-
Medical journals
- Career
Calcifying Pseudoneoplasms of the Neural Axis. Report of Three Cases
Authors: A. M. Rulseh 1,2; J. Keller 1,3; J. Klener 4; J. Šroubek 4; Martin Syrůček 5; I. Zemanová 5; V. Dbalý 4; J. Vymazal 1,6
Authors‘ workplace: Department of Radiology, Na Homolce Hospital, Prague, Czech Republic 1; Department of Radiology, First Faculty of Medicine, Charles University, Prague, Czech Republic 2; Department of Neurology, Third Faculty of Medicine, Charles University, Prague, Czech Republic 3; Department of Neurosurgery, Na Homolce Hospital, Prague, Czech Republic 4; Department of Pathology, Na Homolce Hospital, Prague, Czech Republic 5; Department of Neurology, First Faculty of Medicine, Charles University, Prague, Czech Republic 6
Published in: Cesk Slov Neurol N 2011; 74/107(5): 584-589
Category: Case Report
Overview
Calcifying pseudoneoplasms of the neural axis (CAPNONA) are rare lesions that have been reported in both intra/extra-axial locations intracranially, and in extra-axial locations in the spinal region. Central loss of signal on T1/T2-weighted images with enhancement following contrast administration is most commonly observed on magnetic resonance imaging, however, the histopathological composition of CAPNONA is diverse which may be reflected in the imaging findings. We present three cases of CAPNONA detected by MRI and confirmed by histological evaluation, and the largest review of the MRI characteristics of these lesions to date.
Key words:
calcifying pseudoneoplasm – CAPNONA – fibro-osseous lesion – calcificationIntroduction
Calcifying pseudoneoplasms of the neural axis (CAPNONA), or fibro-osseous lesions as they are alternatively known, are rare, tumor-like lesions that have been reported to occur in both intra-axial (IA) and extra-axial (EA) locations intracranially, and in EA locations in the spinal region. Due to their rarity, CAPNONA are seldom considered in the differential diagnosis of atypical brain or spinal lesions. These lesions carry an excellent prognosis and are generally curable by surgical resection. We present three cases of CAPNONA detected with magnetic resonance imaging (MRI) and confirmed by histological evaluation.
Case Reports
Case 1
A 46-year-old male with a three-year history of headache suffered a skull fracture at the age of eleven in the right parietal region following a blow to the head with a sharp metal object. Current neurological examination did not reveal any abnormalities. MRI at 1.5T showed a wedge-shaped lesion in the right angular region with increased signal intensity and central hypointense areas on proton-density - (PD) and T2-weighted images (Fig. 1a). The wedge-shaped region had significantly decreased signal intensity on T1-weighted images, suggestive of a pseudocystic lesion. Internal nodular enhancement was observed after contrast agent administration (Fig. 1b). Due to the ambiguous character of the clinically symptomatic lesion, microsurgery with navigation was recommended. During the surgery a bony fragment approximately 8 × 10 × 20 mm was observed as the plate of the skull was lifted off. The fragment was adherent to and extended 20 mm into the brain parenchyma. Gross and intraoperative microscopic inspection of the MRI-enhancing nodule revealed a fibrous stroma filled with numerous small calcifications. Histological examination showed a chondromyxoid matrix in a nodular pattern, calcification and osseous metaplasia, scattered psammoma bodies, palisading spindle cells, foreign-body-like reaction with giant cells and a fibrous stroma (Fig. 2). The postoperative course of the patient was uneventful. The patient reported the cessation of his headaches and MRI six months after surgery demonstrated total removal of the lesion (Fig. 1c).
Fig. 1a. T2-weighted TSE sequence. A hypointense nodule is visible within a wedge-shaped lesion in the angular region. Fig. 1b. Post-contrast T1-weighted SE sequence shows an area of nodular enhancement attached to a hypointense lesion. Fig. 1c. Post-contrast T1-weighted SE sequence six months after resection of the lesion. Minimal enhancement was detected in the resected region. 
Fig. 2. Calcifying pseudoneoplasm of the neural axis: fibrillar tissue, amorphous stroma with partial calcification. 
Case 2
A 43-year-old female presented with recurrent pain in the lumbar region with radiation to both legs along the dorsal aspect. She had undergone surgery of the lumbar spine two years previously for isthmic spondylolisthesis and a herniated disc at L5/S1. MRI at 1.5T showed a small nodular extradural lesion at the level of L3. The lesion was hypointense on T1-, T2 - and PD-weighted images (Fig. 3). No contrast agent was administered. During the microsurgery a small, calcified nodule approximately 5 mm in diameter was removed. The nodule was attached to one of the nerve roots accompanied by small tortuous vessels supplying a vascularized fibrous stroma. Histological examination revealed a calcified lesion consisting of primitive bone trabeculae and islets of choroid tissue in a moderately cellular matrix, scattered psammoma bodies and a fibrous stroma. The pathologist confirmed the diagnosis of CAPNONA. The postoperative course of the patient was uneventful. MRI ten months after the surgery showed no evidence of recurrence.
Fig. 3a. T1-weighted TSE sequence showing a small hypointense nodule at the level of L3 (arrow). Fig. 3b. T2-weighted TSE sequence showing the same hypointense nodule (arrow). 
Case 3
A 23-year-old male was referred due to refractory epilepsy. At ten years of age he suffered an episode of serous meningitis and developed epilepsy two years later. At the age of fifteen a pial AVM in the left frontal region was diagnosed and resected. Following the surgery the patient has been seizure-free for four years, after which time the seizures recurred. CT and MRI examination revealed a calcified lesion in the post-resection area with surrounding gliosis (Fig. 4). MRI was acquired at 1.5T and revealed a hypointense lesion on T1-, T2 - and PD-weighted images. No contrast agent was administered. During the surgery a large area of gliosis was identified and resected. The resected area included calcified tissue measuring approximately 7 × 6 × 2 mm, in contact with the frontal horn of the left lateral ventricle. Histological examination of the calcified tissue showed palisading spindle to epithelioid cells, calcification and osseous metaplasia with primarily adipose bone marrow, and a fibrous stroma. The pathologist confirmed the diagnosis of CAPNONA. The patient had two seizures during the first week following the surgery, and since that time (11/2009) is seizure-free.
Fig. 4a. T1-weighted MP-RAGE sequence. A large post-surgical defect may be seen in the left frontal region. Fig. 4b. T2*-weighted FLASH sequence reveals a small hypointense lesion within the defect. Fig. 4c. CT image acquired one day later, following application of subdural strip and grid electrodes. A calcified (500 HU) lesion in the left frontal region is visible. Strip and grid electrodes are visible on the left, and pneumocephalus may be seen anteriorly. 
Discussion
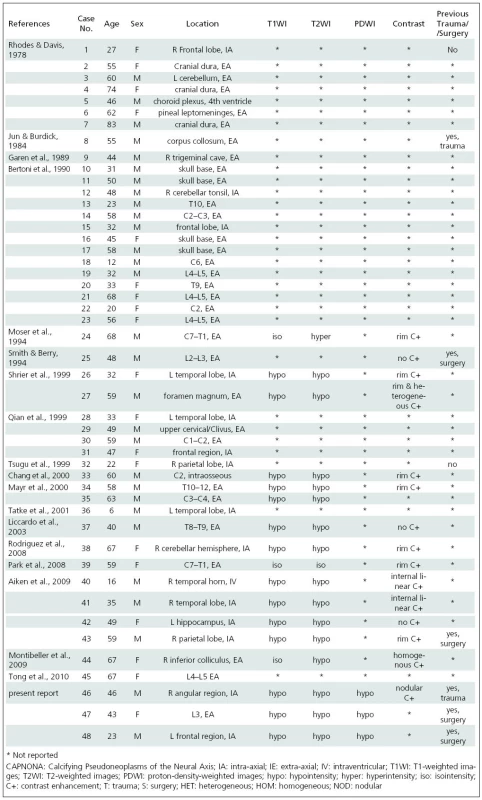
First described by Rhodes and Davis in 1978 [1], only 45 cases of CAPNONA have been reported [1–18]. CAPNONA have been reported more in males than females (1.5 : 1) and in ages ranging from 6–83 years (27 male, 18 female; mean age at diagnosis 47.4 years, SD 17 years) (Table 1).
The histopathological characteristics of CAPNONA are diverse. Aiken et al. [2] summarized the typical findings as the variable appearance of 1. a typical chondromyxoid matrix in a nodular pattern; 2. palisading spindle to epithelioid cells; 3. variable amounts of fibrous stroma; 4. calcification, osseous metaplasia, and scattered psammoma bodies; 5. and foreign-body reaction with giant cells. Only one of our cases demonstrated all five findings. Calcification, osseous metaplasia, scattered psammoma bodies, and a variable amount of fibrous stroma were seen in all three of our cases.
MRI findings in CAPNONA have been previously reported [2,4,7–15] and may be summarized as 1. central low signal intensity on T1/T2/PD-weighted images consistent with calcification, and 2. variable post-contrast enhancement, most often seen as peripheral rim enhancement. There may be a single focus or multiple foci of low signal intensity reflecting the heterogeneous nature of calcification reported in these lesions.
Some exceptions to these typical findings have been reported. Moser et al. [10] reported central T1 isointensity with a margin of slightly increased signal, and central T2 hyperintensity. At surgery the lesion was found to be fluid-filled. Park et al. [11] reported central isointensity on T1/T2-weighted images; the pathology report indicated that the mass was semi-solid and contained only scattered calcification. Montibeller et al. [9] also presented a case with central T1 isointensity; the lesion was shown to be of low cellularity and only partially calcified. All three of the cases we present showed central hypointensity in all imaging sequences.
When a contrast agent has been administered, peripheral rim enhancement is most often reported and reflects the presence of a well-developed fibro-vascular stroma [2,8,11,13,14]. Internal enhancement has been reported to occur as homogeneous [9], heterogeneous [14], or linear [2], and lesions showing no enhancement have been reported as well [2,7,15]. A contrast agent was only administered in one of our patients and showed a nodular pattern of internal enhancement.
The differential diagnosis of calcified intra - and extra-axial lesions is broad, but can be significantly narrowed by reviewing the medical history of the patient and by careful observation of the location and imaging characteristics of the lesion. To the best of our knowledge, CAPNONA have never been reported to occur within the spinal cord. It would appear from the limited amount of data available that CAPNONA occur in three spaces (IA-Intracranial, EA-Intracranial, EA-Spinal), and within each of these three spaces with approximately equal frequency (Table 1).
Primary neoplasms such as oligodendrogliomas, choroid plexus papillomas, ependymomas, astrocytomas, meningiomas, and craniopharyngiomas may all present as calcified intracranial masses [19] and should be included in the differential diagnosis. Other calcifying intracranial processes may also be considered such as chronic hematomas, vascular malformations, and tuberculoma. In the spine, the differential diagnosis of a calcified mass should include herniation of calcified disc material, calcified synovial cyst and psammomatous meningioma, followed by less frequently calcifying processes such as epidural abscess, chronic hematoma and tuberculoma.
CAPNONA are often considered to be a reactive, metaplastic response to injury [1,3,12,13,16]. A history of trauma or surgery was only reported in five cases previously, being positive in three [2,6,15] and negative in two [1,18]. All three of our patients had a history of previous trauma (case 1) or surgery (cases 2 & 3), and in two of these cases this directly impacted the region where CAPNONA later manifested. In one of our cases the previous surgery was at the level of L5/S1 and CAPNONA later manifested at the level of L3. Thus, our cases lend further support to a reactive hypothesis.
In conclusion, CAPNONA are rare, benign lesions that have been reported in IA and EA locations intracranially and in EA locations in the spinal region. The histopathological composition of these lesions is diverse, which may be reflected in the imaging findings. Finally, considering the history of trauma or surgery in the cases presented here and in those reported previously in the literature, it would appear that in at least some cases CAPNONA are a reactive, metaplastic response to injury.
Support
This work was supported by grant NS 9654-4 from the Ministry of Health of the Czech Republic and by Ministry of Education Research Programs MSM 0021620849 and MSM 0021620816.
Prof. Josef Vymazal, M.D., D.Sc.
Na Homolce Hospital
Roentgenova 2
15030 Prague 5
e-mail: Josef.Vymazal@homolka.czAccepted for review: 31. 12. 2010
Accepted for print: 4. 4. 2011
Sources
1. Rhodes RH, Davis RL. An unusual fibro-osseous component in intracranial lesions. Hum Pathol 1978; 9(3): 309–319.
2. Aiken AH, Akgun H, Tihan T, Barbaro N, Glastonbury C. Calcifying pseudoneoplasms of the neuraxis: CT, MR imaging, and histologic features. AJNR Am J Neuroradiol 2009; 30(6): 1256–1260.
3. Bertoni F, Unni KK, Dahlin DC, Beabout JW, Onofrio BM. Calcifying pseudoneoplasms of the neural axis. J. Neurosurg 1990; 72(1): 42–48.
4. Chang H, Park JB, Kim KW. Intraosseous calcifying pseudotumor of the axis: a case report. Spine 2000; 25(8): 1036–1039.
5. Garen PD, Powers JM, King JS, Perot PL jr. Intracranial fibro-osseous lesion. Case report. J Neurosurg 1989; 70(3): 475–477.
6. Jun C, Burdick B. An unusual fibro-osseous lesion of the brain. Case report. J Neurosurg 1984; 60(6): 1308–1311.
7. Liccardo G, Lunardi P, Menniti A, Floris R, Pastore FS, Fraioli B. Calcifying pseudo-tumor of the spine: description of a case and review of the literature. Eur Spine J 2003; 12(5): 548–551.
8. Mayr MT, Hunter S, Erwood SC, Haid RW Jr. Calcifying pseudoneoplasms of the spine with myelopathy. Report of two cases. J Neurosurg 2000; 93 (Suppl 2): 291–293.
9. Montibeller GR, Stan A, Krauss JK, Nakamura M. Calcifying pseudoneoplasm of the inferior colliculus: an unusual location for a rare tumor: case report. Neurosurgery 2009; 65(5): E1005–E1006.
10. Moser FG, Tourje EJ, Pressman BD, Blinderman EE. Calcifying pseudotumor of the cervical spine. AJNR Am J Neuroradiol 1994; 15(3): 580.
11. Park P, Schmidt LA, Shah GV, Tran NK, Gandhi D, La Marca F. Calcifying pseudoneoplasm of the spine. Clin Neurol Neurosurg 2008; 110(4): 392–395.
12. Qian J, Rubio A, Powers JM, Rosenblum MK, Pilcher WH, Shrier DA et al. Fibro-osseous lesions of the central nervous system: report of four cases and literature review. Am J Surg Pathol 1999; 23(10): 1270–1275.
13. Rodriguez FJ, Scheithauer BW, Fourney DR, Robinson CA. Ependymoma and intraparenchymal calcifying pseudoneoplasm of the neural axis: incidental collision or unique reactive phenomenon? Acta Neuropathol 2008; 115(3): 363–366.
14. Shrier DA, Melville D, Millet D, Qian J, Millet D, Nelson C et al. Fibro-osseous lesions involving the brain: MRI. Neuroradiology 1999; 41(1): 18–21.
15. Smith DM, Berry AD 3rd. Unusual fibro-osseous lesion of the spinal cord with positive staining for glial fibrillary acidic protein and radiological progression: a case report. Hum Pathol 1994; 25(8): 835–838.
16. Tatke M, Singh AK, Gupta V. Calcifying pseudoneoplasm of the CNS. Br J Neurosurg 2001; 15(6): 521–523.
17. Tong D, Karunaratne N, Howe G, Spencer D, Manolios N. Clinical images: Calcifying pseudoneoplasm of the neuraxis. Arthritis Rheum 2010; 62(3): 704.
18. Tsugu H, Fukushima T, Takeno Y. Calcifying pseudotumor of the neural axis--case report. Neurol Med Chir (Tokyo) 1999; 39(11): 762–765.
19. Martin F jr, Lemmen LJ. Calcification in intracranial neoplasms. Am J Pathol 1952; 28(6): 1107–1131.
Labels
Paediatric neurology Neurosurgery Neurology
Article was published inCzech and Slovak Neurology and Neurosurgery

2011 Issue 5-
All articles in this issue
- Developmental Coordination Disorder – Developmental Dyspraxia
- Awake Resection of Adult Supratentorial Low-grade Gliomas Located within or Adjacent to Eloquent Areas
- Cognitive Evoked Potentials
- Early-Onset Hereditary Alzheimer’s Disease Caused by p.M139V Mutation in the PSEN1 Gene – a Case ReportAlzheimerova demence je nejčastější demence u pacientů ve starším věku. V některých rodinách může být geneticky podmíněna. Naše kazuistika ukazuje případ 43letého muže, v jehož rodině se vyskytlo dalších šest členů rodiny s manifestací demence ve věku 40–50 let. Genetickým vyšetřením byla u pacienta prokázána patogenní mutace c.415A>G (p.M139V) v exonu 5 genu PSEN1 v heterozygotním stavu. Stejná mutace byla zjištěna u demencí postiženého bratrance. V rodině tak byla potvrzena hereditární predispozice k časné formě Alzheimerovy demence s autozomálně dominantní dědičností na molekulární úrovni. Vývoj onemocnění byl u pacienta sledován po dobu osmi let. Postupně dochází k deterioraci kognitivních funkcí a vývoji atrofických změn mozku dle magnetické rezonance. Obdobné změny jsou pozorovány u jeho bratrance. Genetické vyšetřování v rodinách zasažených demencí může být do budoucna důležité především pro možnost včasné léčby pacientů v riziku.
- Bilateral Ischemic Retinopathy and Optic Neuropathy as an Isolated Ophthalmic Clinical Entity in Altitude Sickness – a Case Report
- Progressing Spasticity, Cognitive Deficit and Non-elicitable Cortical Motor Evoked Potentials as Signs of Probable Primary Lateral Sclerosis – a Case Report
- Quality of Life after Deep Brain Stimulation in Patients with Advanced Parkinson’s Disease
- The Prevention of Venous Thrombosis and Pulmonary Embolism in Neurosurgery
- Experience with a Burr-hole Craniostomy for Chronic Subdural Hematoma
- Cognitive Deficit in Patients with Clinical Isolated Syndrome and Multiple Sclerosis
- Aggregometry in Secondary Prevention of Stroke. Aspirin Resistance
- Eye Movement Examination in Neurological Practice
- Calcifying Pseudoneoplasms of the Neural Axis. Report of Three Cases
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Developmental Coordination Disorder – Developmental Dyspraxia
- Cognitive Evoked Potentials
- Progressing Spasticity, Cognitive Deficit and Non-elicitable Cortical Motor Evoked Potentials as Signs of Probable Primary Lateral Sclerosis – a Case Report
- Experience with a Burr-hole Craniostomy for Chronic Subdural Hematoma
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career