle and normal-tension glaucoma. PLoS One. 2017 Sep 15;12(9):e0184301. doi: 10.1371/journal.pone.0184301. eCollection 2017.
14. Tepelus TC, Song S, Borrelli E. et al. Quantitative Analysis of Retinal and Choroidal Vascular Parameters in Patients With Low Tension Glaucoma. J Glaucoma. 2019 Jun;28(6):557–562.
15. Kim JS, Kim YK, Baek SU. et al. Topographic correlation between macular superficial microvessel density and ganglion cell-inner plexiform layer thickness in glaucoma-suspect and early normal-tension glaucoma. Br J Ophthalmol. 2019 Apr 2. pii: bjophthalmol-2018-313732. doi: 10.1136/bjophthalmol-2018-313732. [Epub ahead of print]
16. Nitta K, Sugiyama K, Wajima R, Tachibana G, Yamada Y.. Associations between changes in radial peripapillary capillaries and occurrence of disc hemorrhage in normal-tension glaucoma. Graefes Arch Clin Exp Ophthalmol. 2019 Sep;257(9):1963–1970.
17. Cheng HC, Chan CM, Yeh SI, Yu JH, Liu DZ. The hemorheological mechanisms in normal tension glaucoma. Curr Eye Res. 2011;36(7):647–653.
Labels
Ophthalmology
Article was published inCzech and Slovak Ophthalmology
2020 Issue 3-
All articles in this issue
- TRAUMA IN OCULOPLASTIC SURGERY A REVIEW
- CHANGES OF THE FOVEAL AVASCULAR ZONE AND MACULAR MICROVASCULATURE WITHIN THE FRAMEWORK OF OCT ANGIOGRAPHY EXAMINATION IN YOUNG PATIENTS WITH TYPE 1 DIABETES (PILOT STUDY)
- OCT ANGIOGRAPHY AND DOPPLER SONOGRAPHY IN NORMAL-TENSION GLAUCOMA
- OPTIC CHIASM WIDTH IN NORMAL-TENSION AND HIGH-TENSION GLAUCOMA
- EXTERNAL OPHTHALMOMYIASIS CAUSED BY OESTRUS OVIS (A CASE REPORT)
- PRES SYNDROME
- Czech and Slovak Ophthalmology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- PRES SYNDROME
- EXTERNAL OPHTHALMOMYIASIS CAUSED BY OESTRUS OVIS (A CASE REPORT)
- TRAUMA IN OCULOPLASTIC SURGERY A REVIEW
- CHANGES OF THE FOVEAL AVASCULAR ZONE AND MACULAR MICROVASCULATURE WITHIN THE FRAMEWORK OF OCT ANGIOGRAPHY EXAMINATION IN YOUNG PATIENTS WITH TYPE 1 DIABETES (PILOT STUDY)
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

