-
Medical journals
- Career
Non-arteritic anterior ischaemic optic neuropathy: treatment and risk factors
Authors: S. Kalábová; K. Marešová; M. Karhanová
Authors‘ workplace: Oční klinika LF UP a FN Olomouc
Published in: Čes. a slov. Oftal., 76, 2020, No. 2, p. 78-87
Category: Original Article
doi: https://doi.org/10.31348/2020/15Overview
Aim: To ascertain whether various therapeutic procedures in non-arteritic anterior ischaemic optic neuropathy (NAION) have an impact on the resulting visual acuity of the affected eye. To assess the prevalence of risk factors that accompany this disease according to the literature.
Methods: The retrospective study enrolled 55 eyes of 53 patients (41 men, 12 women) with an age range of 46 to 85 years (mean 64.9; median 64.0) who were hospitalized at the Department of Ophthalmology of the Faculty of Medicine and Dentistry and the University Hospital in Olomouc with the diagnosis of NAION between 2005 and 2016, and who received systemic treatment with intravenous vasodilators, either alone or in combination with intravenous corticosteroids. Central visual acuity (CVA) prior to treatment and immediately after its termination was evaluated. CVA was measured using the Snellen chart and is presented in decimal values. Using medical history data and medical records, the presence of systemic disease, namely hypertension, type 2 diabetes mellitus, and hypercholesterolaemia, was studied in these patients and evaluated for a possible association with NAION.
Results: In the group of patients who were treated with intravenous vasodilators, the resulting CVA improved by 0.083 on average. In the group of patients who, in addition to vasodilator therapy, also received treatment with corticosteroids, the resulting CVA improved by only 0.03 on average. Although there was a more prominent improvement in CVA in the group treated with intravenous vasodilators alone, this difference was not statistically significant. At least one risk factor was found in the vast majority of the patients (96%). Eighty percent of the patients had hypertension, 43.6% of them were treated for diabetes mellitus, and 72.7% of the patients took drugs for hypercholesterolaemia. A combination of all these conditions was found in 36.4% of the patients. The proportion of smokers and past smokers did not exceed that of non-smokers.
Conclusion: The mean improvement in the resulting CVA in patients after systemic therapy with vasodilators alone was greater than in those treated with a combination of vasodilators and corticosteroids; however, this difference was not statistically significant. In most patients in the group, at least one systemic risk factor was noted, most frequently hypertension. The prevalence rate of systemic risk factors was comparable to that reported in the literature.
Keywords:
corticosteroids – risk factors – non-arteritic anterior ischaemic optic neuropathy – vasodilators
Sworn declaration:
The authors of the article declare that no conflict of interest exists in the compilation, theme and subsequent publication of this professional communication, and that it is not supported by any pharmaceuticals company.
The authors further declare that the study has not been submitted to any other journal or printed elsewhere, with the exception of congress abstracts and recommended procedures.
INTRODUCTION
Non-arteritic anterior ischaemic optic neuropathy – NAION) is the most widespread cause of acute affliction of the optic nerve in patients in the 6th and 7th decades of life. The pathology is caused by occlusion of the short posterior ciliary arteries, which are branches of the arteria ophthalmica [14]. This is not an inflammatory pathology, but a consequence of vascular insufficiency. The pathology can therefore be considered to constitute an infarction of the optic nerve, nevertheless due to its different mechanism of origin it cannot be compared with an infarction of the brain [4]. It is manifested as a rule as a sudden, painless deterioration of visual acuity in one eye. Loss of vision usually appears in the morning after waking, which indicates a possible connection with nocturnal hypotension [8]. Central visual acuity (CVA) may be within the range of 1.0 to mere light perception. Upon examination of the visual field, lower altitudinal scotoma is typically found, although this may also be a central, paracentral, quadrant or arcuate outage of the visual field. In the case of a larger deterioration of central vision, we often find a relative afferent pupillary defect (RAPD). Deterioration of vision progresses for approximately two weeks and then remains stable. In addition to reduction of visual acuity, disorder of colour vision – dyschromatopsia may be expressed, depending on the level of affliction of visual acuity.
A typical symptom of the acute phase of non-arteritic anterior ischaemic optic neuropathy is pale diffuse or sectored infiltration of the optic nerve papilla, often in connection with several peripapillary splintered haemorrhages or cotton-wool ischaemic deposits on the adjacent retina [16,20]. The clinical manifestations of this pathology may be variable, and in many cases it is difficult to determine a definitive diagnosis. Approximately 3 to 4 weeks after the onset of the pathology we may find atrophy and paleness of the papilla, which may be diffuse or sectored. During the course of this pathology there is a loss of nerve fibres, both peripapillary and in the macular region [20]. The risk of occurrence of this pathology in the other eye is 10 % within two years and 15 to 25 % within five years [8,20]. Recurrence of the pathology in the same eye is 6 % [3]. In the case of occurrence of NAION in the other eye, we may observe “pseudo Foster-Kennedy syndrome”, which is atrophy of the optic nerve papilla in one eye and pale edema of the optic nerve in the other eye [16].
Predisposing morphological risk factors for the occurrence of the pathology are small papillas with small or virtually disappeared excavation, and a markedly visible nerve fibre layer (“crowded-disc” or “disc at risk”), and drusens of the optic nerve papilla [9]. These predispositions act with a compressive mechanism on the optic nerve and may contribute to the onset of NAION. The main general risk factors are especially vascular factors – hypertension, diabetes mellitus, hypercholesterolaemia, hypercoagulation states (hyperhomocysteinemia), as well as collagenosis, nocturnal hypotension, obstructive sleep apnoea syndrome, treatment for erectile dysfunction and smoking. Predisposition on the basis of sex has not been unequivocally confirmed, but in our experience men are afflicted more often [18]. Certain other pathologies may also be linked with the occurrence of NAION, for example age-related macular degeneration or retinal vein occlusion [5]. Cataract surgery may also be a risk factor for the occurrence of NAION (postoperatively increased intraocular pressure, intraocular inflammation, retrobulbarly administered anaesthetic) [8].
Unfortunately, no demonstrably effective treatment of NAION exists to date, and the prognosis regarding visual functions is also poor. The basis of therapy is the careful adjustment of all pathological conditions in the body, especially the aforementioned vascular pathologies. Antithrombotic and vasodilator drugs are used in adjuvant therapy [3,11]. The effect of generally administered corticosteroids is stated mainly in alleviating swelling of the optic nerve papilla [10,11,21,22]. Nonetheless, no treatment is able to cure the condition causally, and none so far reduces either the risk of occurrence of NAION in the other eye or relapse in the same eye. A new method being experimented with is intravitreal injection of steroids (triamcinolone) or vascular endothelial growth factor (VEGF) inhibitors, which could reduce vasogenic edema by inhibiting VEGF [3,8]. The use of neuroprotective agents in acute stages of NAION is so far in the phase of trials [8]. For patients with progressive form of NAION it is possible to consider the performance of decompression of the optic nerve sheaths, even if this method is controversial [15,16].
The aim of this study is to determine whether the various therapeutic approaches that we use on patients with NAION at our centre have had an influence on the resulting CVA of the affected eye, and to evaluate the occurrence of the factors that accompany this pathology according to the literature.
COHORT AND METHOD
Design of study
The retrospective study included all the patients who were hospitalised with a diagnosis of NAION at the Department of Ophthalmology at the Faculty of Medicine and Dentistry of Palacký University and University Hospital Olomouc in the period from 2005 to 2016. First of all a list of the patients (total 147) with a diagnosis of NAION was obtained from the department. Of these, 58 reported for a targeted examination for the purpose of this study. Subsequently, all the patients who were provided with general therapy by intravenously administered vasodilator drugs, antithrombotic drugs and in indicated cases also corticosteroids intravenously, were included in the study. According to the applied therapy, the patients were subsequently divided into two groups. The first group comprised patients who received intravenous application of only vasodilation therapy, and the second group vasodilation and corticosteroid therapy. Five patients who could not be treated with general vasodilation therapy for internal reasons were excluded from the study, and other therapy was applied. In 3 cases this concerned monotherapy with intravenously applied corticosteroids, in two cases only outpatient treatment was applied (oral vasodilator drugs) upon the patient’s request. Due to the nature of the study, the interval between the onset of the pathology and the targeted examination was of varying length (3 months to 11 years). For the study we compiled a form in which we recorded all the observed and supplementary information. We recorded the type of therapy, length of complaints before commencement of treatment, we carefully recorded the patient’s personal medical history, in which we focused especially on the presence of hypertension, type 2 diabetes mellitus and hypercholesterolaemia. We also recorded data on whether patients smoked. According to the report from hospitalisation, we determined CVA before treatment and immediately after the completion of treatment upon discharge of the patient. We also recorded current CVA, which we however did not include in the resulting evaluation, due to the possible influence of other ocular pathologies, in particular cataract. CVA was examined on Snellen’s optotypes and recorded in decimal value. In the case of poor CVA within the range of counting fingers in front of the eye or only perception of movement in front of the eye, we used a conversion table (Table 1). We performed computer perimetry (Octopus, Haag Streit, Switzerland) in both eyes (entire visual field) and HRT (HRT 2, Heidelberg Retina Tomograph, Heidelberg Engineering, Germany) examination in order to determine the average size of the optic nerve papilla.
1. Conversion of poor central visual acuity to decimal value 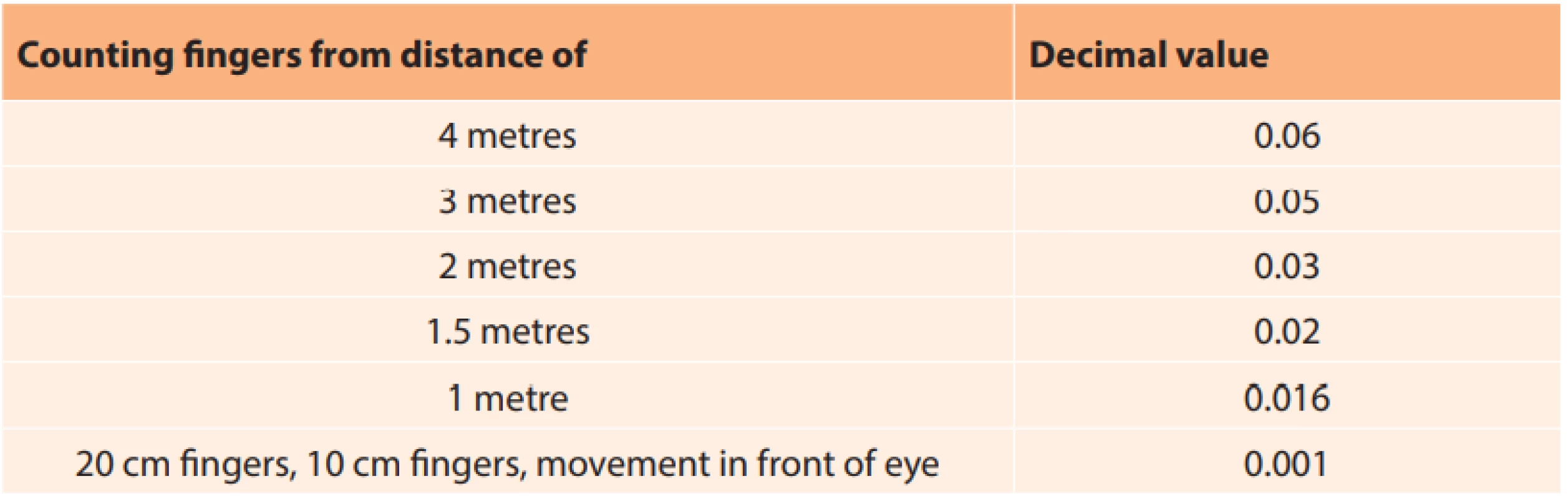
Administered general therapy
At our department, our standard treatment for patients with NAION is to apply an infusion of procaine hydrochloride 0.2 % (Prokain®) 500 ml per day for a period of 5 days, unless their internal condition excludes the possibility of this treatment. In the case of a beneficial grading effect, this treatment is prolonged to up to 9 days. We also administer antithrombotic drugs in a therapeutic dose (Fraxiparine® injection) unless the patient is already using such medications (even if in oral form). In the case of pronounced edema of the optic nerve papilla, marked deterioration of vision or reduction of visual acuity in the other eye for various reasons, we supplement general therapy with the intravenously applied corticosteroid methylprednisolone (SoluMedrol®) 500 mg per day for a period of 3 days in order to accelerate the resorption of the edema.
Characteristics of cohort
The retrospectively observed group comprised 55 eyes of 53 patients, who were hospitalised at the Department of Ophthalmology at the Faculty of Medicine and Dentistry of Palacký University and University Hospital Olomouc with a diagnosis of NAION in the period from 2005 to 2016. The number of eyes treated intravenously with applied vasodilator drugs (Prokain®) was 37. Combined therapy with Prokain® and SoluMedrol® was indicated in 18 cases (Table 2).
2. Number of treated eyes in individual groups 
In total the cohort comprised 41 men (77.4 %) and 12 women (22.6 %). The representation of men and women in the individual groups is presented in Table 3. The age range of the treated patients was from 46 to 85 years (mean 64.9; median 64.0). The age of the men and women in the individual groups (age range, mean, median) is presented in Table 4.
3. Representation of sex in individual groups 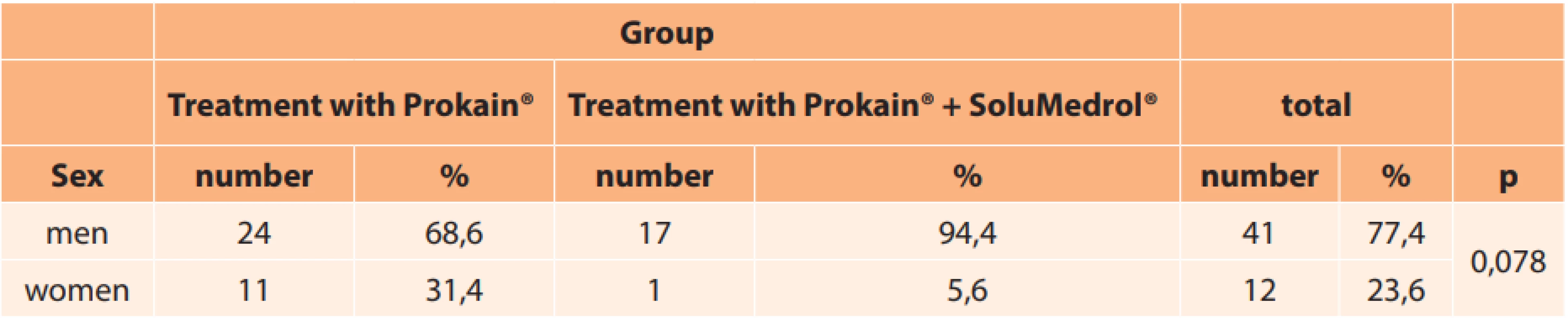
4. Age of men and women in individual groups 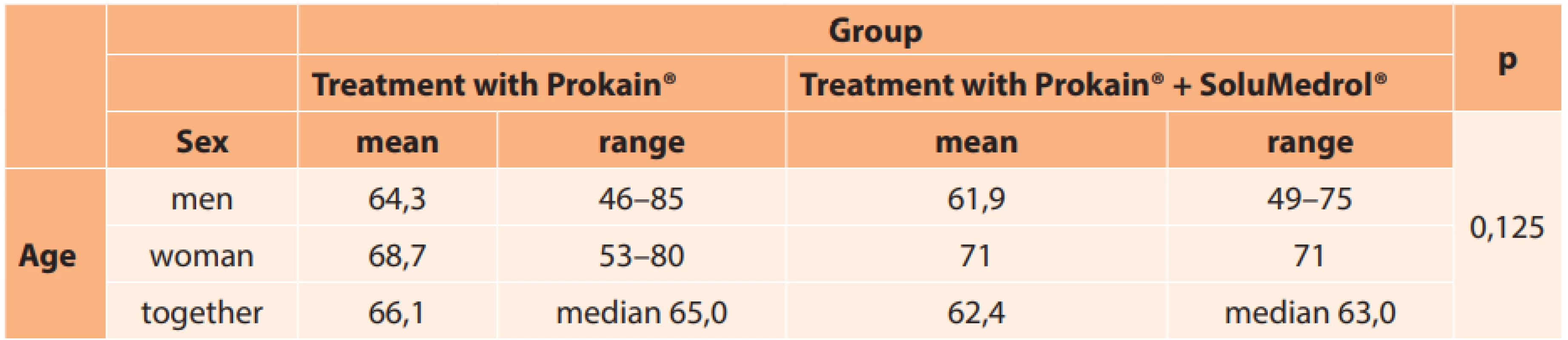
Data analysis and statistical evaluation
The data from each patient was recorded in a pre-prepared form. The data was subsequently converted into electronic form, subjected to a descriptive analysis and statistically processed. Change of CVA was assessed with the aid of a Wilcoxon paired test. The dependency between change of CVA and the length of the complaints was analysed with the aid of a Spearman correlation coefficient. Both groups according to treatment were compared in quantitative parameters with the aid of a Mann-Whitney U test, in categorical parameters with the aid of a chi-quadrant test or Fisher test in the case of small numbers. The normality of the data was tested with the aid of a Shapiro-Wilk test. All the tests were conducted on a level of significance of 0.05. The statistical software IBM SPSS Statistics version 22 was used for the data analysis.
RESULTS
No significant difference was found upon a comparison of both groups according to sex (Table 3; p = 0.078) and age (Table 4; p = 0.125).
In the patients treated only with intravenous application of a vasodilator drug (Prokain®), average CVA at the beginning of the pathology was 0.356, and immediately after the end of therapy 0.439. Resulting CVA improved by an average of 0.083. In the group with combined therapy with Prokain® and SoluMedrol®, average CVA before treatment was 0.398 and immediately after the end of therapy 0.429. Resulting CVA improved by an average of 0.031. In a statistical comparison, the groups did not differ significantly according to treatment, either in baseline CVA or after discharge. No significant difference was determined between the groups in the change of visual acuity (Table 5, p = 0.723).
5. Average values of central visual acuity before and after treatment 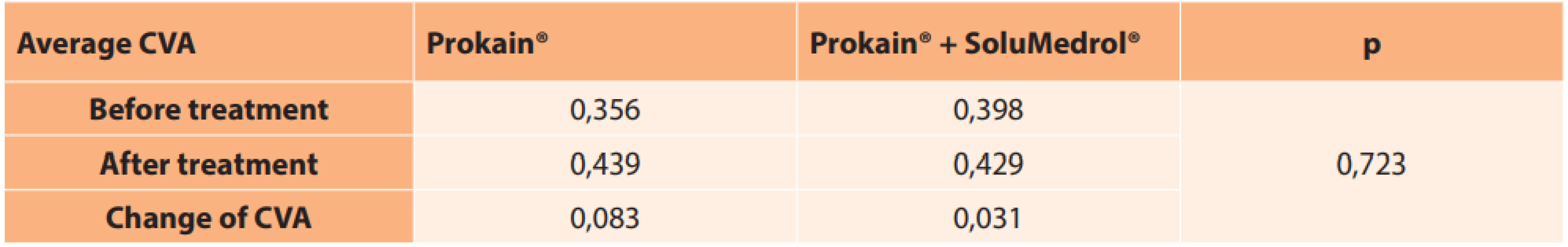
CVA – central visual acuity In the group treated with Prokain®, an improvement was recorded in 15 eyes. There was no change of CVA in the same number of eyes, and in 7 eyes CVA deteriorated (Table 6, Graph 1). In the patients treated with Prokain® and SoluMedrol® an improvement was recorded in 5 eyes, in 11 eyes CVA was unchanged and in 2 cases it deteriorated (Table 6, Graph 2).
6. Change of central visual acuity after treatment in individual groups 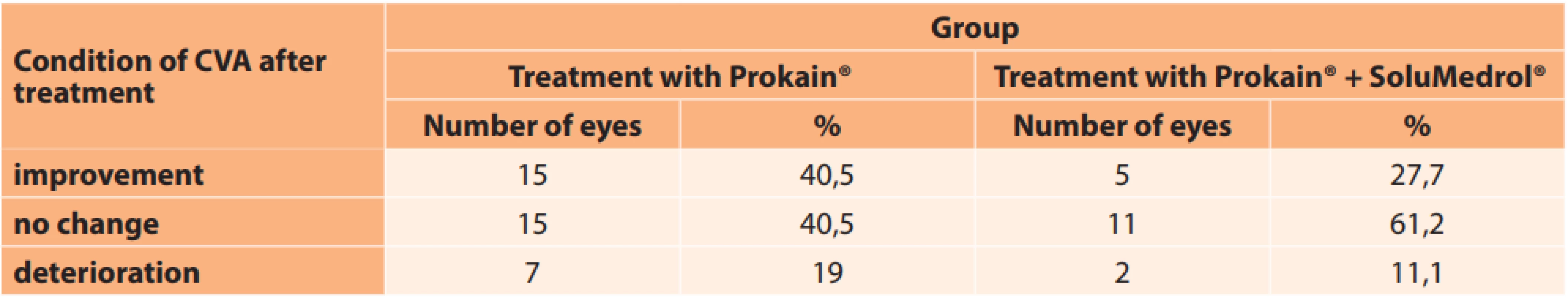
CVA – central visual acuity 1. Change of central visual acuity after treatment in group treated with Prokain® 
2. Change of central visual acuity after treatment in group treated with Prokain® and SoluMedrol® 
In the improved eyes in the group of patients treated only with Prokain®, CVA improved on average by 0.27 (Table 7).
7. Absolute values of central visual acuity in improved patients in group treated with Prokain® 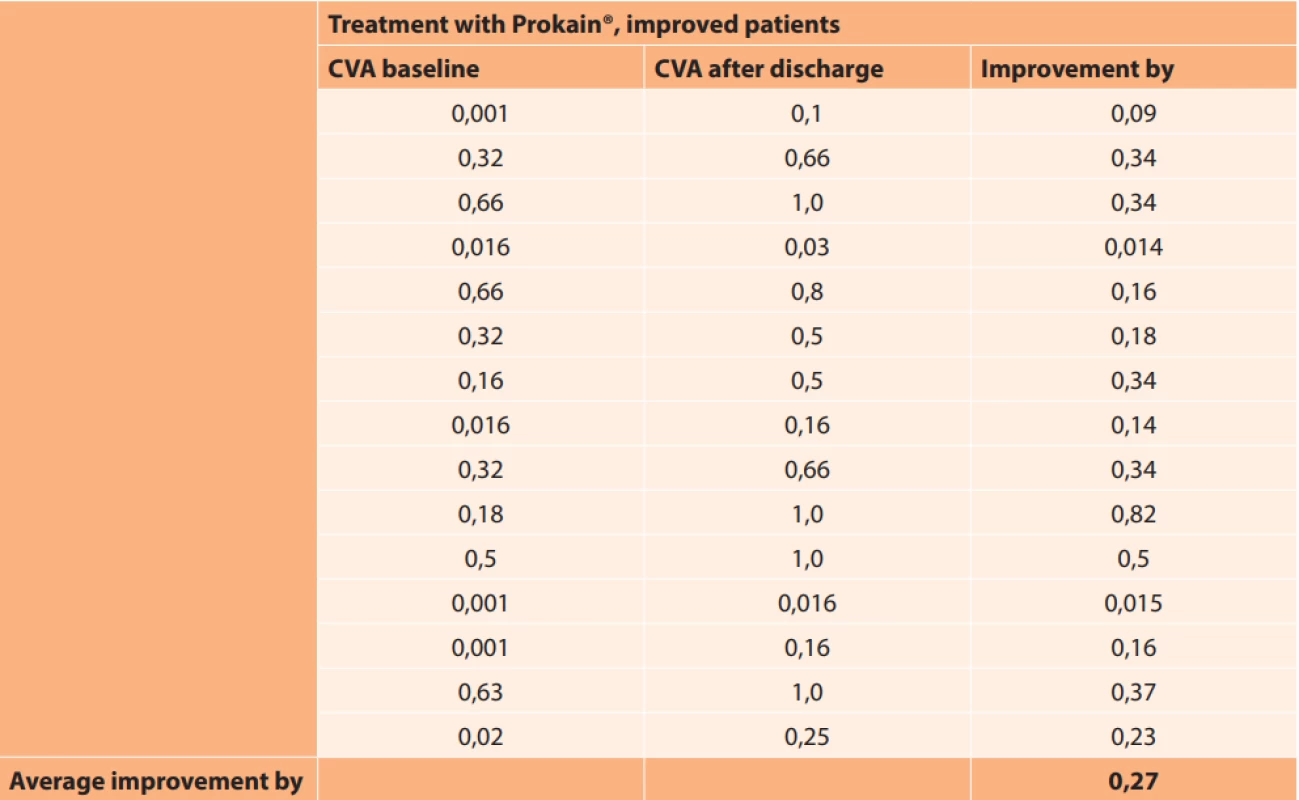
CVA – central visual acuity In the improved eyes in the group of patients treated with Prokain® and SoluMedrol®, CVA improved on average by 0.17 (Table 8).
8. Absolute values of central visual acuity in improved patients in group treated with Prokain® and SoluMedrol® 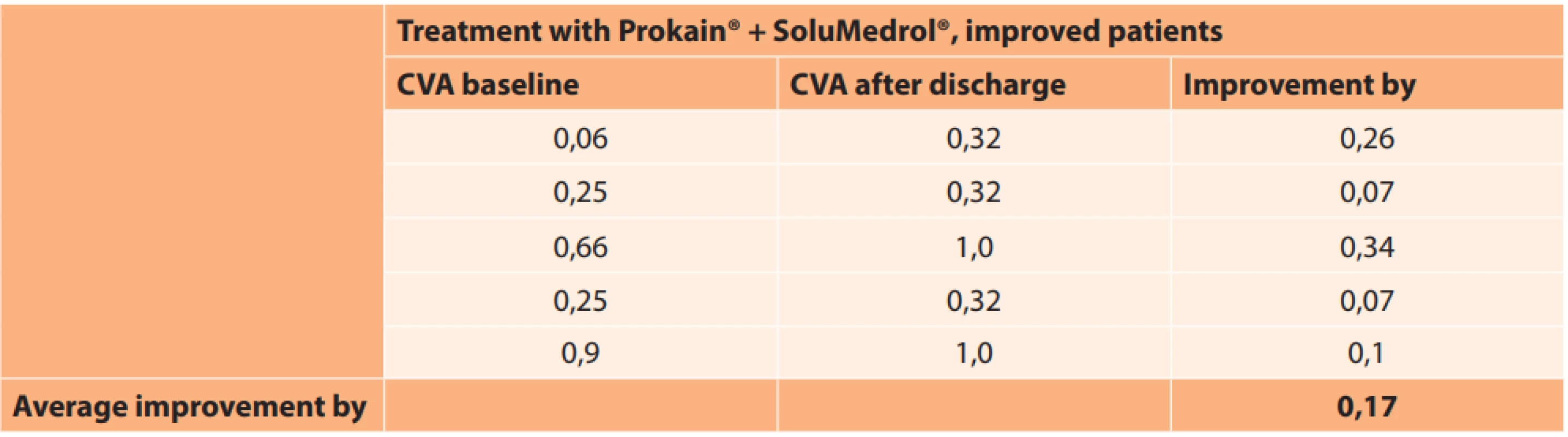
CVA – central visual acuity The average length of the complaints before the commencement of treatment was 9.8 days in the group treated with Prokain®, and 10.6 days in the group with combined therapy, in which the time range was from 1 day to 56 days. The difference in the length of the complaints between the two groups was not statistically significant (Table 9, p = 0.333). No significant correlation was demonstrated between the length of duration of the complaints and the change of visual acuity (either in the entire cohort or in the individual groups).
9. Average length of complaints before treatment 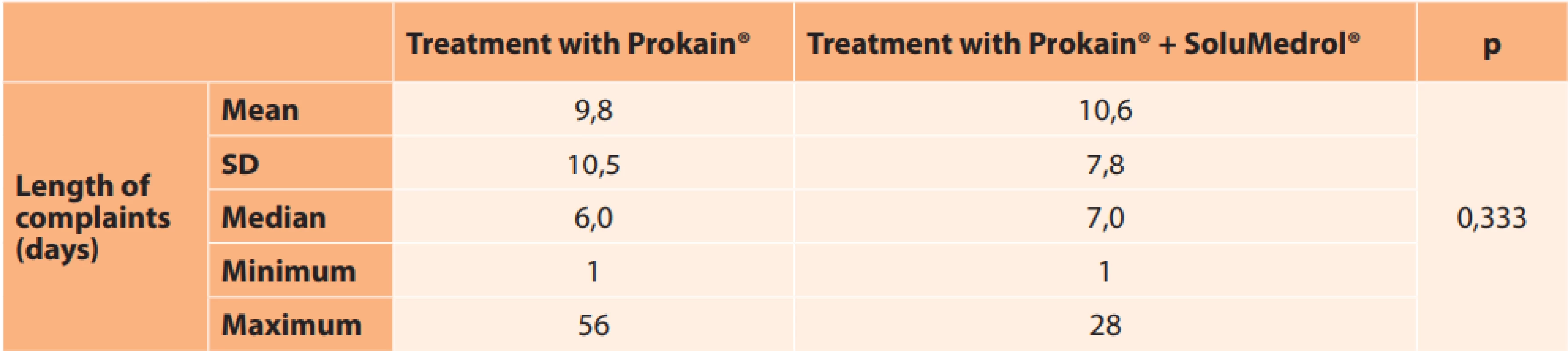
SD – standard deviation In the entire observed cohort, 80 % of patients had hypertension, 43.6 % of patients had type 2 diabetes and 72.7 % of patients were being treated for hypercholesterolaemia. All 3 observed pathologies were present in 36.4 % of patients. The majority of the patients, specifically 96 %, had at least 1 risk factor. Only in 2 cases was no risk factor determined, and these patients were not being treated for any other pathology (Table 10). No significant difference was determined between the two groups in terms of the incidence of risk factors (Table 11).
10. Representation of general risk factors 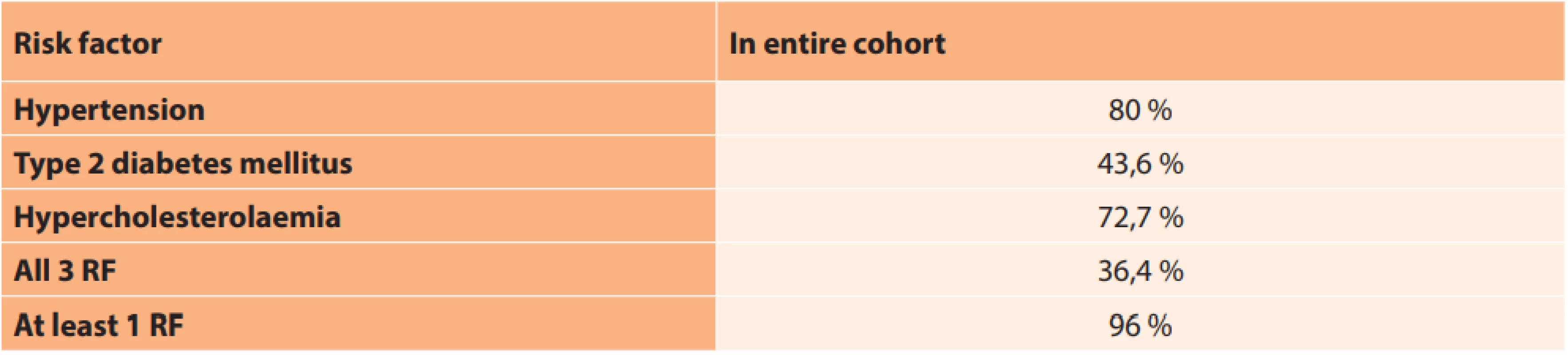
RF – risk factor 11. Representation of risk factors in individual groups according to treatment 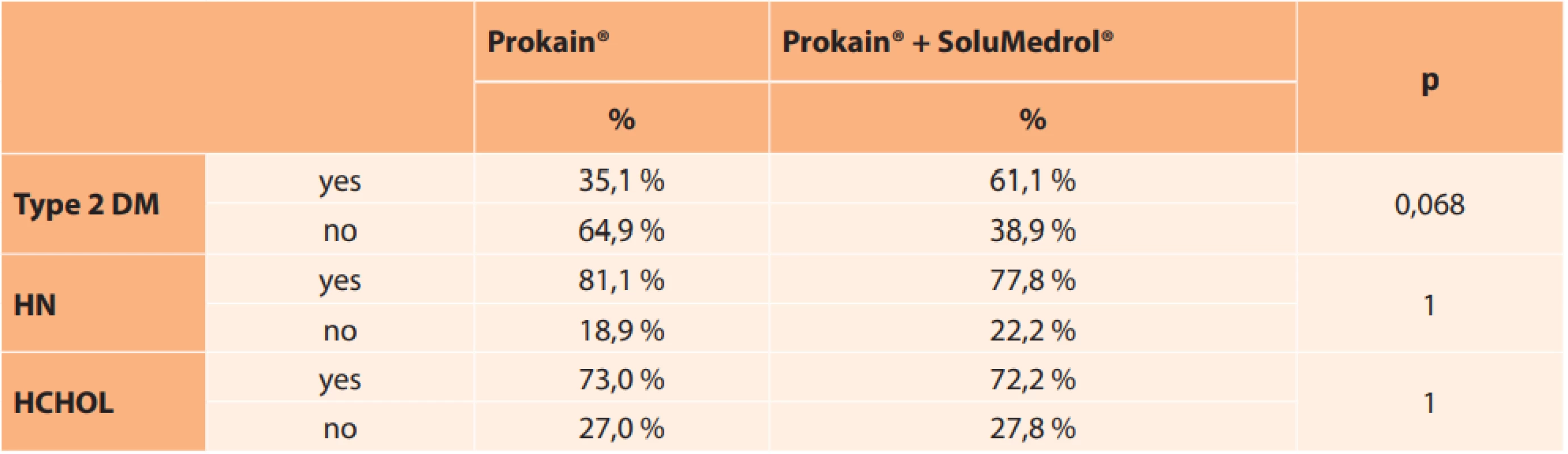
DM – diabetes mellitus; HN – hypertension; HCHOL – hypercholesterolaemia Out of the entire cohort, only 13.8 % of patients were smokers, 20.7 % were former smokers and the remainder were non-smokers (60.3 %) (Table 12, Graph 3). Upon a statistical comparison, no significant difference was found within the groups in terms of smoking.
12. Representation of smokers in entire cohort 
3. Representation of smoking in entire cohort 
The average size of the optic nerve papilla in the affected eye, measured with the aid of HRT, was 1.85 mm2, which came within the normal size range (1.63–2.43). The smallest papilla measured 1.14 mm2, and the largest measured 3.37 mm2. No significant difference was found in the individual groups in terms of the average sizes of the papillae. In 3 eyes we did not succeed in conducting this examination due to insufficient fixation of the eye with poor CVA (Table 13).
13. Size of optic nerve papilla 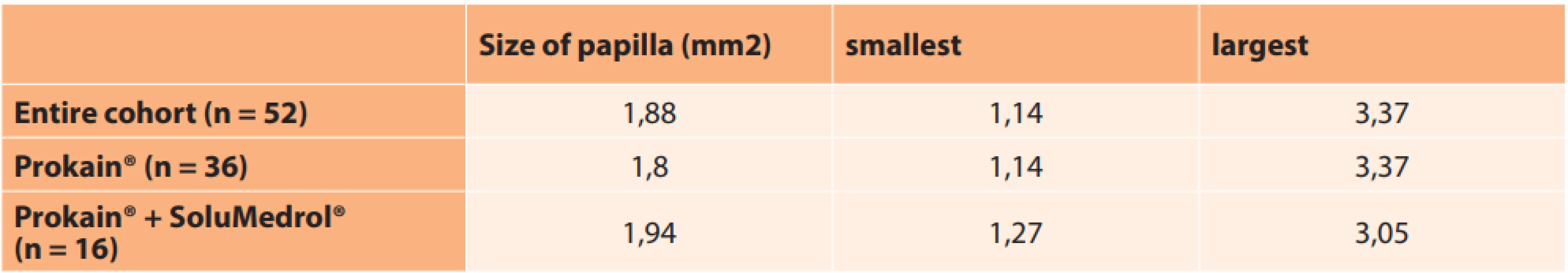
n – number of eyes Out of the entire cohort, the size of the papilla was beneath the norm (less than 1.63) in 25 % of eyes.
We determined drusens of the optic nerve papilla in one patient (in the afflicted eye).
In one patient with bilateral affliction, we recorded all 3 general risk factors (HN, type 2 DM, hypercholesterolaemia). The patient was a smoker. In another patient with bilateral affliction, we recorded familiar hypercholesterolaemia, hypertension and severe sleep apnoea syndrome.
DISCUSSION
Ischaemic Optic Neuropathy is the second most commonly occurring pathology of the optic nerve papilla in patients aged over 50 years, immediately behind glaucoma [14,16]. The papilla of the optic nerve is divided into the superficial nerve fibre layer, the prelaminar region, the region of the lamina cribrosa and the retrolaminar region. The superficial nerve fibre layer is supplied mainly from arterioles originating from the central retinal artery. The prelaminar region is supplied with blood from a circuit of arterioles termed the circulus Halleri and circulus Zinni. This circuit is formed by branches of the short posterior ciliary arteries, which are branches of the ophthalmic artery. The autonomous nervous system contributes to the regulation of retrobulbar and choroidal circulation, but ends in the lamina cribrosa, so the vascular system supplying the anterior part of the optic nerve papilla does not have direct innervation [17].
According to localisation of an ischaemic event, we differentiate between anterior ischaemic optic neuropathy (AION), which is far more common and is characterised by a typical triad – afferent pupillary defect, disorder of visual functions and edema of the optic nerve papilla [14,16]. A rare form is posterior ischaemic optic neuropathy (PION), which is relatively infrequent and is probably caused by occlusion of one or more of the pial branches. In this case we do not opthalmoscopically demonstrate edema of the optic nerve papilla.
AION may be connected with several general pathologies. In practice, however, we differentiate between two basic forms, namely arteritic (AAION) and non-arteritic (NAION). It is of entirely fundamental importance to distinguish between these two forms. Arteritic form of anterior ischaemic optic neuropathy (AAION), known as Horton’s disease (gigantocellular arteritis, temporal arteritis), is a granulomatous necrotising arteritis of the large and medium sized superficial arteries, especially the superficial temporal artery, ophthalmic artery, posterior ciliary artery and proximal vertebral artery. AAION may be its first clinical symptom. It is also manifested by deteriorated vision, headache in the temporal and occipital area, we find sensitivity to touch above the superficial temporal artery, patients state acute pain when chewing, which is caused by ischaemia of the masseter muscle, patients also suffer from loss of weight, loss of energy, pains of the proximal muscle groups, temperatures and nocturnal sweating. Laboratory tests generally record a high level of erythrocyte sedimentation (FW) up to around 100 mm per hour, increased CRP (C-reactive protein), anaemia in the blood count, and in addition the result of a biopsy also contributes to diagnosis. Treatment of AAION consists in systemic administration of corticosteroids intravenously as soon as possible. Biopsy of the temporal artery is performed especially for the purpose of confirming the diagnosis, although in the case of suspicion of AAION treatment with corticosteroids is commenced immediately, without waiting for the result of the biopsy. Timely and correct diagnosis may be decisive in saving not only the patient’s sight but also life, and as a result the diagnosis and treatment of this pathology is always urgent. In untreated patients with unilateral affliction, there is a large risk of loss of visual acuity also in the second eye [18].
Treatment of non-arteritic form of anterior ischaemic neuropathy (NAION) is not so unequivocal, the results are not optimal and new possibilities are constantly being sought. Atkins et al. [3] evaluate various therapeutic methods of NAION as empirically based procedures, which act on the presumed agent in connection with this pathology – blood vessels, thrombosis and swelling of the optic nerve. Other treatment is focused on a neuroprotective effect [8,10,11]. A possible intervention recently proposed is intravitreal injection of steroids and anti-VEGF preparations. However, to date no studies have demonstrated the benefit of medicamentous or surgical therapy of NAION. Similarly, no studies have demonstrated the influence of treatment on the occurrence of the pathology in the other eye.
In regular clinical practice we encounter the two most frequently used therapeutic procedures, namely vasodilation therapy and application of general corticosteroids. These procedures are discussed in the literature, and the conclusions are not unequivocal. According to Atkins et al. [3], treatment of NAION with general corticosteroids is based on a study from 1960 [7], which described the influence of these pharmaceuticals on reducing the permeability of the vascular wall, and therefore on more rapid absorption of papilloedema. This reduces compression of the capillaries in the optic nerve papilla, improves through-flow of blood and restores the function of the “survived” neurons. In 2008, Hayreh and Zimmerman [10] published a prospective study, in which they describe the influence of generally administered corticosteroids on the speed of absorption of edema of the optic nerve papilla. Upon the application of prednisolone orally within 2 weeks of the onset of NAION, papilloedema was absorbed within an average of 6.8 weeks, while in the group without treatment by prednisolone this was 8.2 weeks. The authors came to the conclusion that during the acute phase of NAION, systemically administered corticosteroids bring about a significant improvement of visual acuity and the visual field. On the other hand, Rebolleda et al. [21] in their study did not determine any significant difference in resulting CVA between patients treated with general corticosteroids and a control group. Al-Zubidi et al. [1] in their summary study came to the conclusion that the controversy in the use of corticosteroids for NAION has not yet been resolved. The use of vasodilation therapy administered generally in this diagnosis has a long tradition, either separately or in combination with corticosteroids. Steigerwald et al. [22] published case reports of two patients who were systemically administered corticosteroids in combination with intravenously applied prostaglandin E1 in order to improve visual acuity.
In our observation it was demonstrated that monotherapy with vasodilator preparations resulted in better resulting visual acuity than therapy reinforced by corticosteroids. However, this difference was not statistically significant. The results may also be influenced by the fact that combined therapy was administered to patients with a worse finding on the optic nerve papilla.
Our study is retrospective. We included in our evaluation patients who met the given criteria. A weakness of the study is therefore especially the variability in the interval between the onset of NAION and the administration of treatment, and the difference in the time between termination of the treatment and the final evaluation of the effect of therapy. In our patients we did not indicate decompression of the sheaths of the optic nerve in any case. This method may be indicated in cases of progressive form of NAION. A study conducted by Jirásková et al. [15] describes the results of this surgical method in specific ocular diagnoses, in which decompression was performed in 25 cases of progressive form of NAION. The authors observed visual acuity and changes on the perimeter. They demonstrated a significant improvement of visual acuity and the scope of the visual field after surgery. The most marked improvement was achieved when the operation was performed in a sufficiently timely manner. They also evaluated subjective patient satisfaction, which was positive in the majority of cases. The multicentric randomised clinical trial Ischemic Optic Neuropathy Decompression Trial (IONDT), which was conducted in the USA between 1992 and 1994, observed the safety and efficacy of decompression of the optic nerve in patients with NAION in comparison with a control group, which was left without treatment. In this study there was a high percentage of perioperative and postoperative complications such as pain and diplopia, and by contrast a high percentage of spontaneously improved visual functions in the control group of untreated patients [13]. However, doubts have been cast on the results of this study precisely due to the high percentage of complications in connection with the operations, which could have influenced the results. The selection of patients suitable for decompression is also contentious [16]. Dickersin et al. [6] came to a similar conclusion.
The incidence of risk factors of cardiovascular diseases, which are also risk factors for the occurrence of non-arteritic anterior ischaemic optic neuropathy, is high in the population of the Czech Republic. Hypertension ranks among the most common cardiovascular diseases. It has a high prevalence in the Czech Republic, specifically 60 % in men and 62 % in women aged between 25 and 64 years, with a manifest increase in higher age groups (in the decade from 55 to 64 years 72 % of men and 65 % of women have high blood pressure) [24]. The prevalence of type 2 diabetes mellitus is constantly increasing in the Czech Republic. At present almost 800 thousand patients (8 %) are registered in this country with this chronic metabolic disorder. Worldwide also it is possible to observe an increasing incidence of type 2 diabetes, not only in the adult population but also in adolescents. This is a consequence of decreasing physical activity combined with an incommensurately large energy intake [23]. Authors state the incidence of hypercholesterolaemia at 40 % [25], some studies state an even higher representation. According to statistics from the Ministry of Health of the Czech Republic, this concerns 70 to 80 % of the population. Since these disorders are not accompanied by painful symptoms, a large number of people (as many as 25 %) are entirely unaware of their pathology, and often do not discover it until it is manifested in the heart, or indeed the eyes.
Atkins et al. [3] state in their study that in an observed cohort of patients with NAION, 60 % had at least 1 vascular risk factor, 47 % had hypertension and 24 % had diabetes. In their study smoking was not confirmed as a risk factor for the occurrence of the pathology. The authors state further potential risk factors as sleep apnoea, general hypoperfusion, vasospasms, defective autoregulation, severe anaemia and nocturnal hypotension. They did not confirm any correlation with sex. Gaier et al. [8] in a summary article describe hypertension, diabetes, hyperlipidemia and smoking as vascular factors in the occurrence of NAION. They state hypertension in 39–88 % of patients with NAION, type 2 diabetes mellitus in 15–49 %, and hyperlipidaemia in 48 % of patients. They state smoking in 49 % of affected individuals. Similar results are presented also in an extensive study by Newman et al. [12].
In our cohort at least one risk factor was present in 96 % of patients, most frequently high blood pressure. All three observed pathologies were present in 36.4 % of patients. An interesting secondary finding was that patients with diabetes mellitus received combined treatment more often (61.1 %), therefore their baseline finding was worse.
Our study confirmed that the majority of people with anterior ischaemic optic neruopathy have one of the risk factors, either separately or in combination. Risk factors should therefore not be overlooked, and their thorough compensation should be ensured, in order to bring about a reduction of the incidence of cardiovascular diseases and therefore also non-arteritic anterior ischaemic optic neuropathy. In our study, however, we were not able to evaluate the degree of compensation of general pathologies.
The majority of foreign and domestic authors state a similar representation of these risk factors in patients with anterior ischaemic optic neuropathy [3,8,16,18], namely hypertension (39–88 %) diabetes (15–48 %) and hypercholesterolaemia (47 %). In our cohort we determined a similar incidence of these pathologies. The small differences between the data in the literature and our study may be explained by different lifestyle, a more responsible approach to health on the part of foreigners, but also possibly more rigorously applied healthcare in the Czech Republic, which detects these pathologies in their pre-clinical stage. Smokers were in a minority in our group, only 13.8 % of our patients were active smokers, while 20.7 % were former smokers, with a varying length of time since they had stopped smoking. The majority were non-smokers, with 60.3 % of patients never having smoked.
A whole range of other pathological conditions are stated as further potential risk factors for the occurrence of NAION – hypercoagulation states (hyperhomocysteinemia), collagenosis, nocturnal hypotension, obstructive sleep apnoea syndrome, treatment for erectile dysfunction, polyarteritis nodosa and others. For example, Palombi et al. [19] in their study focused on obstructive sleep apnoea as a risk factor for the occurrence of the disorder. They determined that 89 % of patients with NAION met the clinical criteria for sleep apnoea (the frequency of apnoea and hypopnoea per hour, and the length thereof was observed). Arda et al. [2] in their study also determined a similarly high number of incidences of sleep apnoea in patients with NAION, regardless of sex. By contrast, Cestari et al. [5] determined that obstructive sleep apnoea has no connection with the occurrence of NAION. Obstructive sleep apnoea is therefore more common in patients with anterior ischaemic optic neuropathy, but is not a direct risk factor in the occurrence of the pathology, but contributes to the pathology together with other risk factors. In our study we did not focus on these other potential risk factors.
Predisposing morphological risk factors for the occurrence of NAION include the appearance of optic nerve drusens and a small papilla with small or virtually disappeared excavation and a markedly visible nerve fibre layer (“crowded disc” or “disc at risk”) [9]. In our study, although the average size of the optic nerve papilla in patients with NAION was within the limits of the norm, we nevertheless verified small papilla in 25 % of eyes and optic nerve drusens in 1 patient. This morphological predisposition in combination with general risk factors may play an important role, especially for the risk of occurrence of NAION in the other eye.
In a number of risk patients from the cohort, NAION appeared in the other eye after an interval of several years, even despite intravenous vasodilation therapy and long-term antithrombotic medication. This fact confirms data in the literature, which evaluates vasodilation treatment only as adjuvant therapy, which is not capable of curing the pathology and preventing the occurrence of the disease in the second eye.
CONCLUSION
Anterior ischaemic optic neuropathy is a serious sight-deteriorating pathology, which significantly influences the life of the affected individual. It is the second most commonly occurring pathology of the optic nerve in patients aged over 50 years, after glaucoma [16]. To date it is not possible to eliminate the risk of occurrence of this pathology in the other eye or recurrence in the affected eye. Well known risk factors for the occurrence of NAION are hypertension, diabetes mellitus and hypercholesterolaemia, which were demonstrated to be highly represented also in our own observed cohort. With regard to the increasing prevalence of all these diseases, including at a lower age, we may expect the incidence of NAION also to increase. To date no causal medicamentous therapy exists which could cure NAION. We have only adjuvant therapy available, which acts systemically but has a disputable effect. Strict compensation for affiliated internal pathologies is recommended in treatment.
Sources
1. Al-Zubidi, N. et al.: Systemic corticosteroids in nonarteritic ischemic optic neuropathy. Indian J Ophthalmol, 62(10);2014 : 1022–1024.
2. Arda, H., Birer, S., Aksu, M. et al.: Obstructive sleep apnoea prevalence in nonarteritic anterior ischaemic optic neuropathy. Br J Ophthalmol,97;2013 : 206-209.
3. Atkins, EA., Beau, BB., Newman, NJ., Biousse, V.: Treatment of Nonarteritic Anterior Ischemic Optic Neuropathy, Surv Ophthalmol,55(1);2010 : 47–63.
4. Biousse, V., Newman, NJ.: Ischemic optic neuropathies. N Engl J Med,372;2015 : 2428–2436.
5. Cestari, DM., Gaier, ED., Bouzika P. et al.: Demographic, systemic and ocular factors associated with non-arteritic anterior ischemic optic neuropathy. Ophthalmology,123(12);2016 : 2446–2455.
6. Dickersin, K., Li, T.: Surgery for nonarteritic anterior ischemic optic neuropathy. Cochrane Database Syst Rev,12(3);2015: CD001538.
7. Foulds, WS.: Visual disturbances in systemic disorders: optic neuropathy and systemic disease. Trans Ophthalmol Soc UK,89; 1970 : 125–46.
8. Gaier, ED., Turon, N.: The enigma of nonarteritic anterior ischemic optic neuropathy, Curr Opin Ophthalmol,27(6);2016 : 498–504.
9. Hauptvogelová, M., Šustykevičová, Z.: Non-arteritická predná ischemická optická neuropatia pri drúzách zrakového nervu. Cesk Slov Oftalmol,66 (4);2010 : 184–187.
10. Hayreh, SS., Zimmerman, MB.: Non-arteritic anterior ischemic optic neuropathy: role of systemic corticosteroid therapy. Graefes Arch Clin Exp Ophthalmol,246(7);2008 : 1029–46.
11. Hayreh, SS.: Anterior ischemic optic neuropathy. III. Treatment, prophylaxis and differential diagnosis. Br J Ophthalmol,58(12); 1974 : 981–9.
12. Newman, JN. et al.: (Research group):Characteristics of patients with nonarteritic anterior ischemic optic neuropathy eligible for the Ischemic Optic Neuropathy Decompression Trial. Arch Ophthalmol,114;1996 : 1366–1374.
13. Kaufman, D. et al. (Research group): Ischemic optic neuropathy decompression trial: twenty-four-month update. Arch Ophthalmol,118;2000 : 793–798.
14. Jirásková,N., Studnička, J., Rozsíval, P.: Papiledém a ischemický edém terče optiku, Čas Lék Čes,151;2012 : 527–530.
15. Jirásková, N., Rozsíval, P.: Výsledky 62 dekompresí obalů zrakového nervu,Cesk Slov Oftalmol,3;1999 : 136–144.
16. Jirásková, N.: Neurooftalmologie, minimum pro praxi, Triton, 2001,26–30 s.
17. Kuchynka, P. et al.: Oční lékařství, Grada, 2007,262 s.
18. Kuchynka, P. et al.: Oční lékařství, Grada, 2007,513–514 s.
19. Palombi, K.,Renard, E., Levy, P. et al.: Nonarteritic anterior ischaemic optic neuropathy is nearly systematicaly associated with obstructive sleep apnoea. Br J Ophthalmol,90;2006 : 879–882.
20. Pazderová, M., Novák, J.: Změny v tloušťce nervových vláken u non-arteritické formy AION na OCT,Cesk Slov Oftalmol,65(3); 2009 : 87–90.
21. Rebolleda, G., Pérez-López, M., Casas-LLera, P.: Visual and anatomical outcomes of non-arteritic anterior ischemic optic neuropathy with high-dose systemic corticosteroids. Graefes Arch Clin Exp Ophthalmol,251;2013 : 255–60.
22. Steigerwalt, RD., Cesarone, MR., Belcaro, G.: Arteritic anterior ischemic optic neuropathy treated with intravenous prostaglandin E1 and steroids.Int J Angiol, 19(3);2010 : 113–115.
23. Špinar, J., Lábrová, R.: Dapagliflozin a studie DECLARE – budoucnost léčby diabetes mellitus, Kardiol Rev Int Med,18(2);2016 : 119–124.
24. Štejfa, M.: Progresivní kontinuum – hypertenze, ischemická choroba srdeční, srdeční selhání a náhrada srdeční funkce, Kardiol Rev Int Med,13(1);2011 : 7–8.
25. Žák, A. a kolektiv: Ateroskleróza: Nové pohledy, Grada, 2011,96 s.
Labels
Ophthalmology
Article was published inCzech and Slovak Ophthalmology

2020 Issue 2-
All articles in this issue
- Zemřela prof. MUDr. Jarmila Boguszaková DrSc.
- Vzpomínka na doc. MUDr. Cigánka, CSc.
- EPIGENETIC CHANGES IN MALIGNANT UVEAL MELANOMA AND POSSIBILITIES OF THEIR THERAPEUTIC TARGETING
- Primary Intrabulbar Neurofibroma
- Does adjuvant intracameral triamcinolone acetonide increase the effectiveness of phacotrabeculectomy? A Case-Control Study
- Non-arteritic anterior ischaemic optic neuropathy: treatment and risk factors
- Aflibercept for Vascularised Serous Pigment Epithelial Detachment: One-Year Anatomical and Functional Results
- Betaxolol, Brimonidin and Carteolol in the Therapy of Normal-Tension Glaucoma
- Czech and Slovak Ophthalmology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Non-arteritic anterior ischaemic optic neuropathy: treatment and risk factors
- Vzpomínka na doc. MUDr. Cigánka, CSc.
- Betaxolol, Brimonidin and Carteolol in the Therapy of Normal-Tension Glaucoma
- Aflibercept for Vascularised Serous Pigment Epithelial Detachment: One-Year Anatomical and Functional Results
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

