-
Medical journals
- Career
Expression Analysis of the Long Non-Coding RNA LINC01433 in Lung Cancer
Authors: Z. Niazi 1 1,2; E. Garazhian 1,2; F. Esfandi 3; Z. M. Hassani 3,4; M. Taheri 5; S. Ghafouri-Fard 6
Authors‘ workplace: Department of Basic Medical Sciences, Neyshabur University of Medical Sciences, Neyshabur, Iran 1; Neyshabur Endemic Diseases Research Center, Neyshabur University of Medical Sciences, Neyshabur, Iran 2; Geniran Lab, Tehran, Iran 3; Department of Cell and Molecular Biology, Faculty of Life Sciences, Kharazmi University, Tehran, Iran 4; Urogenital Stem Cell Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran 5; Department of Medical Genetics, Shahid Beheshti University of Medical Sciences, Tehran, Iran 6
Published in: Klin Onkol 2019; 32(6): 453-455
Category: Original Articles
doi: https://doi.org/10.14735/amko2019453Overview
Background: Lung cancer is one of the most fatal human cancers both in males and females. This type of cancer is categorized to different subtypes among them is non-small cell lung cancer (NSCLC). NSCLC accounts for about 80% of all cases. Long non-coding RNAs (lncRNAs) have been shown to influence the pathogenic course of lung cancer. However, the contribution of LINC01433 lncRNA in this type of cancer in Iranian patients is not clear.
Purpose: In the current project, we evaluated expression of LINC01433 in 42 NSCLC samples and their paired non-tumoral tissues using quantitative real time polymerase chain reaction method. Samples were collected from patients admitted to Labbafinejad Hospital during 2016–2017.
Results: There was no significant difference in the expression of LINC01433 between tumoral and non-tumoral tissues (expression ratio 0.67, p = 0.42). Expression of this lncRNA was not associated with any of clinical and demographic data including age, gender, smoking history, stage or cancer subtype.
Conclusion: Based on the similar expression levels of this lncRNA between tumoral and non-tumoral tissues and lack of association between expression levels and clinical data, this lncRNA is not a possible contributor to lung cancer in Iranian patients. However, expression analysis of this lncRNA in larger sample sizes is needed to verify our results.
Keywords:
lung cancer – expression – Long non-coding RNA
Introduction
Lung cancer is one of the most fatal human cancers accounting for the first cause of cancer-related mortality in male and the second one in female patients [1]. This type of cancer is categorized based on the histological features to non-small cell lung cancer (NSCLC) and small cell lung cancer. The major types of NSCLC are adenocarcinoma, squamous cell carcinoma and large cell carcinoma [2]. Based on the close relationship between cancer stage and overall survival [3], biomarkers for early diagnosis of lung cancer are needed. Expression profiling of tumoral tissues vs. non-tumoral tissues is expected to facilitate identification of such biomarkers. Long non-coding RNAs (lncRNAs) include a major part of human gene transcripts. Their participations in various cellular processes, for instance proliferation, metastasis and stem cell renewal, has potentiated them as diagnostic biomarkers in NSCLC [4]. We have recently reported dysregulation of some lncRNAs in NSCLC tissues of Iranian patients [5,6]. A previous study, which aimed at identification of lung-cancer-related lncRNAs, has detected alterations in lncRNA profile in the human bronchial epithelial cell line after transformation of cells by anti-benzo (a) pyrene-7,8-diol-9,10-epoxide. Among the altered lncRNAs was the long intergenic non-protein coding RNA 1433 (LINC01433 or alternatively named LOC728228). This lncRNA has been up-regulated in transformed cells compared with control untransformed cells [7]. Its silencing has suppressed cell proliferation, induced cell-cycle arrest, inhibited cellular migration and repressed tumorigenic potential both in vitro and in vivo. Consequently, author suggested this lncRNA as an oncogenic lncRNA in lung cancer [7]. A recent study has reported over-expression of LINC01433 in NSCLC samples compared with the normal non-tumoral tissues. Their in vitro experiments also verified the results of the previous study regarding the oncogenic role of this lncRNA [8]. Based on these studies, we aimed to confirm these results in Iranian NSCLC patients. Therefore, we designed the current investigation to compare expression of LINC01433 between tumoral and non-tumoral tissues from NSCLC patients.
Materials and Methods
Patients
Forty-two patients with NSCLC have been enrolled in the current study. Patients were referred to the Labbafinejad Hospital during 2016–2017. Tumoral and non-tumoral samples were gathered prior to radio/chemotherapy from each person. Informed consent forms were obtained from all study participants. The study protocol was approved by the local ethical committee.
Expression assays
Total RNA was isolated from tumoral and non-tumoral tissues using the TRIzol™ material (Invitrogen, Carlsbad, CA, USA). Next, the quantity and quality of the extracted RNA was appraised using NanoDrop equipment (Thermo Scientific, USA). The ratio of absorbance at 260 nm and 280 nm was measured. The ratios between 1.8–2.0 were regarded as acceptable for further steps. The High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) was used for complementary DNA (cDNA) production. Approximately 500 ng – 1μg of RNA was reverse transcribed. Then, 20–40 ng of the synthesized cDNA was used for each reaction. Transcript levels of LINC01433 were compared between tumoral and non-tumoral tissues in Real-Time PCR System. B2M was used as the endogenous control. Each run had a no template control (comprised of all polymerase chain reaction reagents and primers but no cDNA template) used as negative control. Moreover, a positive control was included in each run. All reactions were performed in duplicate. The nucleotide sequences of primers are shown in Tab. 1.
1. The sequences of primers used for assessment of LINC01433 levels. 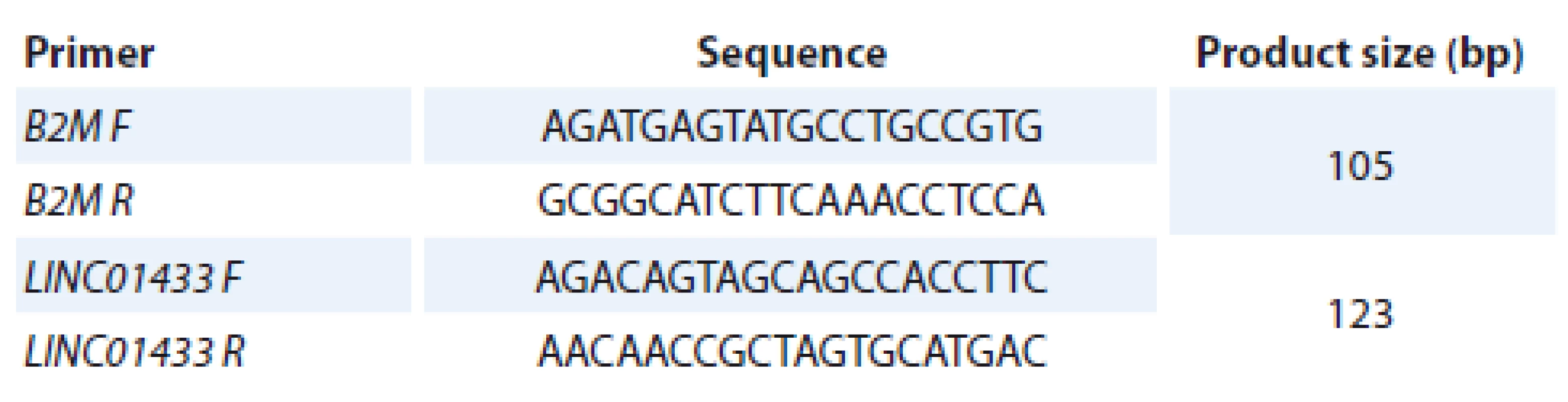
Statistical analysis
Transcript levels of LINC01433 were compared between tumoral and non-tumoral considering the efficiency values. The association between LINC01433 expression and clinicipathologic data was appraised using Chi-square test. Statistical analyses were executed in SPSS Statistics version 18 (Chicago, IL, USA).
Results
General data of patients
General information of patients is summarized in Tab. 2.
2. General information of patients. 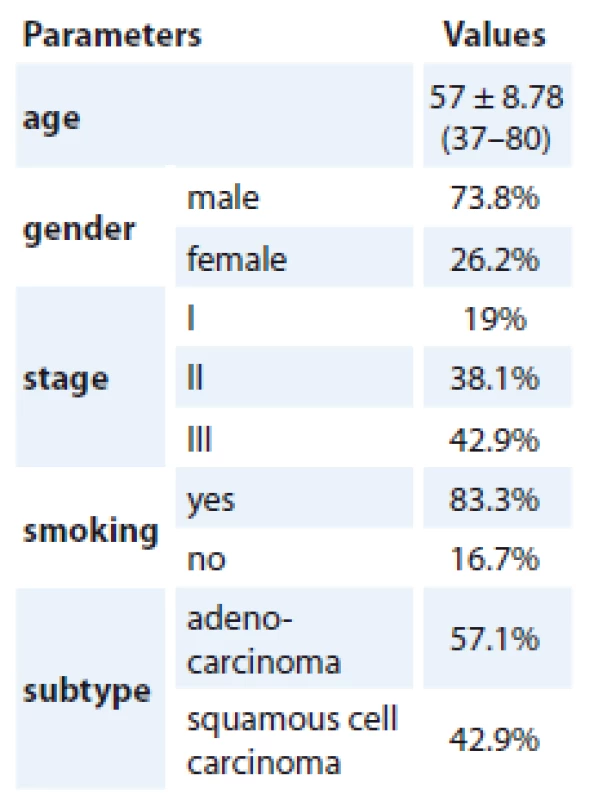
Expression assay
There was no significant difference in the expression of LINC01433 between tumoral and non-tumoral tissues (expression ratio 0.67, p = 0.42). Expression of this lncRNA was not associated with any of clinical and demographic data including age, gender, smoking history, stage or cancer subtype. Tab. 3 shows the results of association analysis between expression levels of LINC01433 and patients’ characteristics.
3. The results of association analysis between expression levels of LINC01433 and patients’ characteristics. 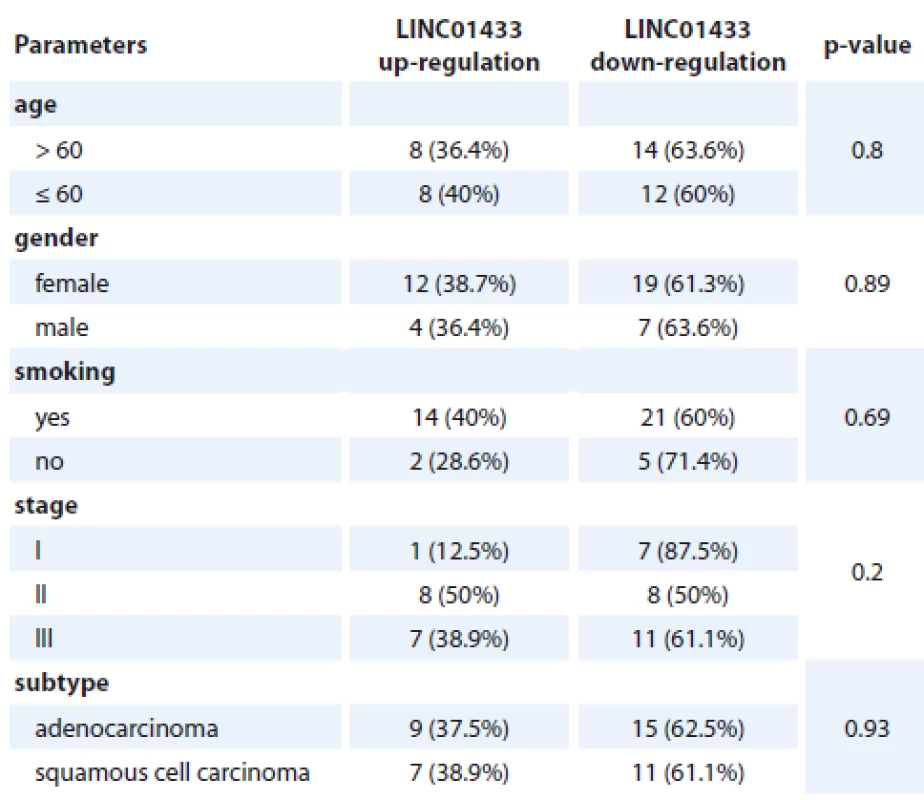
Discussion
LncRNAs have been regarded as crucial regulators of cell survival and apoptosis in different tissues such as lung tissue. Among lncRNAs with putative roles in lung carcinogenesis are long intergenic RNAs. Zhao et al. reported significant up-regulation of LINC00152, LINC00691 and LINC00578 in lung cancer tissues. They also demonstrated down-regulation of LINC00668, LINC00710 and LINC00607 in these tissues compared with paired non-tumoral tissues [9]. Another long intergenic RNA with possible participation of lung carcinogenesis has been LINC01433. Two independent groups have demonstrated oncogenic roles of this lncRNAs via in vitro assays [7,8]. Moreover, the results of in vivo experiments support such role in animal models [7]. LINC01433 up-regulation in cancer cell lines has enhanced cell proliferation, migration, and invasion, and induced epithelial-mesenchymal transition [8]. Based on these studies, we supposed that expression of LINC01433 would be higher in tumoral tissues obtained from Iranian NSCLC patients as well. However, expression assays indicated similar expression levels of this lncRNAs between tumoral and non-tumoral tissues of these patients. The discrepancy between our results and the results of Qian et al. study [8] might be due to different origin of included patients and their dissimilar genetic background. Consequently, we propose conduction of expression assays in samples from different ethnic groups to unravel whether genetic/environmental factors contribute in dysregulation of expression of this lncRNA. Similar to our results, Qian et al. did not report any association between expression of LINC01433 and any of clinical data including tumor size, lymph node metastasis and clinical stage [8], which reduces the possibility of participation of this lncRNA in the pathogenesis of lung cancer. However, Qian et al. reported association between expression of LINC01433 and smoking history [8], which was not revealed in our study.
In brief, our study did not verify the over-expression of LINC01433 in NSCLC Iranian patients despite the reported oncogenic role of this lncRNA. In our recent study of assessment of OIP5-AS1 expression in lung cancer tissues, we also reported down-regulation of this lncRNA [5], despite the reported oncogenic role of OIP5-AS1 in other studies [10]. Such results necessitate assessment of expression profile of genes in each ethnic group and potentiate the contribution of genetic/environmental factors in regulation of gene expression in tumoral tissues. Consequently, this note should be considered in adoption of transcript biomarkers for detection of cancer.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE recommendation for biomedical papers.
Mohammad Taheri, MD, PhD
Urogenital Stem Cell Research Center
Shahid Beheshti University of Medical Sciences
Bldg No. 2 SBUMS
Arabi Ave, Daneshjoo Blvd, Velenjak
Tehran, Iran
e-mail: mohammad_823@yahoo.com
Soudeh Ghafouri-Fard, MD, PhD
Department of Medical Genetics
Shahid Beheshti University of Medical Sciences
Bldg No. 2 SBUMS
Arabi Ave, Daneshjoo Blvd, Velenjak
Tehran, Iran
e-mail: s.ghafourifard@sbmu.ac.ir
Submitte: 18. 6. 2019
Accepted: 8. 8. 2019
Sources
1. Mao Y, Yang D, He J et al. Epidemiology of lung cancer. Surg Oncol Clin N Am 2016; 25 (3): 439–445. doi: 10.1016/ j.soc.2016.02.001.
2. Zarogoulidis K, Zarogoulidis P, Darwiche K et al. Treatment of non-small cell lung cancer (NSCLC). J Thorac Dis 2013; 5 (Suppl 4): S389–S396. doi: 10.3978/j.issn.2072-1439.2013.07.10.
3. Chansky K, Sculier JP, Crowley JJ et al. The International Association for the Study of Lung Cancer Staging Project: prognostic factors and pathologic TNM stage in surgically managed non-small cell lung cancer. J Thorac Oncol 2009; 4 (7): 792–801. doi: 10.1097/JTO.0b013e3181a7716e.
4. Lu T, Wang YY, Chen D et al. Potential clinical application of lncRNAs in non-small cell lung cancer. Onco Targets Ther 2018; 11 : 8045–8052. doi: 10.2147/Ott.S178431.
5. Esfandi F, Kholghi Oskooei V, Taheri F et al. Expression analysis of OIP5-AS1 in non-small cell lung cancer. Klin Onkol 2018; 31 (4): 260–263. doi: 10.14735/amko2018260.
6. Esfandi F, Taheri M, Omrani MD et al. Expression of long non-coding RNAs (lncRNAs) has been dysregulated in non-small cell lung cancer tissues. BMC cancer 2019; 19 (1): 222. doi: 10.1186/s12885-019-5435-5.
7. Hu G, Yang T, Zheng J et al. Functional role and mechanism of lncRNA LOC728228 in malignant 16HBE cells transformed by anti-benzopyrene-trans-7,8-dihydrodiol-9,10-epoxide. Mol Carcinog 2015; 54 (Suppl 1): 192–204. doi: 10.1002/mc.22314.
8. Qian B, Wang X, Mao C et al. Long non-coding RNA linc01433 promotes migration and invasion in non-small cell lung cancer. Thorac Cancer 2018; 9 (5): 589–597. doi: 10.1111/1759-7714.12623.
9. Zhao B, Xu H, Ai X et al. Expression profiles of long noncoding RNAs in lung adenocarcinoma. Onco Targets Ther 2018; 11 : 5383–5390. doi: 10.2147/OTT.S167633.
10. Wang M, Sun X, Yang Y et al. Long non-coding RNA OIP5-AS1 promotes proliferation of lung cancer cells and leads to poor prognosis by targeting miR-378a-3p. Thorac Cancer 2018; 9 (8): 939–949. doi: 10.1111/1759-7714.12767.
Labels
Paediatric clinical oncology Surgery Clinical oncology
Article was published inClinical Oncology

2019 Issue 6-
All articles in this issue
- Tranzice péče o onkologické pacienty z dětského do dospělého věku
- Sarcopenia in Metastatic Colorectal Carcinoma
- Rare Hereditary Burden associated with a Hypercalcemic Small-Cell Carcinoma of Cervix in a Young Female Patient
- Extravasation (Paravasation) of Chemotherapy Drugs – Recommendations for Standard Care in the Czech Republic based on Consolations between Representatives of the Supportive Care Group of the Czech Society for Oncology, Oncology Section of the Czech Nurses Association, and the Society for Ports and Permanent Catheters
- Aktuality z odborného tisku
- Spomienka na prof. MUDr. Ľudovíta Milana Jurgu, DrSc.
- Maligní onemocnění, psychika a stres. Příběhy pacientů s komentářem psychologa
- Benign Tumours and Pseudotumours Within the Porta Hepatis Masquerading as Perihilar Cholangiocarcinoma
- Epidemiological Trends for Childhood and Adolescent Cancers in the Period 1994–2016 in the Czech Republic
- Ukraine Data on Prognostic Factors and Treatment Outcomes in Patients with Peripheral T-Cell Lymphomas
- Bortezomib and Thalidomide Treatment Results in Newly Diagnosed Transplant-Ineligible Multiple Myeloma Patients are Comparable in Long-Term Follow-Up
- Expression Analysis of the Long Non-Coding RNA LINC01433 in Lung Cancer
- Clinical Oncology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Extravasation (Paravasation) of Chemotherapy Drugs – Recommendations for Standard Care in the Czech Republic based on Consolations between Representatives of the Supportive Care Group of the Czech Society for Oncology, Oncology Section of the Czech Nurses Association, and the Society for Ports and Permanent Catheters
- Rare Hereditary Burden associated with a Hypercalcemic Small-Cell Carcinoma of Cervix in a Young Female Patient
- Tranzice péče o onkologické pacienty z dětského do dospělého věku
- Bortezomib and Thalidomide Treatment Results in Newly Diagnosed Transplant-Ineligible Multiple Myeloma Patients are Comparable in Long-Term Follow-Up
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

