-
Medical journals
- Career
Change in Quality of Life Measured over Time in Czech Women with Breast Cancer
Authors: L. Anderkova 1; N. Elfmarkova 1,2; T. Sverak 1; H. Peterkova 2; D. Brancikova 3; M. Bendová 4; M. Protivánková 3; K. Benesova 5; L. Dusek 5; Jiří Jarkovský 5; L. Minar 4; K. Skrivanova 2
Authors‘ workplace: Applied Neuroscience Research Group, CEITEC – Central European Institute of Technology, Masaryk University, Brno, Czech Republic 1; Department of Psychology and Psychosomatics, Faculty of Medicine, Masaryk University, Brno, Czech Republic 2; Department of Internal Medicine, Hematology and Oncology, University Hospital Brno and Masaryk University, School of Medicine, Brno, Czech Republic 3; Department of Gynaecology and Obstetrics, University Hospital Brno and Masaryk University, School of Medicine, Brno, Czech Republic 4; Institute of Biostatistics and Analyses, Faculty of Medicine and Faculty of Science, Masaryk University, Brno, Czech Republic 5
Published in: Klin Onkol 2016; 29(2): 113-121
Category: Original Articles
doi: https://doi.org/10.14735/amko2016113Overview
Background:
This study examined the impact of breast cancer on quality of life (QOL) of Czech women by comparing the QOL of breast cancer patients with that of age-matched healthy controls.Methods:
The sample consisted of 74 breast cancer patients who filled in self-assessment questionnaires retrospectively before treatment and at the time of the study. In addition, 73 healthy controls completed the same battery of questionnaires. QOL was assessed using the Rand 36-Item Health Survey, the Life Satisfaction Questionnaire, and the Czech research version of Functional Assessment of Breast Cancer Therapy. The Wilcoxon paired test and Mann-Whitney U test were used for data analysis.Results:
A statistically significant decline in QOL in breast cancer patients was found for the following components: Physical Functioning (p = 0.021), Role Functioning-Physical (p < 0.001), Bodily Pain (p = 0.001), General Health (p = 0.031), Role Functioning-Emotional (p = 0.023), and Physical Well-being (p = 0.001). The only significant increase over time was observed in Social/Family Well-being (p = 0.024). For most of the components, patients showed a statistically significant lower QOL than that of healthy controls. A recent diagnosis, advanced disease stage, more comorbidities, a higher BMI, and other sociodemographic characteristics were associated with a higher incidence of a lower QOL over time.Conclusion:
Perceived QOL decreased over time in breast cancer patients mainly in components such as physical and emotional functioning, bodily pain, and general health, with several risk factors strongly influencing this change. The QOL of patients was lower than that of the non-cancer population, indicating that subsequent care should be improved to minimize the adverse effects that breast cancer has on QOL.Key words:
breast cancer – quality of life – change over time – retrospective study – risk factorsIntroduction
Breast cancer is the most common cancer type among Czech women, which is in line with other economically developed countries. The Czech Republic is characterized by a growing incidence of diagnosed breast cancer in women over the last decades. Fortunately, due to early detection of tumors (proportionally more women detected in stage I or II) and improved therapeutical strategies, the mortality rate has stabilized and the number of breast cancer survivors is growing [1]. With this growing amount of women who experienced diagnosis and successful treatment of breast cancer, analysis of the psychosocial context of the course of disease and recovery from it has begun to receive more attention. The most interesting questions are how struggles with breast cancer influence quality of life (QOL) of these women, what factors and characteristics are connected with better QOL and how they could be influenced by further supportive care.
QOL is a multidimensional construct that can be described as the perceived satisfaction or well-being of several life domains (such as physical, psychological, social, and spiritual). Subjective assessment of QOL is particularly essential because objective characteristics of the disease can be the same but QOL that women state can differ significantly [2]. Determination of factors related to differences in measured components of QOL is crucial in order to better understand the impact of breast cancer on QOL and possibly improve intervention and rehabilitation processes.
Previously, several factors were associated with changes in QOL in breast cancer patients. Generally, patients with breast cancer have comparable QOL [3 – 8] to healthy controls with detected improvement of QOL over time [9,10]. But some studies also showed worse QOL in patients with breast cancer compared to healthy controls [10 – 12] or even a decrease in global QOL over time [13]. Some researchers [12,14] observed an association between stage of disease and QOL, while others [15] did not find any connection. Recurrence [4,6,14] and comorbidity [11,12,16,17] are related to worse QOL among cancer survivors. Being either underweight [12] or obese [18] was associated with poorer QOL in breast cancer survivors especially in the white female demographic [19]. Fatigue, depression/ psychological distress, and anxiety adversely influence QOL among women with breast cancer [11,20 – 25].
Age is one of the most frequently mentioned factors that affects QOL in breast cancer survivors with younger women usually reporting worse QOL [3,8,26,27]. It has also been observed that older groups of breast can-cer survivors showed poor QOL in the physical domain, while younger groups had worse QOL in social or material domains [14,28]. Some researchers [14] found that unmarried or single women had worse QOL but others [11,29,30] found no effect of marital status on QOL. Similarly, some studies [11,14] showed a connection between better QOL in breast cancer survivors with higher education but another [30] revealed no connection. Women with higher income reported higher QOL [10,12,14,31]. There were also reported complaints about worsening financial situations at follow-ups [9,32]. Altogether marital, educational, and employment status seem to be only partially associated with QOL in breast cancer patients [17,33].
The aims of the study are to examine the impact of breast cancer and its treatment on QOL of Czech women and to detect the changes of subjectively perceived QOL resulting from the development of the disease and its treatment. The second goal was to explore to what extent QOL of Czech breast cancer patients differs in comparison to healthy controls. This is the first study to focus on broad psychosocial and disease-related factors that can be associated with QOL in a sample of Czech women after breast cancer treatment as part of a four-year, on-going, complex research project.
Patients and methods
Participants
A total of 74 breast cancer patients participated in this retrospective study of QOL. Patients were recruited during their scheduled medical appointment in the University Hospital Brno. The eligible criteria for the patient group were breast cancer diagnosis, age 30 – 90, Czech language proficiency, and completion of primary oncological treatment. Women were excluded if they were unable to speak Czech. Socio-demographic data were collected via a face-to-face interview conducted by trained psychologists, and disease-related data from medical chart abstraction by medical doctors using structured questionnaires. These data were twofold: the first cluster included socio-demographic and lifestyle-related factors, such as age, educational level, employment status, monthly income, residence size, marital status, number of children, age at first birth, age at menarche, duration of breastfeeding, smoking habits, spirituality, and others. The second cluster included medical factors, such as weight and height (BMI), time of diagnosis and beginning of treatment, current pharmacotherapy, menopausal status, stage of breast cancer at the time of primary diagnosis, comorbidity, used therapeutical modality (surgery, chemotherapy, radiotherapy, biological and hormonal treatment), toxicity of anticancer therapy, clinical immunological parameters, blood count after operation, recurrence of disease, and others.
Patients completed a self-assessment of their QOL retrospectively before their treatment and at the time of the study using questionnaires described in detail below. The refusal rate was less than 1%. Additionally, 73 age-matched Czech women without psychiatric disorder were recruited as healthy controls (HC) and completed the same battery of self-assessment questionnaires. All parti-cipants gave their informed consent prior to inclusion in the sample, and the study was approved by local ethics committee.
QOL methods
QOL was assessed using the Rand 36 – Item Health Survey (SF-36), the Life Satisfaction Questionnaire (LSQ) and the Czech research version of the Functional Assessment of Breast Cancer Therapy (FACT-B). SF-36 [34,35] is a survey constructed to study health-related QOL and consists of eight health concepts: Physical Functioning, Role Functioning – Physical, Body Pain, General Health, Vitality, Social Functioning, Role Functioning – Emotional, and Mental Health. Total scores can vary from 0 to 100, with higher scores representing a more favorable QOL. SF-36 is a tool frequently used to study QOL in patients with breast cancer, and many studies have established this questionnaire in this patient population [2,4,5,10,18,19,31]. A Czech translation of the SF-36 survey was used for the purpose of our research; this translation was subject to a validation which confirmed that the Czech version of SF-36 is as effective as the original US version [36]. Scores were calculated according to the original US version. LSQ [37] is a tool used to study life satisfaction on a seven-point scale and it contains ten dimensions of life: Health, Employment, Financial Situation, Leisure Time, Marriage and Partnership, Children, Personality, Sexuality, Friends and Relatives, Housing and Total Life Satisfaction. This questionnaire was used previously in a sample of Czech women with breast cancer [38]. FACT-B [39] is a questionnaire used for self-assessment which was specifically developed for use in women with breast cancer, and includes the assessment of Physical, Social, Emotional and Functional Wellbeing plus the breast cancer subscale (Additional Concerns). Each item is rated on a five-point scale (0 – not at all; 1 – a little bit; 2 – somewhat; 3 – quite a bit; 4 – very much) with a higher total score indicating better functional status. This instrument was also previously used in several studies with breast cancer patients [22,27]. An authorized Czech translation of FACT-B was used for the purpose of our research; this translation was provided by the FACIT.org as the copyright holder; the internal con-sistency reliability based on Cronbach‘s α is 0.741. We used the Czech research version of the FACT-B.
Statistical methods
Detailed patients’ characteristics were summarized using standard descriptive statistics. Absolute and relative frequencies were used for categorical variables, and mean with standard deviation (SD) or median supplemented by 5th – 95th percentile range were used for continuous variables and scores. Internal consistency reliability for the Czech research version of FACT-B was tested using Cronbach‘s α. Statistical significance of differences among groups of subjects was tested by means of Mann-Whitney U test or Kruskal-Wallis test; statistical significance of changes over time in QOL scores in patients were tested using the Wilcoxon paired test. Statistical analysis was computed using SPSS 22 (IBM Corporation, 2013), regarding standard setting of the tests with α = 0.05. A difference of about 10% of the QOL instrument range is usually interpreted as a clinically significant change in patient-reported outcomes [40].
Results
Description of the sample
Our sample consisted of 74 women (median age 63) with histologically confirmed breast cancer that were recruited and then participated in an interview. Patients were mostly postmenopausal (81.1%), with a median BMI of 27.5, had children (90.5%) and had breastfed them in the past (83.8%), and depression was found in 20.3% of patients. Common comorbidities were hypertension (51.4%), diabetes mellitus (24.3%), and vascular brain disease (21.6%). Disease stage was II + III in 67.6% of patients, and 0 + I in 32.4%. All patients had undergone surgery and some of them had additional radiotherapy (86.5%), hormone therapy (82.4%), chemotherapy (66.2%), and/ or biological therapy (18.2%). Progression was found in 8.8% and relapse in 19.4% of patients. There were 172 women recruited. Follow-up QOL questionnaires were completed by 74 women. The age-matched control group consisted of 73 women with median age 67. The detailed basic, sociodemographic, and medical characteristics of the sample are provided in Tab. 1.
1. Sample description. 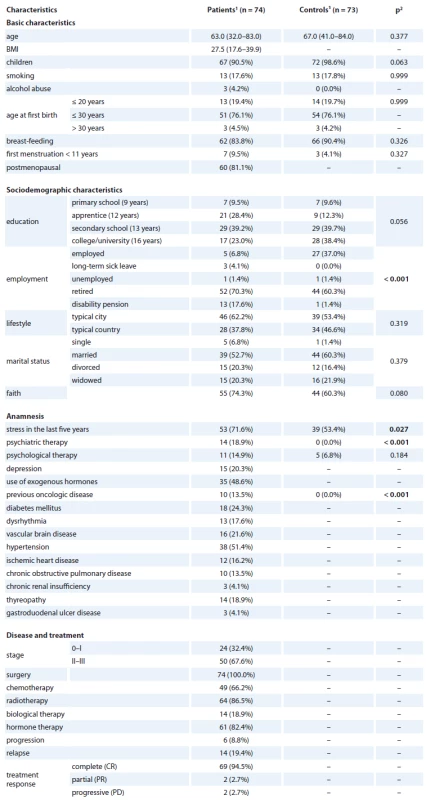
1 median (min.–max.) or n (column %) 2 p-value of Mann-Whitney U test for continuous variables and p-value of Fisher’s exact test for categorical variables Overall QOL change over time in patients
Overall, we found a decrease over time in some components of QOL in patients with breast cancer. A statistically significant decrease in QOL was found for the following components of SF-36: Physical Functioning (p = 0.021), Role Functioning – Physical (p < 0.001), Body Pain (p = 0.001), General Health (p = 0.031) and Role Functioning – Emotional (p = 0.023). A similar decline was also found for Physical Well-being (p = 0.001). On the other hand, there was a slight increase in Social/ Family Well-being (p = 0.024). The detailed results are displayed in Tab. 2.
2. QOL – overall change over time in breast cancer patients. 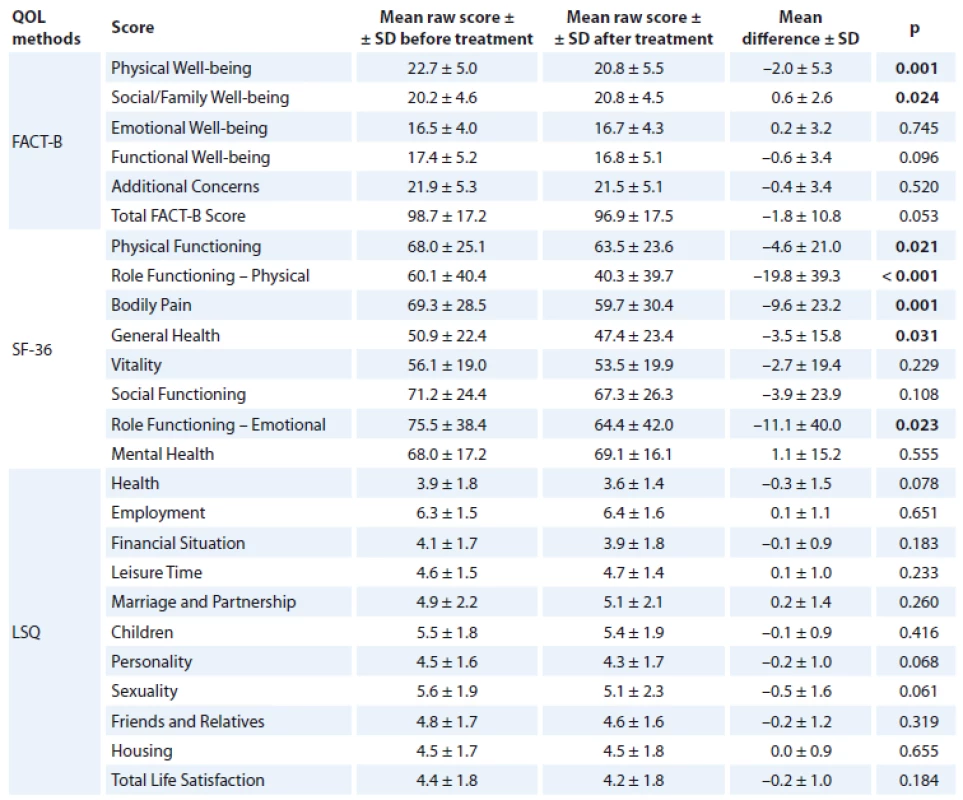
p-value of paired Wilcoxon test QOL in patients vs. controls
In most of the measures, Czech breast cancer patients perceive their QOL at the time of the study (after treatment) to be significantly worse compared to age-matched healthy controls. The most profound differences were in the physical domain of QOL in each scale. Breast cancer patients scored significantly lower on all SF36 and FACT-B subscales except: Body Pain, Vitality, Social/ Family Well-being and Emotional Well-being (EWB). Moreover, in LSQ, subjects with breast cancer scored significantly lower on Health (p < 0.001), Financial Situation (p = 0.017), Children (p = 0.032), Housing (p = 0.044) and Total Life Satisfaction (p = 0.012). For more details, see Tab. 3.
3. Comparison of patients and controls. 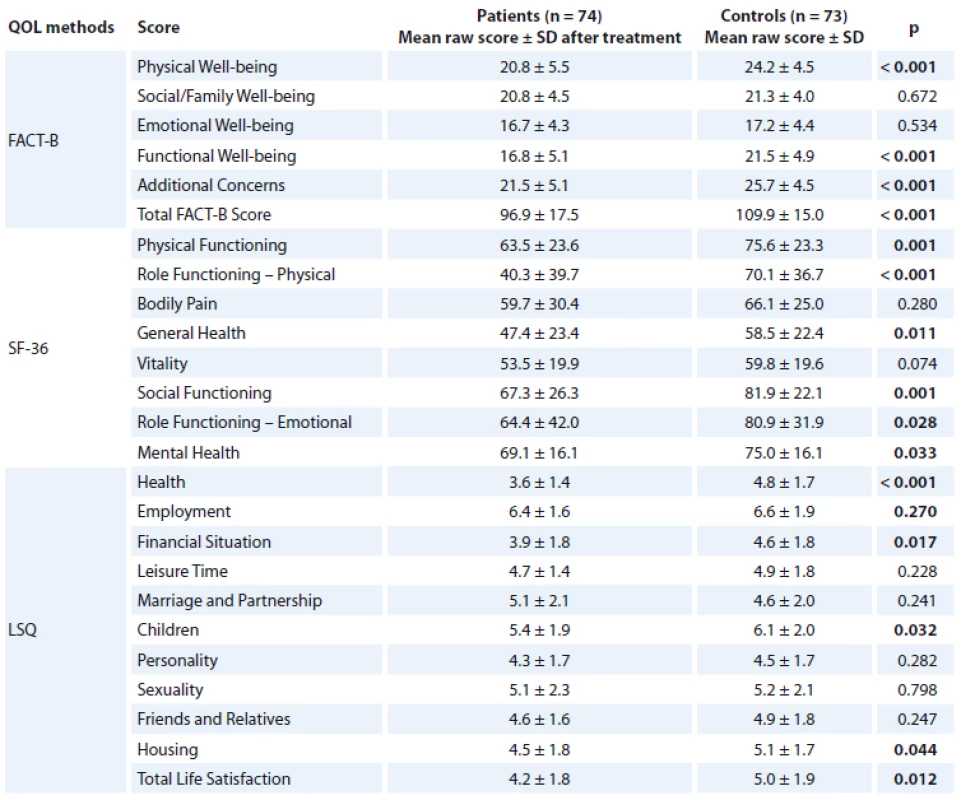
p-value of Mann-Whitney test (patients after treatment vs. controls) Years since diagnosis
In our analysis, patients were also divided into two groups: long-term cancer survivors (> 5 years since diagnosis; n = 20; 142 ± 66.1 months between the diagnosis and the commencement of study) and patients with recent diagnosis (≤ 5 years since diagnosis; n = 54; 22 ± 16.3 months between the diagnosis and the commencement of study). Patients with recent diagnoses showed a statistically significant decrease over time in the following QOL components: Role Functioning – Physical (p = 0.001) and Bodily Pain (p = 0.004). Both long-term cancer survivors and patients with recent diagnoses reported a statistically significant decrease in Physical Well-being (p = 0.008; p = 0.028, resp.).
Stage of the disease
We also divided the patients into two groups according to the stage of their disease (0 + I; II + III). Patients in both groups demonstrated a statistically significant decrease in QOL components Role Functioning – Physical (p = 0.013; p = 0.003), Bodily Pain (p = 0.013; p = 0.01) and Physical Wellbeing (p = 0.010; p = 0.023), resp. Women in stage II + III showed a statistically significant decrease in QOL components Personality (p = 0.026) and Social/ Family Well-being (p = 0.004), whereas the group of women in stage 0 + I were characterized by a decrease in Role Functioning – Emotional (p = 0.041).
Comorbidity
A general trend was observed in which patients with more comorbidities scored lower on QOL scales. Solely in patients with comorbidities was there a statistically significant decrease over time in QOL components: Personality (p = 0.017), Role Functioning – Emotional (p = 0.048) and Bodily Pain (p = 0.002). Only in patients without comorbidities there was a statistically significant increase over time in Social/ Family Well-being (p = 0.009). Patients both with and without comorbidities reported a statistically significant decrease in Role Functioning – Physical (p = 0.008; p = 0.005) and Physical Well-being (p = 0.031; p = 0.025, resp.).
BMI
In the Role Functioning – Physical domain, each BMI group displayed a statistically significant decrease over time. Both overweight (BMI 25 – 30) and obese (BMI > 30) women with breast cancer report a statistically significant decrease over time in Physical Well-being (p = 0.048; p = 0.012) and Bodily Pain (p = 0.022; p = 0.007, resp.). Overweight patients also showed a decrease in Sexuality (p = 0.012), whereas obese patients also exhibited decreases in General Health (p = 0.032), Physical Functioning (p = 0.030), Role Functioning – Emotional (p = 0.046), Functional Well-being (p = 0.017), Additional Concerns (p = 0.028) QOL components and Total FACT-B Score (p = 0.015). The group with normal weight reported a statistically significant increase over time in QOL component Social/ Family Well-being.
Education level
Overall, we noticed a trend that patients with higher education level had a better QOL. A statistically significant decrease over time was found most frequently in patients with secondary school in components: Role Functioning – Physical (p = 0.002), Health (p = 0.033), Role Functioning – Emotional (p = 0.014), Physical Well-being (p = 0.011), Financial Situation (p = 0.019) and Total FACT-B Score (p = 0.004). Patients with primary education significantly worsened in Physical Functioning (p = 0.039), patients with apprentice-level education worsened in Personality (p = 0.047), Bodily Pain (p = 0.033) and Role Functioning – Physical (p = 0.029) and patients with university degree worsened in Bodily Pain (p = 0.028) and improved in Emotional Well-being (p = 0.013).
Living alone vs. living in a relationship
Women with breast cancer that were living in a relationship decreased significantly in QOL components: Physical Functioning (p = 0.024), Role Functioning – Physical (p = 0.001), Bodily Pain (p = 0.001), Role Functioning – Emotional (p = 0.008), Physical Well-being (p = 0.004). The patients living alone reported a statistically significant decrease in Role Functioning – Physical (p = 0.037), only. However, we found a trend that women living alone showed overall worse QOL scores compared to the women living in a relationship.
Income
For lower income group, there was a statistically significant decrease in QOL components: Physical Functioning (p = 0.014), Role Functioning – Physical (p = 0.001), Bodily Pain (p = 0.003), Role Functioning – Emotional (p = 0.019), Social Functioning (p = 0.023), Physical Well-being (p = 0.005), Functional Well-being (p = 0.036) and Total FACT-B score (p = 0.005). Patients with higher income showed a statistically significant increase in QOL component Emotional Well-being (p = 0.019). We also observed a tendency in patients with higher income to exhibit overall higher QOL scores compared to those with a lower one.
Discussion
The presented study aims to contribute to the knowledge about the changes in the QOL of patients with breast cancer and factors that may be connected to this progress. Overall, we found that patients with breast cancer felt that their QOL worsened over time in several main components, such as general Physical and Emotional Functioning, Bodily Pain, and General Health. Conversely, we also found a mild improvement in the Social/ Family Well-being component.
A persistent decrease in QOL over time of global QOL in patients with breast cancer has been reported in previous studies [13]. In a recent prospective study on a cohort of Australian women [41], a decrease in SF-36 components Bodily Pain, General Health, Physical Functioning, Role functioning physical, and Vitality was found in newly diagnosed patients, which corroborates our findings. In the Australian study, however, at a three-year follow-up, QOL had increased to a point close to its pre-diagnosis state. Although the retrospective design of this study is not suitable for continuously monitoring this change, if we compare the QOL in components of the SF-36 in the group of long-term cancer survivors (> 5 years since diagnosis) to those of patients with recent diagnoses, the current state of long-term survivors shows no significant improvement. This may indicate that QOL in the sample of Czech women does not increase back to the baseline (before diagnosis) but instead remains deteriorated. Nevertheless, in order to verify this observation, a prospective study with fixed follow-up measurements on the Czech sample is needed. The improvement in the Social/ Family Well-being component that we found may be associated with a higher engagement in life and posttraumatic growth which was noticed in a previous study [42] in domains such as relationships with others, personal strength, and appreciation of life.
The QOL of women with breast cancer was significantly worse in most measured components compared to healthy controls with similar demographic characteristics. This has been reported in some previous studies [10 – 12] and leads to the conclusion that there is still room to improve care and interventions in order to improve the QOL of cancer patients and survivors to that of the general population. The fact that the median age in our patient group was 65 and we did not have a very robust group of young women with breast cancer did prevent us from making conclusive statements about age and perceived QOL in connection with change over time. Further research is needed to map the changes in QOL of a younger cohort of Czech women with breast cancer. Prior studies have found that advanced stages of the disease are associated with a decrease in QOL mainly in Social Well-being [12,14] which is in line with our results. The results of our study, as well as those that preceded it, underline the importance of using supportive care to improve the social engagement in women suffering from advanced stages of the disease.
In general, the findings of this study indicate a decrease in QOL over time in numerous components in patients with recent diagnoses, advanced stages, and more comorbidities than in long-term survivors, early stages, and patients without comorbidities. The negative impact of these factors has been shown before [4]. Additionally, we also noticed a trend in patients with comorbidities to score generally lower on QOL scales than patients without them. This research adds to the increasing amount of evidence that a BMI > 25 (overweight and obesity level) has a negative impact on QOL, as well as its change over time in patients with breast cancer [18,19]. The efforts to maintain or reduce one’s weight to a normal level may lead to better QOL outcomes in women with breast cancer in continuous care.
In terms of social factors, we noticed that patients with higher education usually tend to have better QOL as shown previously [11,14]. In our sample, a statistically significant decrease over time was most visible in the group of patients with a secondary school education. Moreover, we found that women with breast cancer that were living in a relationship displayed a statistically significant decrease in QOL components, yet also that they tend to have higher QOL scores than those living alone, which has been shown previously [14]. Women with higher incomes tended to exhibit a higher QOL, which is in line with previous findings [10,12,14,31], while there was a significant decrease in QOL in several components only observed among members of the lower-income group. Appropriate future interventions should be addressed especially to women with breast cancer with worse financial situation because their QOL may worsen more over time than in patients with a better financial background.
The main strength of this study is that we collected complex data about studied subjects, including detailed medical records, socio-demographic, and lifestyle-related factors, together with extensive psychological self-reported data from QOL, coping strategies, personality, and other questionnaires as a part of a four-year ongoing, complex research project. This is a unique study designed to capture QOL change over time in a sample of Czech women with breast cancer. This study has several limitations. The retrospective design of the study allows for the possibility that our data may be altered by subjective bias. Moreover, the period between the examined time before treatment and the time of the study varies across patients and may influence the results. Type and extent of surgery were not recorded for the purposes of this study. These variables may represent important confounds that influence QOL of these patients and should be included in the forthcoming studies. We are aware that a longitudinal prospective study may yield different results and should be preferred in the future.
Conclusion
In conclusion, the present study shows that perceived QOL decreased over time in patients with breast cancer, mainly in components such as: physical and emotional functioning, bodily pain, and general health with several factors that strongly influence this change (recent diagnosis, stage of disease, comorbidity, BMI, and some sociodemographic characteristics). Moreover, we have observed that QOL of breast cancer patients is significantly lower compared to healthy controls. With the number of cancer survivors on the constant incline, it is very important to try to improve the subsequent care and minimize the adverse effects that breast cancer has on QOL.
Acknowledgments
We deeply thank our participants for their commitment to our research project. We would like to acknowledge James Bredley (FACIT.org) who provided us with the Czech research version of the questionnaire FACT-B to use this tool for the purpose of our research study.
This study was supported by grant P407/12/0607 of the Czech Science Foundation.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE recommendation for biomedical papers.
Submitted: 31. 8. 2015
Accepted: 6. 10. 2015
Mgr. Katerina Skrivanova, PhD.
Department of Psychology and Psychosomatics
Faculty of Medicine
Masaryk University Kamenice 126/3
625 00 Brno Czech Republic
e-mail: kskrivan@med.muni.cz
Sources
1. Dusek L, Muzik J, Maluskova D et al. Cancer incidence and mortality in the Czech Republic. Klin Onkol 2014; 27(6): 406 – 423. doi: 10.14735/amko2014406.
2. Bloom JR, Stewart SL, Chang S et al. Then and now: quality of life of young breast cancer survivors. Psychooncology 2004; 13(3): 147 – 160.
3. Koch L, Jansen L, Herrmann A et al. Quality of life in long-term breast cancer survivors – a 10-year longitudinal population-based study. Acta Oncol 2013; 52(6): 1119 – 1128. doi: 10.3109/0284186X.2013.774461.
4. Helgeson VS, Tomich PL. Surviving cancer: a comparison of 5-year disease-free breast cancer survivors with healthy women. Psychooncology 2005; 14(4): 307 – 317.
5. Tomich PL, Helgeson VS. Five years later: a cross-sectional comparison of breast cancer survivors with healthy women. Psychooncology 2002; 11(2): 154 – 169.
6. Dorval M, Maunsell E, Deschenes L et al. Long-term quality of life after breast cancer: comparison of 8-year survivors with population controls. J Clin Oncol 1998; 16(2): 487 – 494.
7. Mosconi P, Apolone G, Barni S et al. Quality of life in breast and colon cancer long-term survivors: an assessment with the eortc qlq-c30 and sf-36 questionnaires. Tumori 2002; 88(2): 110 – 116.
8. Arndt V, Merx H, Sturmer T et al. Age-specific detriments to quality of life among breast cancer patients one year after diagnosis. Eur J Cancer 2004; 40(5): 673 – 680.
9. Hsu T, Ennis M, Hood N et al. Quality of life in long-term breast cancer survivors. J Clin Oncol 2013; 31(28): 3540 – 3548. doi: 10.1200/JCO.2012.48.1903.
10. Klein D, Mercier M, Abeilard E et al. Long-term quality of life after breast cancer: a french registry-based controlled study. Breast Cancer Res Treat 2011; 129(1): 125 – 134. doi: 10.1007/s10549-011-1408-3.
11. Romito F, Cormio C, Giotta F et al. Quality of life, fatigue and depression in italian long-term breast cancer survivors. Support Care Cancer 2012; 20(11): 2941 – 2948. doi: 10.1007/s00520-012-1424-9.
12. Lu W, Cui Y, Zheng Y et al. Impact of newly diagnosed breast cancer on quality of life among chinese women. Breast Cancer Res Treat 2007; 102(2): 201 – 210.
13. Montazeri A, Vahdaninia M, Harirchi I et al. Quality of life in patients with breast cancer before and after diagnosis: an eighteen months follow-up study. BMC Cancer 2008; 8 : 330. doi: 10.1186/1471-2407-8-330.
14. Cui Y, Shu XO, Gao YT et al. The long-term impact of medical and socio-demographic factors on the quality of life of breast cancer survivors among chinese women. Breast Cancer Res Treat 2004; 87(2): 135 – 147.
15. Petersen LR, Clark MA, Novotny P et al. Relationship of optimism-pessimism and health-related quality of life in breast cancer survivors. J Psychosoc Oncol 2008; 26(4): 15 – 32.
16. Vissers PA, Thong MSY, Pouwer F et al. The impact of comorbidity on health-related quality of life among cancer survivors: Analyses of data from the profiles registry. J Cancer Survivorship 2013; 7(4): 602 – 613. doi: 10.1007/s11764-013-0299-1.
17. Engel J, Kerr J, Schlesinger-Raab A et al. Predictors of quality of life of breast cancer patients. Acta Oncol 2003; 42(7): 710 – 718.
18. Blanchard CM, Stein K, Courneya KS. Body mass index, physical activity, and health-related quality of life in cancer survivors. Med Sci Sports Exerc 2010; 42(4): 665 – 671. doi: 10.1249/MSS.0b013e3181bdc685.
19. Paxton RJ, Phillips KL, Jones LA et al. Associations among physical activity, body mass index, and health-related quality of life by race/ ethnicity in a diverse sample of breast cancer survivors. Cancer 2012; 118(16): 4024 – 4031. doi: 10.1002/cncr.27389.
20. Yen JY, Ko CH, Yen CF et al. Quality of life, depression, and stress in breast cancer women outpatients receiving active therapy in taiwan. Psychiatry Clin Neurosci 2006; 60(2): 147 – 153.
21. Sarenmalm EK, Ohlen J, Oden A et al. Experience and predictors of symptoms, distress and health-related quality of life over time in postmenopausal women with recurrent breast cancer. Psychooncology 2008; 17(5): 497 – 505.
22. So WK, Marsh G, Ling WM et al. Anxiety, depression and quality of life among chinese breast cancer patients during adjuvant therapy. Eur J Oncol Nurs 2010; 14(1): 17 – 22. doi: 10.1016/j.ejon.2009.07.005.
23. Karakoyun-Celik O, Gorken I, Sahin S et al. Depression and anxiety levels in woman under follow-up for breast cancer: relationship to coping with cancer and quality of life. Med Oncol 2010; 27(1): 108 – 113. doi: 10.1007/s12032-009-9181-4.
24. Kim SH, Son BH, Hwang SY et al. Fatigue and depression in disease-free breast cancer survivors: prevalence, correlates, and association with quality of life. J Pain Symptom Manage 2008; 35(6): 644 – 655. doi: 10.1016/j.jpainsymman.2007.08.012.
25. Badger TA, Braden CJ, Mishel MH et al. Depression burden, psychological adjustment, and quality of life in women with breast cancer: patterns over time. Res Nurs Health 2004; 27(1): 19 – 28.
26. Hopwood P, Haviland J, Mills J et al.The impact of age and clinical factors on quality of life in early breast cancer: an analysis of 2,208 women recruited to the ukstart trial (standardisation of breast radiotherapy trial). Breast 2007; 16(3): 241 – 251.
27. So WK, Choi KC, Chan CW et al. Age-related differences in the quality of life of chinese women undergoing adjuvant therapy for breast cancer. Res Gerontological Nurs 2011; 4(1): 19 – 26. doi: 10.3928/19404921-20101201-01.
28. Cimprich B, Ronis DL, Martinez-Ramos G. Age at diagnosis and quality of life in breast cancer survivors. Cancer Pract 2002; 10(2): 85 – 93.
29. Lee CO. Quality of life and breast cancer survivors – psychosocial and treatment issues. Cancer Pract 1997; 5(5): 309 – 316.
30. Nagel GC, Schmidt S, Strauss BM et al. Quality of life in breast cancer patients: a cluster analytic approach. Breast Cancer Res Treat 2001; 68(1): 75 – 87.
31. Casso D, Buist DS, Taplin S. Quality of life of 5–10 year breast cancer survivors diagnosed between age 40 and 49. Health Qual Life Outcomes 2004; 2 : 25.
32. Fehlauer F, Tribius S, Mehnert A et al. Health-related quality of life in long term breast cancer survivors treated with breast conserving therapy: impact of age at therapy. Breast Cancer Res Treat 2005; 92(3): 217 – 222.
33. Awadalla AW, Ohaeri JU, Gholoum A et al. Factors associated with quality of life of outpatients with breast cancer and gynecologic cancers and their family caregivers: a controlled study. BMC Cancer 2007; 7 : 102.
34. Hays RD, Morales LS. The rand-36 measure of health-related quality of life. Ann Med 2001; 33(5): 350 – 357.
35. Ware JE, Sherbourne CD. The mos 36-item short-form health survey (sf-36) 1. Conceptual-framework and item selection. Med Care 1992; 30(6): 473 – 483.
36. Sobotík Z. Zkušenosti s použitím předběžné verze amerického dotazníku o zdraví (sf-36). Zdravotnictví v České republice 1998; 1 – 2(1): 50 – 54.
37. Fahrenberg J, Myrtek M, Schumacher J et al (eds). Dotazník životní spokojenosti. Praha: Testcentrum 2001.
38. Spurná Z, Dražan L, Foretová L et al. The effect of prophylactic mastectomy with recontruction on quality of life in brca positive women. Klin Onkol 2012; 25 (Suppl 1): S74 – S77. doi: 10.14735/amko2012S74.
39. Brady MJ, Cella DF, Mo F et al. Reliability and validity of the functional assessment of cancer therapy-breast quality-of-life instrument. J Clin Oncol 1997; 15(3): 974 – 986.
40. Ringash J, O‘Sullivan B, Bezjak A et al. Interpreting clinically significant changes in patient-reported outcomes. Cancer 2007; 110(1): 196 – 202.
41. Leung J, Pachana NA, McLaughlin D. Social support and health-related quality of life in women with breast cancer: a longitudinal study. Psychooncology 2014; 23(9): 1014 – 1020. doi: 10.1002/pon.3523.
42. Mols F, Vingerhoets AJ, Coebergh JW et al. Well-being, posttraumatic growth and benefit finding in longterm breast cancer survivors. Psychol Health 2009; 24(5): 583 – 595. doi: 10.1080/08870440701671362.
Labels
Paediatric clinical oncology Surgery Clinical oncology
Article was published inClinical Oncology

2016 Issue 2-
All articles in this issue
- Discordance between Clinical and Pathological TNM Classifications in Patients with Oropharyngeal Cancer – Influence on Treatment and Prognosis
- Enzalutamide and Abiraterone in the Treatment of Metastatic Castration-resistant Prostate Cancer after Chemotherapy
- The Role of BRAF/MEK Inhibition in Metastatic Malignant Melanoma – a Case Study
- Extravasation of Cytostatic Drugs – Prevention and Best Practices
- Anticancer Effect of Fish Oil – a Fable or the Truth?
- News in Adjuvant Therapy of Non-seminomatous Germ Cell Testicular Tumors of Stage I
- Comment – Active Surveillance vs. Adjuvant Therapy in Clinical Stage I Non-seminomatous Germ Cell Testicular Cancer
- Change in Quality of Life Measured over Time in Czech Women with Breast Cancer
- Papillary Carcinoma of Thyroid Gland in a Two-year-old Child
- Clinical Oncology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Extravasation of Cytostatic Drugs – Prevention and Best Practices
- Anticancer Effect of Fish Oil – a Fable or the Truth?
- Enzalutamide and Abiraterone in the Treatment of Metastatic Castration-resistant Prostate Cancer after Chemotherapy
- The Role of BRAF/MEK Inhibition in Metastatic Malignant Melanoma – a Case Study
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

