-
Medical journals
- Career
Enucleation after Stereotactic Radiosurgery in Patients with Uveal Melanoma
Authors: P. Zahorjanová 1; J. Sekáč 2; P. Babál 3; M. Štubňa 1
Authors‘ workplace: Očné oddelenie, FNsP Žilina, Primár: MUDr. Michal Štubňa, Ph. D. 1; Klinika Oftalmológie LF UK a UNB Ružinov, Prednosta: doc. MUDr. Vladimír Krásnik, Ph. D. 2; Ústav patologickej anatómie LF UK a UNB, Prednosta: prof. MUDr. Ľudovít Danihel, Ph. D. 3
Published in: Čes. a slov. Oftal., 76, 2020, No. 1, p. 46-51
Category: Original Article
doi: https://doi.org/10.31348/2020/6Overview
Introduction: In the past enucleation was the treatment of choice for all the patients with uveal melanoma. Nowadays, we prefer glope-sparing treatment modalities, except for large tumors, tumors with extrascleral extension and painful blind eyes. Most of the patients perform radiotherapy or local resection techniques. In Slovak Republic, the only one possibility is a stereotactic radiotherapy on a linear accelerator LINAC. Nevertheless, enucleation after radiotherapy is necessary for some patients. The causes are postradiation complications, mainly neovascular glaucoma, tumor recurrence, tumor progression or patient´s decision.
Material and methods: The retrospective non-randomised study of 168 eyes of the patients with choroidal or ciliary body melanoma, who performed one-day session stereotactic radiosurgery at the linear accelerator LINAC during period 2007-2016. The data about postradiation complications were analysed based on the medical records of the patients and the data about enucleated eyes based on the histopathological findings.
Results: The occurence of enucleation after radiotherapy in our cohort was 17 % (28 patients), with median time period after radiotherapy 21,5 months. The most common cause was neovascular glaucoma (82 %), then tumor progression (14 %) and patient´s decision (4 %). The most common histopathological finding was spindle-cell melanoma.
Discussion: Others authors describe similar enucleation rate and causes. The histopathological findings indentified more viable melanoma cells in eyes enucleated for tumor progression in comparison with enucleation for other causes. Enucleation may be more difficult and the occurence of postoperative complications can be higher in the eyes after radiotherapy rather than primary enucleation.
Conclusion: The management of the patients with uveal melanoma is difficult, and requires the cooperation of ophthalmologist, oncologist, radiation physicist and pathologist. Even if we make effort to preserve the eye, enucleation after radiotherapy is necessary in some patients.
Keywords:
uveal melanoma – enucleation – histopathological findings
INTRODUCTION
In the past, enucleation was the treatment of choice for practically all patients with malignant uveal melanoma, in which it was assumed that this was the only possibility of ensuring the survival of patients. A change took place after the large randomised multicentric Collaborative Ocular Melanoma Study, which was conducted in the USA in the period from 1987-1998. The patients in the study were divided on the basis of tumour size, in which those patients with a medium-sized melanoma were divided into two groups – one group had undergone brachytherapy, the second enucleation. It was demonstrated that there was no significant difference between the survival of these two groups of patients [1,3,4,6,8,14].
At present the trend in the treatment of uveal melanoma is radiotherapy. In other countries local resection of the tumour is also practised, sometimes in combination with radiotherapy. Enucleation as the primary treatment is chosen for patients with large tumours, with extrascleral spreading or with a dolorous blind eye [5,10,17,19]. In the Collaborative Ocular Melanoma Study, patients with large melanomas were randomised into two groups, in which the patients in one group had undergone external radiotherapy before enucleation, and the patients in the second group had not. After statistical evaluation, there was no difference in survival between these two groups, and as a result, on the basis of this study, neoadjuvant external radiotherapy is not recommended before enucleation [15]. However, on the basis of a non-randomised prospective trial by Kilic et al., which included 167 patients, the survival rate was better in those patients who had undergone external radiotherapy before enucleation than in patients who had undergone enucleation only [18].
Enucleated eyeballs are subsequently histopathically evaluated, in which the finding after primary enucleation is often differentiated from previously irradiated eyes. In each tumour the type of cells is examined, in which a distinction is made between spindle cell type A and B, epithelioid type, mixed type and necrotic type. Spindle cell type A typically occurs in choroidal nevi. The presence of spindle cells in the melanoma attests to a better prognosis. Epithelioid type morphologically resembles the cells of the epithelium. A finding of these cells attests to a worse prognosis, in which it is often associated also with other adverse prognostic factors such as monosomy of the 3rd chromosome. If spindle or epithelioid cells are present in various proportions, this concerns a mixed type of melanoma. The necrotic type contains places of necrosis, which may cause a secondary inflammatory reaction. Its prognosis is similar to the epithelioid type.
Other negative prognostic factors designated by histopathological examination include large nucleoli, a larger number of mitoses and certain types of extravascular matrix, such as the presence of kinks and meshes.
Further prognostic factors are determined by immunohistological examination. Microvascular density as evidence of more pronounced vascularisation is associated with a high risk of metastases, as well as a higher number of macrophages and lymphocytes in the sample under examination [16,20,25]. Among the antigens of cell proliferation attesting to a worse prognosis are PCNA (proliferating cell nuclear antigen) and Ki-67 antigen [24].
The causes of enucleation following irradiation include neovascular glaucoma, which is generally difficult to manage by treatment, and is etiopathogenically linked with ischemia of the anterior segment or retina. Further causes are recurrence or progression of the tumour even after irradiation, and personal decision of the patient due to fear of the disease [7,9,11,13,23].
Upon histopathological examination of eyes enucleated following radiotherapy, we generally find more necrotic areas, and the microvascular density is lower due to radiosensitivity of the blood vessels. Of the cells we find more epithelioid here, which is surprising, since epithelioid cells are considered more radiosensitive. In tumours after irradiation, melanoma cells are often present and viable. A typical finding is obliteration of the blood vessels and degenerative changes such as the presence of balloon cells [1].
COHORT AND METHOD
We retrospectively analysed a set of 168 eyes in patients with malignant melanoma of the choroidea or corpus ciliare, who had undergone stereotactic radiosurgery at the Oncological Institute of St. Elizabeth in Bratislava, in co-operation with the Department of Ophthalmology at the Faculty of Medicine of Comenius University and Ružinov University Hospital, in the period from 2007-2016. The observation period was 13-122 months. We examined the patients’ health documentation, where we obtained data about post-radiation complications, and we also reviewed the histopathological findings of the enucleated eyeballs. We have already published the results regarding maculopathy in this cohort in the journal Czech and Slovak Ophthalmology [26]. In this article we are focusing on the number and causes of enucleation, in which we are using descriptive statistics, and we are also focusing on a histopathological evaluation of the enucleated eyeballs, which we have processed in co-operation with a pathologist.
RESULTS
Our cohort comprised patients aged from 20 to 92 years, in which most were within the age group of 60-69 years (33 %). The cohort contained 85 women (51 %) and 83 men (49 %). 82 patients (49 %) had a tumour in the right eye, and 86 patients (51 %) in the left eye. In 21 patients (12.5 %) the tumour grew from the region of the corpus ciliare, in 147 patients (87.5 %) from the choroidea. Tumours localised pre-equatorially – thus growing from the corpus ciliare or from the pre-equatorial part of the choroidea – were found in 41 patients (24 %), and growing from the post-equatorial part of the choroidea in 127 patients (76 %). The average volume of the tumour before irradiation was 0.4402 cm3. The dose of irradiation to the area of the tumour was from 35.0 to 62.05 Gy, the median dose was 40.13 Gy.
During the course of the entire observation period, 28 patients in our cohort underwent enucleation (17 %). The most common cause was neovascular glaucoma, which occurred in 23 patients (82 %). In 4 eyes (14 %) enucleation was necessary due to progression of the local finding, in which in all patients this concerned large tumours with a volume of more than 1 cm3. In 1 patient (4 %), the reason for enucleation was the patient’s decision, due to fear of the disease. The time interval between irradiation and enucleation was from 1 to 99 months, on average 26 months, median 21.5 months.
The total incidence of secondary glaucoma (Fig. 1, Fig. 2) in our cohort was 49 patients (29 %). Of these, 12 had melanoma of the corpus ciliare (24 %) and 37 had melanoma of the choroidea (76 %). The treatment of glaucoma was medicamentous in all patients, and 12 patients underwent cyclocryocoagulation, which had a good effect in 4 patients (33 %).
1. Patient with post-radiation cataract and neovascular glaucoma 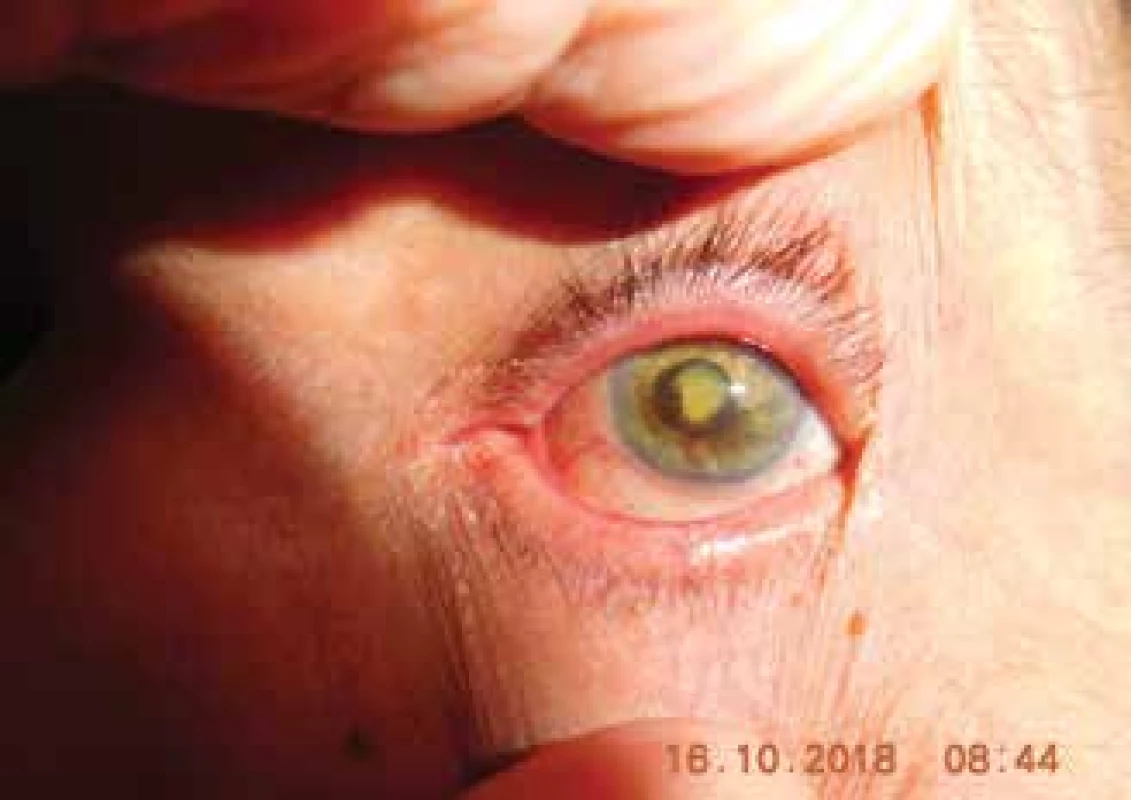
2. Patient with post-radiation optic neuropathy and maculopathy 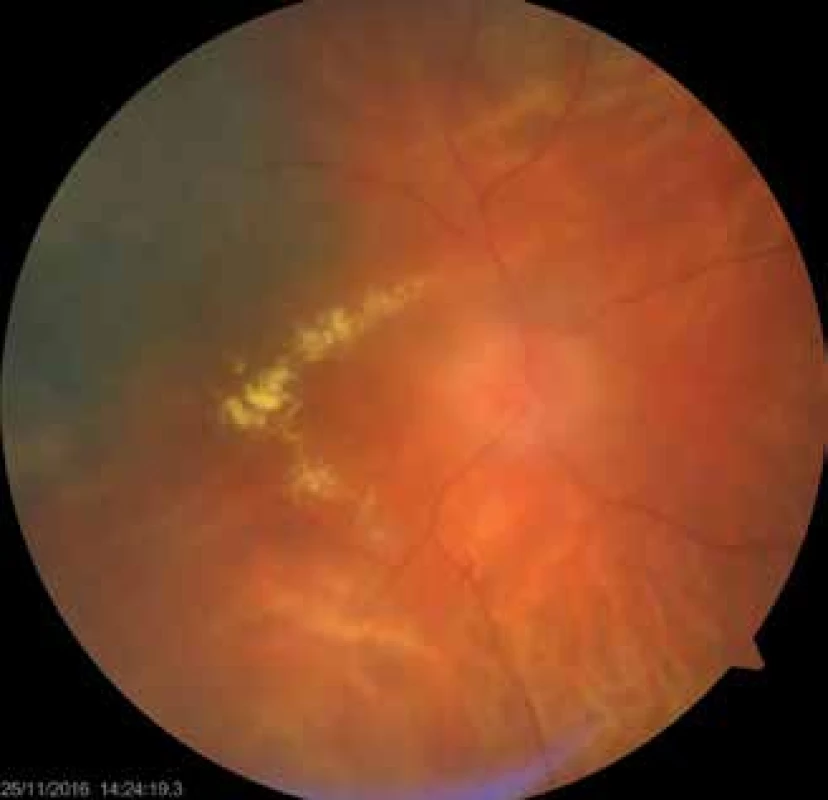
After enucleation, eyes with MUM were histopathologically evaluated. The most widely represented were tumours composed of spindle cells, of which there were 13 (46 %). Of these 4 were of spindle cell type A, 7 were of spindle cell type B, and 2 were mixed spindle cell type A+B. Epithelioid type was found in 5 tumours (18 %). Mixed spindle cell and epithelioid type was found in 5 tumours (18 %). In 5 patients (18 %) the histopathological type of tumour was unknown, most often because the patients underwent enucleation at a different centre (Table 1).
1. Overview of enucleated eyeaballs 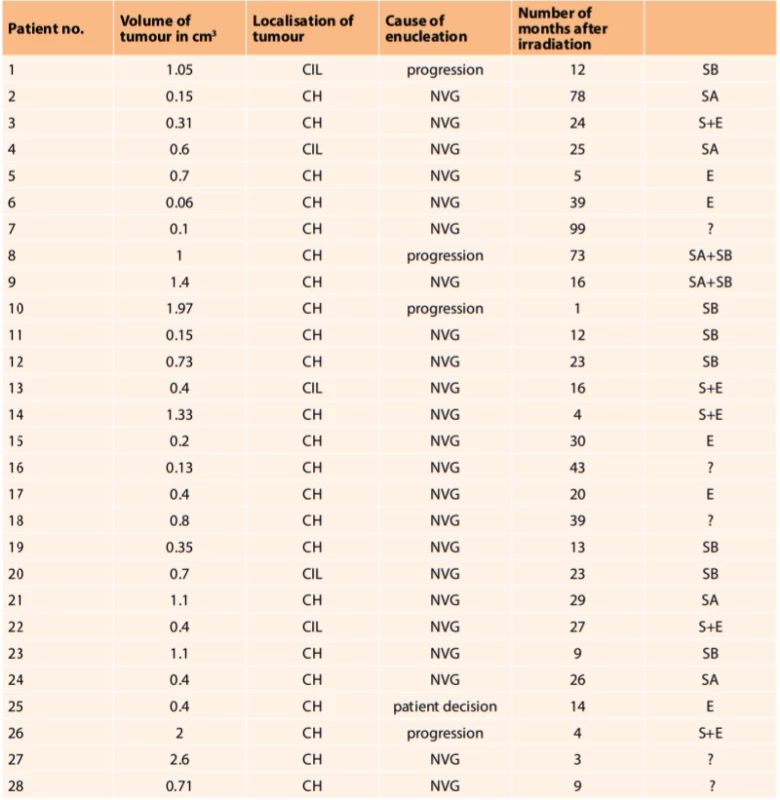
(notes: volume of tumour in cm3 – at time of diagnosis of disease, CH – tumours originating from choroidea, CIL – tumours originating from corpus ciliare, NVG – neovascular glaucoma, S – spindle cell type, SA – spindle cell type A, SB – spindle cell type B, E – epithelioid type, ? - unknown type) Fig. 3 and 4 show the histopathological finding of a recurring melanoma of the corpus ciliare in a female patient in our cohort, with infiltration of the lateral wall of the eyeball, spindle cell type B, haemotoxylin-eosin staining in 25x and 100x enlargement.
3. Recurring melanoma of the corpus ciliare, spindle cell type B, enlarged 25x 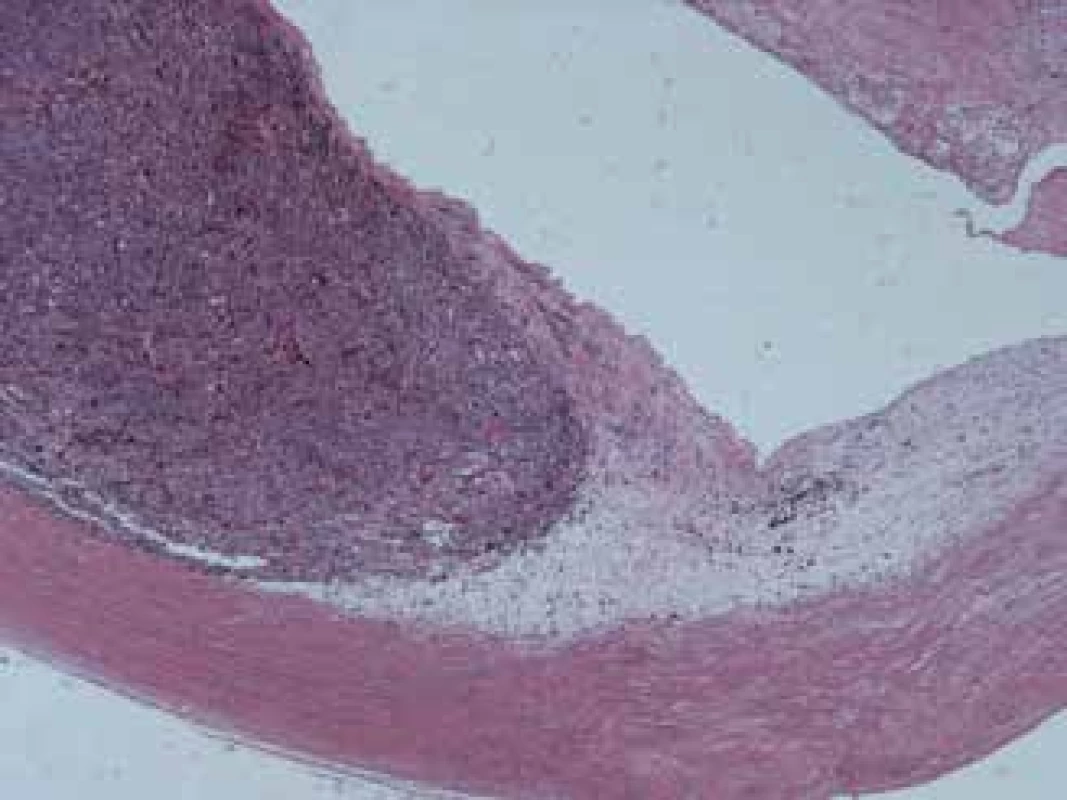
4. Same melanoma, enlarged 100x 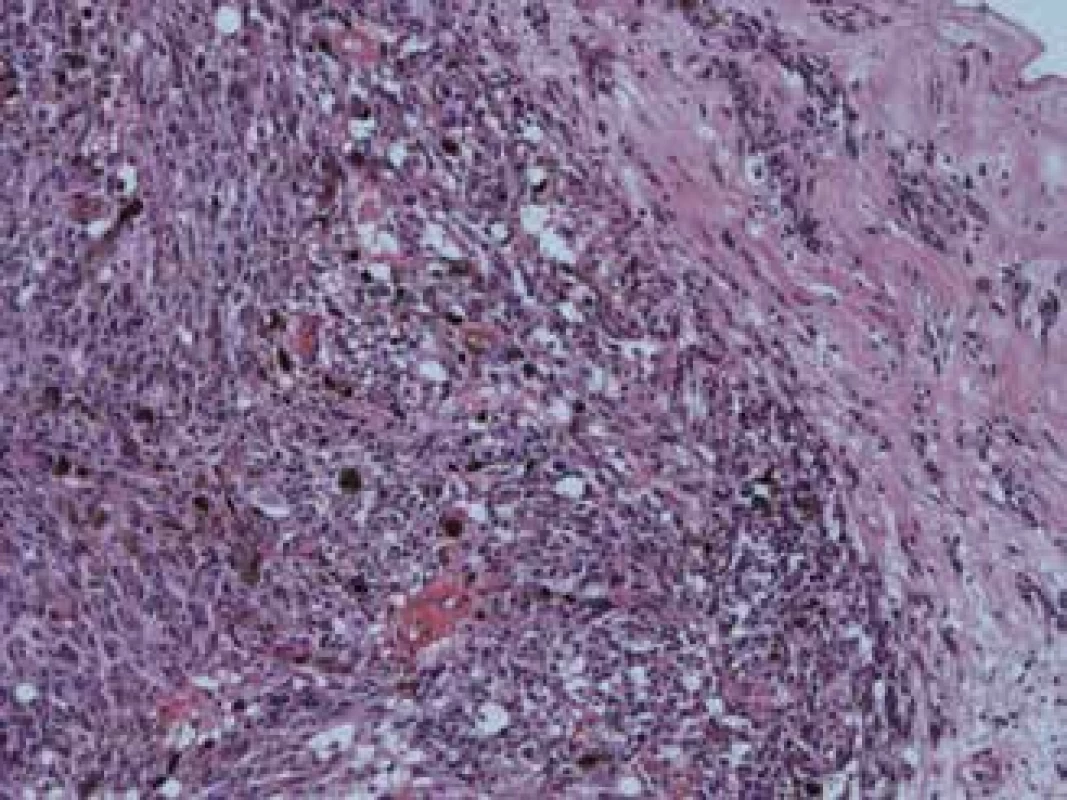
DISCUSSION
Authors from the London Ocular Oncology Service analysed enucleations in patients following radiotherapy in the period from 2008-2014. This concerned a cohort of 99 enucleated eyes, of which 21 were localised in the corpus ciliare (21 %), 31 were with juxtapapillary localisation (31 %), and 47 tumours were in other parts of the choroidea (48 %). The primary treatment used was brachytherapy with the use of Ru106 in 85 patients, proton therapy in 11 patients and transpupillary thermotherapy in 3 patients. The indications for secondary enucleation were: recurrence of tumour in 60 eyes (61 %), neovascular glaucoma in 21 eyes (21 %) and absence of response to treatment in 18 eyes (18 %). In 20 patients (20 %) metastases were diagnosed, in which 12 of them exited. On the basis of a multivariate analysis, juxtapapillary localisation of the tumour was demonstrated to be a risk factor for absence of response to treatment (p = 0.004), and also absence of response to treatment was associated with a higher risk of the development of metastases (p = 0.04). Juxtapapillary localised tumours were therefore demonstrated to be more radioresistant, and also involved a higher risk of the formation of metastases [2].
Furdová et al. analysed the number of secondary enucleations and survival in a cohort of 150 patients who underwent irradiation on a LINAC linear accelerator in the period from 2001-2015. Enucleation after irradiation was necessary in 20 patients (13.3 %), especially due to neovascular glaucoma. Survival of patients was 96 % after 1 year of treatment, 93 % after 2 years, 84 % after 5 years, 80 % after 7 years and 53 % after 11 years [12].
Seregard et al. examined the viability of posterior uveal melanomas following brachytherapy by ruthenium emitters on the basis of a histopathological evaluation of secondarily enucleated eyes. In a cohort of 266 patients, 46 were secondarily enucleated. The causes were: recurrence of tumour in 27 patients (64 %), adverse effects of irradiation in 12 (29 %) and patient’s personal decision in 3 cases (7 %). Of the 46 enucleated eyeballs, 42 were histopathologically evaluated. The median time interval from radiotherapy to enucleation was 23 months in the group with a recurring tumour, and 19 months in the patients without recurrence. Despite the fact that a certain degree of remission was found in all melanomas on the histopathological finding, only 5 were completely necrotic, and viable melanoma cells were found in the remaining 37 previously irradiated tumours (88 %). Cell proliferation was found in 17 out of 23 eyes (74 %) enucleated due to recurrence. This finding was similar as in eyes with melanoma which were primarily enucleated without prior radiotherapy. In contrast with this, cell proliferation was found in only 4 out of 13 tumours (31 %) without clinical recurrence, which attests to the fact that treatment by radiotherapy is effective [22].
Pham et al. analysed the perioperative course and postoperative results of primary enucleation and enucleation following brachytherapy for MUM. There was no difference between the group of primarily enucleated (n=54) and enucleated after brachytherapy (n=34) in terms of age, sex and laterality. Greater perioperative difficulties were experienced in the group enucleated after radiotherapy (28 out of 32 patients; 87.5 %) than in the group of primarily enucleated (1 out of 54 patients; 1.8 %), p < 0.0001. The duration of the operation was more than 2 hours in 3 out of 51 primarily enucleated (6 %) and in 8 out of 32 enucleated after radiotherapy (25%), p = 0.02. The average size of the implant was similar in both groups (20.6 mm), although 2 out of 34 enucleated after radiotherapy (6 %) required a graft of subcutaneous fat. Ptosis of the eyelid was present in 8 out of 49 primarily enucleated (16 %) and 13 out of 30 enucleated after radiotherapy (43 %), p = 0.02. Anophthalmos occurred in 0 primarily enucleated and 5 out of 30 enucleated after radiotherapy (17 %), p = 0.006. The conclusion of the study was that enucleation following brachytherapy was technically more demanding and had more complications than primary enucleation. It is a suitable therapeutic modality for patients who are contraindicated for radiotherapy [21].
CONCLUSION
At present, primary enucleation is chosen as a treatment for uveal melanoma only in the case of an advanced finding. If possible, we decide in favour of therapeutic methods that spare the eyeball, which include especially radiotherapy and also resection of the tumour. However, even after treatment of the melanoma by irradiation, some patients are unable to avoid enucleation. The causes are post-radiation complications such as secondary glaucoma, recurrence and progression of the growth of the tumour, and some patients decide in favour of enucleation themselves due to fear of the disease. The incidence of secondary enucleation in our cohort was 17 %, with a median time interval from irradiation to enucleation of 21.5 months. The most frequent cause was neovascular glaucoma, and the most common finding on the histopathological examination of the enucleated eyeballs was spindle cell type melanoma. Care for patients with uveal melanoma is demanding and requires the co-operation of an ophthalmologist, radiation oncologist and radiation physicist, as well as co-operation with a pathologist.
The authors of the study declare that no conflict of interest exists in the compilation, theme and subsequent publication of this professional communication, and that it is not supported by any pharmaceuticals company.
The authors further declare that the study has not been submitted to any other journal or printed elsewhere, with the exception of congress abstracts and recommended procedures.
Sources
1. Damato, B., Singh, A.D: Clinical Ophthalmic Oncology: Uveal Tumors. Berlin, Springer, 2014, 382 p.
2. Fabian, I.D., Tomkins-Netzer, O., Stoker, I., et al: Secondary Enucleations for Uveal Melanoma: A 7-Year Retrospective Analysis. Am J Ophthalmol, 160(6); 2015 : 1104-1110.
3. Furdová, A., Babál, P.: Giant Orbital Melanoma in a Heroin Abuser. Case Rep Ophthalmol, 8(1); 2017 : 288-293.
4. Furdová, A., Babál, P., Kobzová, D., et al.: Uveal melanoma suvrival rates after single dose stereotactic radiosurgery, 65(6); 2018 : 965-971.
5. Furdová, A., Horkovičová, K., Justusová, P., et al.: Is it sufficient to repeat linear accelerator stereotactic radiosurgery in choroidal melanoma? Bratisl Lek Listy, 117(8); 2016 : 456-462.
6. Furdová, A., Oláh, Z.: Nádory oka a okolitých štruktúr. Akademické nakladatelství CERM, 2010, 152 s.
7. Furdová, A., Růžička, J., Šramka, M., et al.: Choroidal melanoma stage T1 – comparison of the planning protocol for stereotactic radiosurgery and proton beam irradiation. Cesk Slov Oftalmol, 68(4); 2012, 156-161.
8. Furdová, A., Slezák, P., Chorváth, M., et al.: No differences in outcome between radical surgical treatment (enucleation) and stereotactic radiosurgery in patients with posterior uveal melanoma. Neoplasma, 57(4); 2010 : 377-381.
9. Furdová, A., Strmeň, P., Šramka, M.: Complications in patients with uveal melanoma after stereotactic radiosurgery and brachytherapy. Bratisl Lek Listy, 106(12); 2005 : 401-406.
10. Furdová, A., Strmeň, P., Waczulíková, I., et al.: One-day session LINAC-based radiosurgery of posterior uveal melanoma. European Journal of Ophthalmology, 22(2); 2012 : 226-235.
11. Furdová, A., Šramka, M.: Uveal malignant melanoma and stereotactic radiosurgery. Saarbrucken, LAP LAMBERT Academic Publishing, 2014, 181 s.
12. Furdová, A., Šramka, M., Chorváth, M., et al.: Clinical experience of stereotactic radiosurgery at a linear accelerator for intraocular melanoma. Melanoma Res, 27(5); 2017 : 463-468.
13. Furdová, A., Šramka, M., Chorváth, M., et al.: Stereotactic radiosurgery in intraocular malignant melanoma – retrospective study. Neuro Endocrinol Lett, 35(1); 2014 : 28-36.
14. Furdová, A., Waczulíková, I., Šramka, M., et al.: Relative survival rates and presence of complications in uveal melanoma patients after stereotactic radiosurgery. Adv Ophthalmol Vis Syst, 8(6); 2018 : 283-289.
15. Group TCOMS: The Collaborative Ocular Melanoma Study (COMS) randomized trial of large choroidal melanoma: IV. Ten-year mortality findings and prognostic factors. COMS report number 24. Am J Ophthalmol, 138(6); 2004 : 936-51.
16. Chen, X., Maniotis, A.J., Majumdar, D., et al.: Uveal melanoma cell staining for CD34 and assessment of tumor vascularity. Invest Ophthalmol Vis Sci, 43(8); 2002 : 2533-9.
17. Justusová, P., Štubňa, M., Veselovský, M., et al.: Exenterácia orbity u pacienta s generalizovaným choroidálnym melanómom. Cesk Slov Oftalmol, 72(3); 2016 : 92-96.
18. Kilic, E., Stijnen, T., de Jong, P.T., et al.: Reduced melanoma-related mortality in uveal melanoma by preenucleation radiotherapy. Arch Ophthalmol, 123(10); 2005 : 1363-7.
19. Krantz, B.A., Dave, N., Komatsubara, K.M., et al.: Uveal melanoma: epidemiology, etiology and treatment of primary disease. Clin Ophthalmol. 2017;11 : 279-289.
20. Mäkitie, T., Summanen, P., Tarkkanen, A., et al.: Tumor-infiltrating macrophages (CD68+ cells) and prognosis in malignant uveal melanoma. Invest Ophthalmol Vis Sci, 42(7); 2001 : 1414-21.
21. Pham, C.M., Custer, P.L., Couch, S.M.: Comparison of primary and secondary enucleation for uveal melanoma. Orbit, 36(6); 2017 : 422-7.
22. Seregard, S., Lundell, G., Lax, I., et al.: Tumour cell proliferation after failded ruthenium plaque radiotherapy for posterior uveal melanoma. Acta Ophthalmol Scand, 75; 1995 : 148-154.
23. Sekáč, J., Ferková, S.L., Kollárová, A., et al.: Secondary glaucoma in small versus large uveal melanoma patients treated with stereotactic radiosurgery on linear accelerator. Bratisl Med J, 120(12); 2019 : 945-949.
24. Singh, A.D., Kalyani, P., Topham, A.: Estimating the risk of malignant transformation of a choroidal nevus. Ophthalmology, 112(10); 2005 : 1784-9.
25. Smolková, B., Horváthová Kajabová, V., Zmetáková, I., et al.: Role of epigenetic deregulation in hematogenous dissemination of malignant uveal melanoma. Neoplasma, 65(6); 2018 : 840-854.
26. Zahorjanová, P., Furdová, A., Waczulíková, I., et al.: Postradiačná makulopatia u pacientov s malígnym melanómom corpus ciliare a chorioidey po stereotaktickej rádiochirurgii. Cesk Slov Oftalmol, 75(1); 2019 : 3-10.
Labels
Ophthalmology
Article was published inCzech and Slovak Ophthalmology
2020 Issue 1-
All articles in this issue
- Transscleral Extraction of an Intraocular Foreign Body from the Posterior Segment of the Eye without Pars Plana Vitrectomy
- Visual Training in Virtual Reality in Adult Patients with Anisometric Amblyopia
- Our experience with micropulse cyclophotocoagulation in the therapy of glaucoma
- Current use of the automatic retinal oximetry. Review
- Importance of PET/CT examination in patients with malignant uveal melanoma
- Enucleation after Stereotactic Radiosurgery in Patients with Uveal Melanoma
- Czech and Slovak Ophthalmology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Importance of PET/CT examination in patients with malignant uveal melanoma
- Our experience with micropulse cyclophotocoagulation in the therapy of glaucoma
- Visual Training in Virtual Reality in Adult Patients with Anisometric Amblyopia
- Transscleral Extraction of an Intraocular Foreign Body from the Posterior Segment of the Eye without Pars Plana Vitrectomy
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

