-
Medical journals
- Career
Platelet-rich plasma improves esthetic postoperative outcomes of maxillofacial surgical procedures
Authors: Yuliya Menchisheva 1; Ulmeken Mirzakulova 1; Dildora Usupova 2; Gulzhan Yermukhanova 3; Zhanagul Rysbayeva 4
Authors‘ workplace: Department Surgical Dentistry, State Hospital №5, S. D. Asfendiyarov Kazakh National Medical University, Almaty, Kazakhstan 1; Department of the Diseases and Traumatology of the Maxillofacial Region, Tashkent State Dental Institute, Tashkent, Uzbekistan 2; Department of Pediatric Dentistry, S. D. Asfendiyarov Kazakh National Medical University, Almaty, Kazakhstan 3; Department of Clinical Disciplines, Al Farabi Kazakh National University, Almaty, Kazakhstan 4
Published in: ACTA CHIRURGIAE PLASTICAE, 63, 3, 2021, pp. 118-126
doi: https://doi.org/10.48095/ccachp2021118Introduction
Platelet-rich plasma (PRP) is the fraction of plasma containing higher concentrations of platelets compared to whole blood [1]. The platelets are rich in growth factors which boosts healing and repair process [2]. Growth factors contained in PRP – platelet-derived growth factor (PDGF), transforming growth factor β (TGF-β), vascular endothelial growth factor (VEGF or PDAF), insulin-like growth factor (IGF) and epidermal growth factor (EGF) – activate cells migration, stimulate fibroblasts, osteoblasts, endothelial cells and keratinocytes proliferation, enhance the production of collagen and fibronectin, increase vascular permeability and stimulate angiogenesis [3].
PRP is widely applied in different clinical applications to promote healing of damaged tissues. Most of the studies described in the literature are devoted to the use of PRP in traumatology and sports medicine (repairing of acute muscle, tendon, ligament, nerve and cartilage injury and relieve pain in tendonitis, arthritis, ligament sprains and tear) [4–7], gynecology (use of PRP in vulvar lesions, genital prolapse and genital fistulas, in gynesthetics treatment) [8,9], surgery (healing of acute and chronic wounds, burns, defects) [10,11], neurosurgery (treatment of disc tissue pathology, spinal cord injury) [12,13], ophthalmology (treatment of symptomatic dry eye, corneal ulcers and ocular burns) [14,15], dentistry and oral surgery (healing after tooth extraction, treatment of periodontitis, use in implantology) [16,17]. PRP is used in dermatology for purposes including the treatment of ulcers, scars, and alopecia [18].
Thus, the clinical use of PRP is treatment of soft tissue injuries, burns and hard to heal wounds. Also, PRP might be applied to initiate repair of bone lesion in case of reduced osteoblasts proliferation or delayed chondrogenesis [19].
According to the results of conducted studies [1–19] of the use of PRP in surgery and other specialties of medicine, the application of autologous platelet-rich plasma is indicated for the induction of normal wound healing, for promoting the healing of hard to heal or non-healing wounds, ulcers and burns.
Due to the widespread use of PRP in esthetic medicine, plastic surgery and dermatology, it is reasonable to believe that the use of PRP is also indicated for improving the results of surgical treatment.
After plastic and reconstructive surgery, postoperative facial scars have a substantial impact on the quality of life through psychological distress and depression, which affects patients’ working capacity and social adaptation [20,21].
Platelet-rich plasma (PRP) releases numerous growth factors that may be invaluable in treatment [22,23]. The effects of growth factors may be beneficial as a therapy for wounds with delayed healing [24,25]. Complications in the early postoperative period, such as suppuration of the wound, divergence of sutures and delayed healing of patients with comorbid conditions often lead to adverse outcomes with scarring in the late postoperative period. Previous studies have assessed the efficiency of PRP in wound healing [26,27] although few of them provide an assessment of the influence on the skin or shed light on patient satisfaction. To evaluate esthetic outcomes, there is a tendency for physicians to use questionnaires [28–30]. In some studies, outcomes are presented with the use of pictures but without objective analysis of quantitative data, or samples are presented too indistinctly to be verifiable and trustworthy. The aim of this study was to evaluate surgical outcomes and esthetic effects in patients after plastic and reconstructive surgery in the maxillofacial area.
Wound healing studies have demonstrated that scars usually develop 6–8 weeks following re-epithelization, and that a period of 6–18 months is required for scar maturation [31,32]. Healing and remodeling are largely complete by 8–12 months [33], and the evaluation of the scars might be delayed until 1-year post-surgery [34]. Therefore, to draw an appropriate conclusion, observation time is critical.
Study hypothesis: esthetic outcomes and the quality of life are better in patients whose surgical incision was injected with PRP.
Material and methods
Patients
One hundred hospital patients aged 18–60 years (City Hospital 5, Almaty, Kazakhstan) who were undergoing plastic and reconstructive surgery in the maxillofacial region and were considered to be at high risk for scaring were included in this randomized controlled trial. Patients were randomly allocated into two groups. Fifty patients (26 males and 24 females aged 43 ± 6 (21–60) years) in the treatment group received PRP injections at the time of surgery, whereas 50 patients (27 females and 23 males aged 41 ± 5 (19–60) years) in the control group did not receive any injections. The patients underwent the following soft tissue procedures (Tab. 1).
1. Distribution of patients with postoperative wounds of soft tissues of the maxillofacial region after reconstructive plastic and esthetic operations (N = 100) depending on the type of the operation performed. 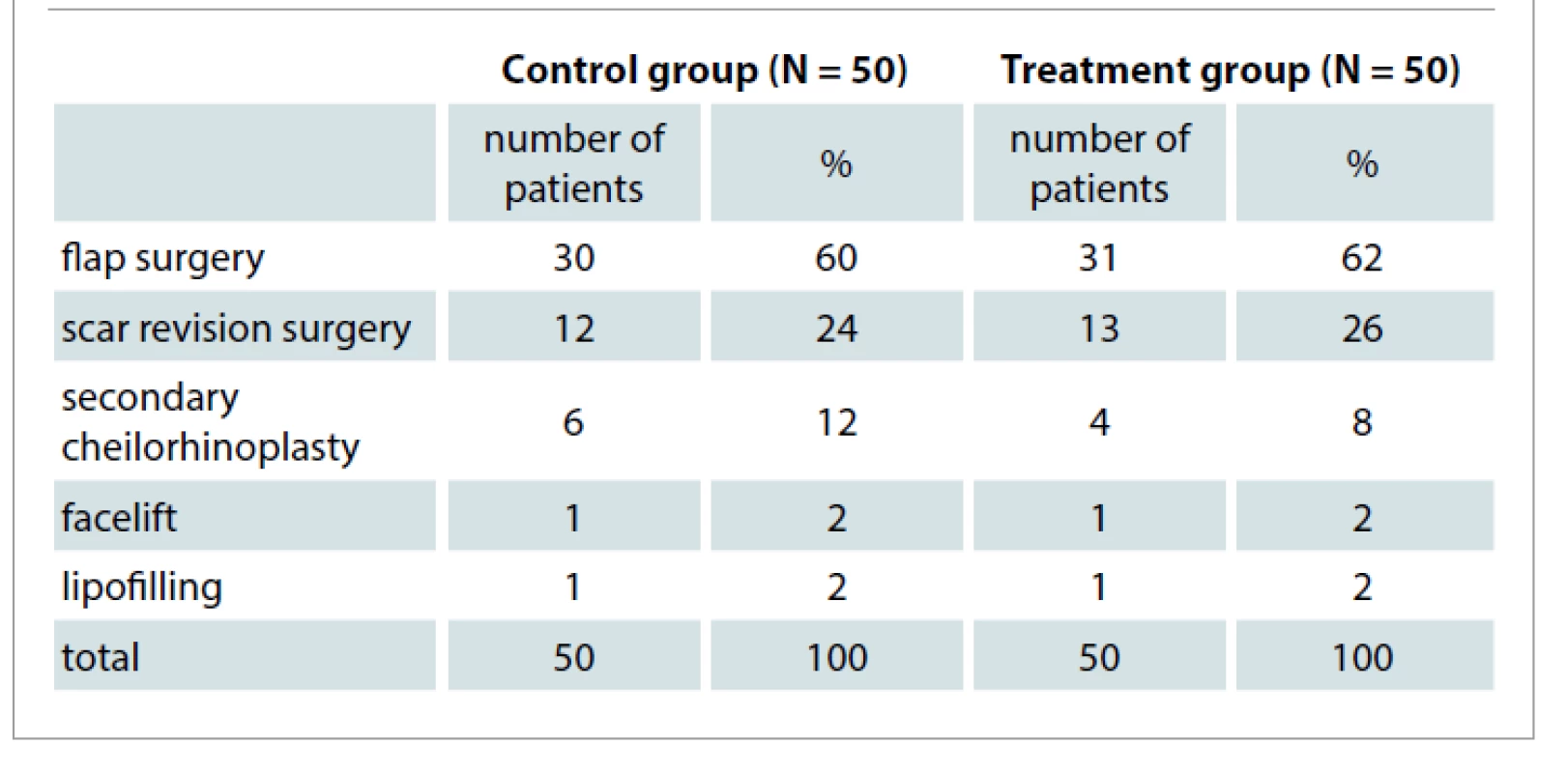
All enrolled patients signed informed consent forms to be eligible for research purposes. This randomized controlled trial was based on the revised CONSORT statement [35]. The study is pre-registered in clinical trials registry.
The inclusion criteria were for patients undergoing plastic and reconstructive operation in the maxillofacial area with high risk for scaring. Exclusion criteria were used for patients with platelet dysfunction syndrome, haemodynamic instability, local infection at the site of the procedure, systemic use of corticosteroids within 2 weeks, recent fever, and cancer. To identify patients considered to be at high risk for scaring, we used regression analysis of the results of clinical and laboratory research methods. By the stepwise logistic regression, we identified factors that significantly increase the risk for scaring after surgical procedures (Tab. 2). All factors were obtained during the study of archival material of medical cases of the patients (with and without excessive scaring) who had plastic and reconstructive procedures.
2. Coefficients of the discriminant function of factors contributing to the development of scaring in patients after plastic and reconstructive surgery. 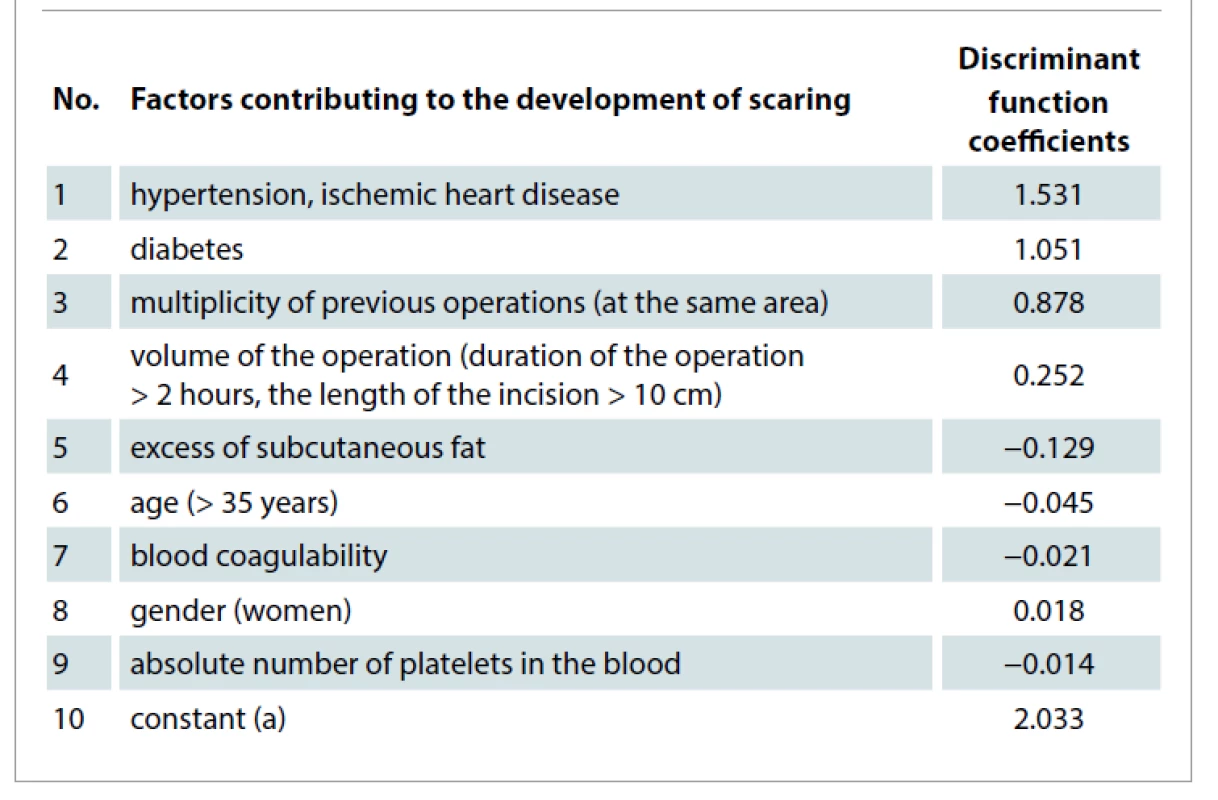
The resulting logistic regression equation for predicting the risk for scaring in the postoperative period is the following:
where d is the value of the discriminant function: d= 2.033 + 1.531x hypertension, ischemic heart disease + 1.051x diabetes + 0.239 x the volume of the operation + 0.878 x multiplicity of previous operations – 0.129 x excess of subcutaneous fat – 0,045 x age – 0.021x blood coagulability – 0.018 x gender – 0.014 x the absolute number of platelets in the blood.
If the P-value is < 0.5, then it can be assumed that the "event" (the development for scaring) will not occur; otherwise, an increased risk for scaring is assumed.
To assess the effectiveness of the method for predicting the development of high risk for scaring, the receiver operating characteristic (ROC) analysis was performed with the construction of a ROC curve. The value of the area under the ROC-curve was 0.98 (95% confidence interval), which indicates the informativeness of the proposed forecasting method based on logistic regression (Graph 1).
Graph 1. Receiver operating characteristic curve. 
PRP preparation and method of injection
All patients in the treatment group received PRP during their surgical operations to improve the healing of postoperative soft tissue wounds in the maxillofacial area. Vacuum tubes (9–27 mL) were used for venous blood sampling. On average, one tube of 9 mL was required for wounds < 10 cm in length, two tubes for 10–20 cm wounds, and three tubes for large wounds (> 20 cm). The tubes filled with venous blood were centrifuged for 5 minutes at 3,000 rpm. Thereafter, two fractions of blood samples were visible in the tubes: an erythrocyte-leukocyte clot, and a layer of plasma enhanced with platelets. The lower third of the plasma layer contained 600,000 platelets, the middle of the layer 200,000 platelets, and the top of the layer 50,000 platelets per 1 μL. A syringe was used to take the lower third of the plasma layer, which was injected intradermally, 0.5 cm from the edge of the wound after suturing. Injections of autologous plasma (0.1–0.2 mL) were performed with a syringe using a 30G needle. The distance between injections was 1.5–2 cm. The remaining plasma was applied to a sterile gauze and put over the postoperative wound.
The treatment of patients in the control group was identical to that of patients in the treatment group in the postoperative period and included the following: daily dressings with antiseptic solutions, antibacterial therapy and administration of analgesics.
Evaluation methods
All patients in the treatment and control groups underwent planimetry to determine the width of postoperative scars 1, 3, 5, 7, 10, 30 and 90 days after surgery using a micrometer and Image J program. To record the width of wounds, photographs were taken using a Nikon camera (D5100, 50 mm lens).
The assessment of postoperative scars was carried out after 30 and 90 days by conducting a questionnaire that used the Patient and Observer Scar Assessment Scale (POSAS) with all patients and doctors. The POSAS questionnaire has 6 indicators using a 1–10 scoring scale, with 1 being normal skin and 10 being the least normal skin possible.
The Dermatological Quality of Life Index (DQLI) was used to determine and assess the negative impact of the results of treatment on various aspects of patients’ lives 30 and 90 days after surgery. DQLI has 10 questions, with up to 3 points for each question, thus allowing a minimum of 0 points and a maximum of 30 points. A higher score indicates the postoperative scars had a greater negative impact on the patient’s quality of life.
The results of histological and ultrasound examination, determination of interleukins in the postoperative wound were published earlier. This study is aimed only at assessing the esthetic component of the results of the use of PRP.
Statistical analysis
The statistical analysis was performed using the SPSS software package (IBM Corp., Released 2012, IBM SPSS Statistics for Windows, Version 21.0, Armonk, NY). The distribution of the parameters was tested using the Kolmogorov–Smirnov method. The variables between two groups were compared by the Mann–Whitney U test as the resultant distribution of parameters in two groups was not normal. The statistical data were presented as the mean with the standard error (SE) and the median with 25–75% limits. The difference of parameters with P value < 0.05 was set as statistically significant. The statistical analysis is performed in consultation with a certified biostatistician from the Department of Biostatistics (S. D. Asfendiyarov KazNMU, Almaty)
Results
Scars planimetry
Fifty patients in the treatment group and 50 patients in the control group underwent planimetry using a micrometer to determine the width and expansion of postoperative scars. There were no statistically significant differences in the width of postoperative wounds in the first day after the operation.
The median of postoperative wounds width in the control group was 2.0 mm (Р25 = 1.0; Р75 = 3.0) which is greater than the median width in the treatment group 1.0 mm (Р25 = 1.0; Р75 = 1.5) on 3.5 days after surgery (P < 0.05). On the 7th day after surgical procedure, the median widths of postoperative wounds were 2.0 mm (Р25 = 1.0; Р75 = 3.0) and 1,0 mm (Р25 = 1.0; Р75 = 2.0) in the control and treatment groups, respectively (P < 0.05).
The most noticeable changes were on the 10th and 30th days after operation. The scars of the patients in the control group (Fig. 1, 2) were distinguishable from the normal surrounding skin on the 10th and 30th days after operation as opposed to the treatment group patients (Fig. 3, 4), who received PRP injections. So, on the 10th day after surgery, the median scar widths were 1.0 mm (Р25 = 1.0; Р75 = 2.5) and 2.0 mm (Р25 = 1.0; Р75 = 3.0) in the treatment and control groups, respectively (P < 0.05). On the 30th day, this indicator was 3.0 mm (Р25 = 2.0; Р75 = 4.0) in the control group, which is greater than the median value of the treatment group – 1.5 mm (Р25 = 1.0; Р75 = 3.5) (Graph 2).
Fig. 1. Appearance of scars in patients of the control group on day 10 after surgery. 
Fig. 2. Appearance of scars in patients of the control group on day 30 after surgery. 
Fig. 3. Appearance of scars in patients of the treatment group on day 10 after surgery. 
Fig. 4. Appearance of scars in patients of the treatment group on day 30 after surgery. 
Graph 2. The expansion of postoperative scars (mm) at: A) 3 days; B) 5 days; C) 7 days; D) 10 days; E) 30 days after surgery. 
Using the Image J program, we measured the width of the scars in pixels on the 10th and 30th days. The median widths of the postoperative scar were 57.6 pixels (P25 = 44.0; P75 = 92.7) and 62.8 pixels (P25 = 46.7; P75 = 120.1) in the treatment and control groups on 10th day after surgery, respectively (P < 0.05). One month after the surgical procedure, the postoperative scar width in patients of the treatment group was Me = 80.1 pixels (P25 = 47.0; P75 = 113.4) which is less than the median of the scar width in patients of the control group Me = 99.3 pixels (P25 = 71.1; P75 = 130.4), P < 0.05 (Graph 3).
Graph 3. The expansion of postoperative scars (pixels) at: A) 10 and B) 30 days after surgery. 
Evaluation of scars
POSAS observer scale
We used the POSAS questionnaire 0 and 90 days after surgery to evaluate the quality of postoperative scars. The POSAS questionnaire (observer part) includes six indicators (vascularity, pigmentation, thickness, relief, pliability, surface area) which were assessed by physicians using a 1–10 scoring scale.
Thirty days after surgery, the mean score value of all six indicators of the scale was 5.8 ± 0.14 in the control group, which was about 2.3x greater than the treatment group mean. The mean value in the treatment group was 2.5 ± 0.14 (P < 0.05). Ninety days after surgery, the mean score value of the control group was 3.7 ± 0.23 and the mean value in the treatment group was 1.6 ± 0.07 (P < 0.05) (Tab. 3).
3. The mean observer POSAS scores in the control ad treatment groups 30 and 90 days after surgical procedures. 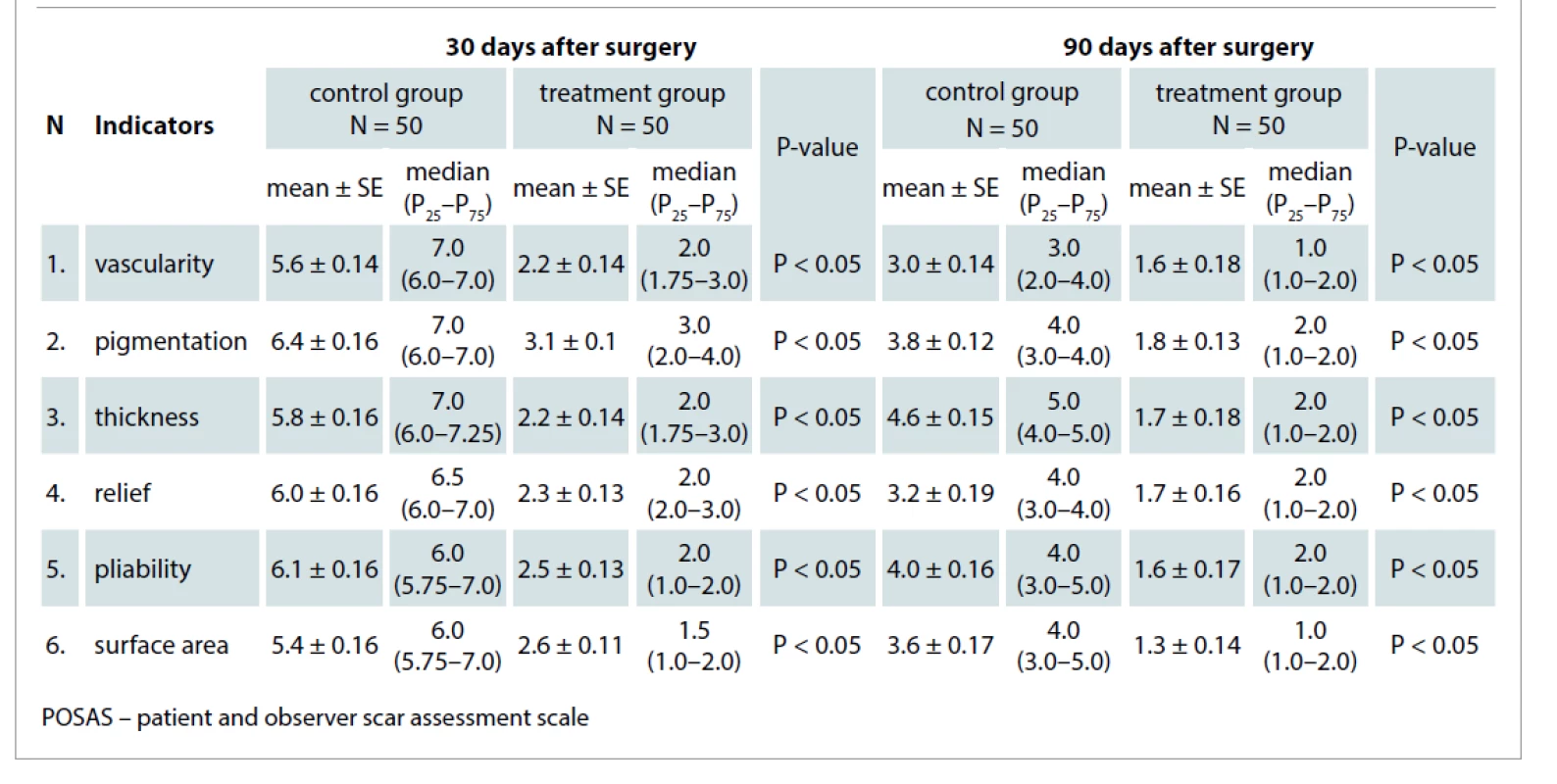
POSAS patient scale
The POSAS questionnaire (patient part) included six questions which were assessed by patients using a 1–10 scoring scale.
Thirty days after surgery, the mean patient POSAS score of the control group was 5.0 ± 0.75, which was about 1.9times greater than the treatment group mean. The mean value in the treatment group was 2.7 ± 0.35 (P < 0.05).
Ninety days after surgery, the mean score value of the control group was 2.7 ± 0.48, which was about 1.8times more than the treatment group mean. The mean value in the treatment group was 1.5 ± 0.14 (P < 0.05). The differences between the mean values in the two groups are displayed in Tab. 4.
4. The mean patient POSAS scores in the control and treatment groups 30 and 90 days after surgical procedures. 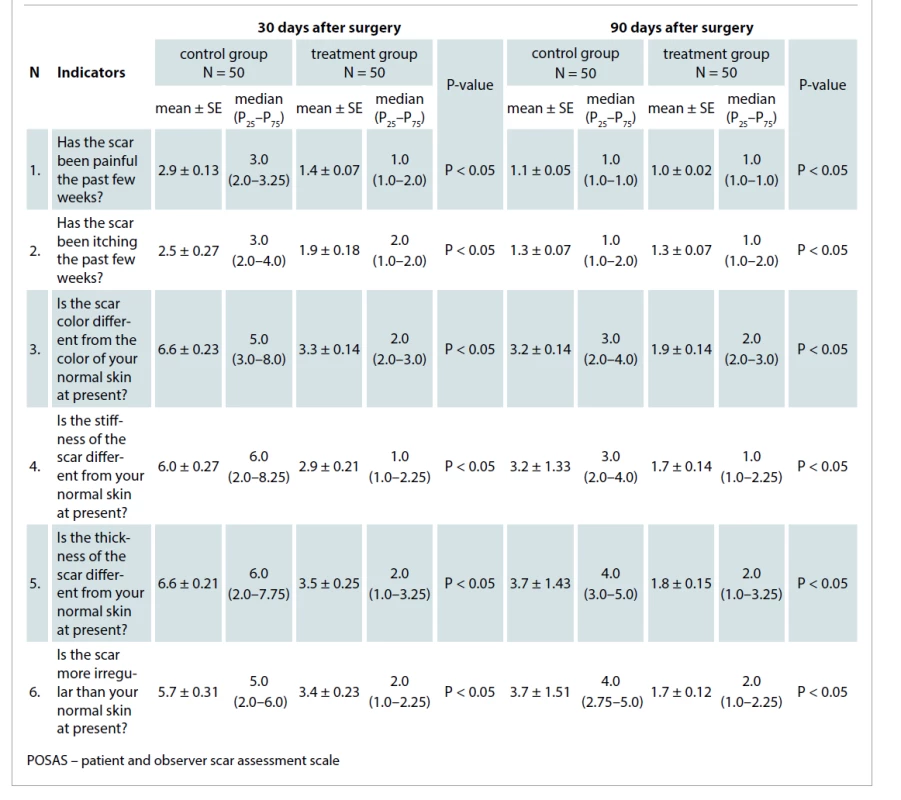
Results of the DQLI
Thirty days after surgical procedures, the mean score value in the control group was 12.7 ± 6.7, which was approx. 4times more than in the treatment group, i.e. 3.1 ± 4.25 (P < 0.05). According to the interpretation of DQLI in the control group, the postoperative outcomes of maxillofacial surgical procedures had a strong negative impact on the patients’ lives, while in the treatment group, these outcomes had a slight negative impact. Ninety days after surgery, the mean values of scores were 4.3 ± 2.91 and 1.7 ± 1.82 points in the control and treatment groups, respectively (P < 0.05). According to the interpretation in the control group, the treatment results had a slightly negative effect, while in the treatment group, they did not have a negative effect. The differences between the mean values of DQLI on the 30th and 90th days after surgery in the two groups are displayed in Graph 4.
Graph 4. The mean values of the dermatological quality of life index in patients of the control and the treatment groups at 1 and 3 months after surgical procedures. 
Discussion
The main results of the study are the 2-fold reduction of the scar width on the 90th day after surgery and higher patient satisfaction obtained from questionnaires.
There is a number of studies offering different speed and time of centrifugation to obtain PRP. The methods of preparation of PRP are different in many ways [36–39], which explains the lack of standardized methods of obtainment and application of PRP. The therapeutic effect of PRP could be achieved by increasing the concentration of platelets twice [40]. We were guided by the method of Akhmerov et al [41] in choosing the time and speed of rotation to PRP (specifically 5 min at a speed of 3,000 rpm). Regarding the choice of the frequency of injection of PRP several studies reported about multiplicity of PRP application [43–45] or injection [46] during or after surgery.
We used a single plasma injection, since we believe that a single plasma injection as a stimulator of regeneration is sufficient to start the process of normal wound healing. A single PRP injection has also been suggested in the studies of Eichler et al [47].
Considering the anatomical features of blood supply and innervation on the face and neck, we propose to inject PRP leaving 0.5 cm from the edge of the wound. We were guided by the regulation of the Republic of Kazakhstan (No. 666, November, 2009) on the procurement and processing of blood components, choosing the amount of blood taken from the patient. According to the recommendations of Akhmerov et al [41], in the treatment of various diseases (in such fields of medicine as surgery, traumatology, gynecology), it is necessary to inject 3–9 mL of PRP, depending on the clinical case. A total of 9 mL of blood was required to obtain 3 mL of PRP. In current study, one tube of 9 mL was required for wounds < 10 cm in length (3 mL of PRP), two tubes for 10–20 cm wounds (6 mL of PRP), and three tubes for wounds > 20 cm (9 mL of PRP).
The goal of PRP is to minimize wound complications and attain better esthetic outcomes. Previous studies have shown the efficiency of PRP in different wound healing processes, but few have provided an assessment of the influence of PRP on skin quality or assessed patient satisfaction with treatment results.
Several studies have evaluated the potential of platelet rich plasma to treat scar tissues. Willemsen et al reported that platelet rich plasma reduced recovery time and improved esthetic outcomes in facial rejuvenation [48]. They observed when the patients could return to work or restart their social activities after surgery. The authors conducted questionnaires about the appearance of 82 patients’ faces after 4 weeks. They used three questions with a scale 1–10 and surveyed only surgeons. Although they used a different scale than the one used in our study, the results were similar: both studies found that scarring was less pronounced in the treatment group relative to the control group. Our study also surveyed patients. We consider this a strength of our study because a surgeon’s perspective alone may not be objective, and because the patient perspective on their scarring is ultimately the most important in relation to patients’ quality of life.
Many studies evaluate the use of PRP in combination with other treatment methods. These studies have had very similar designs, often being presented in a couple of groups, where researchers compare results of treatment methods, one of which includes the use of PRP. Majani et al write about the treatment of patients with traumatic scars [49]. In this study which used a small sample size, and the Manchester Scar Scale, it was found that PRP was associated with better treatment results.
It was our aim to find studies not limited to only facial surgeries or dermatology, where authors describe the esthetic results after surgeries. The most extensive study in our search was made by Balbo et al, where a five-year analysis of the results of 115 patients with the amputations or wounds of fingers treated with platelet gel was presented [50]. The difference of this study from the study conducted at our hospital is the application of the platelet rich gel, not the injection of PRP directly into the soft tissues as it was done in our case. Balbo et al reported that the recovery of soft tissues of all patients ranged from 80 to 100% (median time 3 weeks) and the esthetic results were satisfactory in nearly all cases that were shown in numerous photos after surgeries. According to the article, patients who have undergone surgeries were satisfied with the results of the treatment afterwards, but these claims were not supported by objective quantitative data.
None of the studies found in the literature were directly comparable to our study in terms of design or methodology. However, all studies found similar results. A strength of our study was that we measured outcomes from different perspectives (both that of the patient and the surgeon) and triangulated results from different methods of measurement. We feel that this makes our study more objective than those conducted previously.
Conclusion
The current study demonstrates two findings: the first was that the use of PRP improves postoperative wound healing and results in better esthetic outcomes in the postoperative period; the second finding was that the patient satisfaction with the results and quality of life was higher in the treatment group where PRP was used.
Role of authors: Yuliya Menchisheva: originate concept and design of the study, operation of the patients, measurement of the scars, PRP preparation, acquisition, analysis and interpretation of the data, critical revision of the manuscript, crafting of the manuscript.
Ulmeken Mirzakulova: operation of the patients, analysis and interpretation of the data, crafting of the manuscript, statistical analysis.
Dildora Usupova: operation of the patients, measurement of scars of patients, data analysis.
Gulzhan Yermukhanova: review of the literature, critical revision of the manuscript, crafting of the manuscript.
Zhanagul Rysbayeva: review of the literature, crafting of the manuscript, statistical analysis.
Ethical considerations: All participating patients signed informed consent forms to be eligible for research. Ethics approval was obtained from the local ethics Committee. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Conflict of interest: We are declaring that no competing interests exist. We declare that we received no financial support for the research, authorship or publication of this article.
Yuliya Menchisheva, MD, PhD
S. D. Asfendiyarov Kazakh National Medical University
Tole bi 94
Almaty, 050000
Kazakhstan
e-mail: menchisheva.y@kaznmu.kz
Submitted: 30. 4. 2021
Accepted: 24. 7. 2021
Sources
1. Borrione P., Gianfrancesco AD., Pereira MT., et al. Platelet-rich plasma in muscle healing. Am J Phys Med Rehabil. 2010, 89(10): 854–861.
2. Chicharro-Alcántara D., Rubio-Zaragoza M., Damiá-Giménez E., et al. Platelet rich plasma: new insights for cutaneous wound healing management. J Funct Biomater. 2018, 9(1): 10.
3. Sclafani AP., Azzi J. Platelet preparations for use in facial rejuvenation and wound healing: a critical review of current literature. Aesth Plast Surg. 2015; 39(4): 495–505.
4. Hall MP., Band PA., Meislin RT., et al. Platelet-rich plasma: current concepts and application in sports medicine. J Am Acad Orthop Surg. 2009, 17(10): 602–608.
5. Li X., Xu C., Hou YL., et al. Are Platelet concentrates an ideal biomaterial for arthroscopic rotator cuff repair? A meta-analysis of randomized controlled trials. Arthroscopy 2014, 30(11): 1483–1490.
6. Sanchez M., Delgado D., Sґanchez P., et al. Platelet rich plasma and knee surgery. Biomed Res Int. 2014, 2014 : 890630.
7. Creaney L., Hamilton B. Growth factor delivery methods in the management of sports injuries: the state of play. Br J Sports Med. 2008, 42(5): 314–320.
8. Dawood AS., Salem HA. Current clinical applications of platelet-rich plasma in various gynecological disorders: An appraisal of theory and practice. Clin Exp Reprod Med. 2018, 45(2): 67–74.
9. Galal M., Khalifa A., Abd El Hafez M., et al. Platelet-rich plasma (PRP) in obstetrics and gynecology. Egypt J Hosp Med. 2021, 83(1): 889–894.
10. de Vos RJ., van Veldhoven PL., Moen MH., et al. Autologous growth factor injections in chronic tendinopathy: a systematic review. Br Med Bull. 2010, 95 : 63–77.
11. Ahmed M., Reffat SA., Hassan A., et al. Platelet-rich plasma for the treatment of clean diabetic foot ulcers. Ann Vasc Surg. 2017, 38 : 206–211.
12. Ji-Jun H., Hui-Hui S., Qing L., et al. Efficacy of using platelet-rich plasma in spinal fusion surgery-a preferred reporting items for systematic reviews and meta-analyses-compliant meta-analysis. World Neurosurg. 2020, 139: e517–e525.
13. Kaplan N. A study of platelet rich plasma commonly used in neurosurgery practice. Merit Res J Med Sci. 2019, 7(1): 1–7.
14. Ronci C., Ferraro AS., Lanti A., et al. Platelet-rich plasma as treatment for persistent ocular epithelial defects. Transfus Apher Sci. 2015; 52(3): 300–304.
15. Alio JL., Arnalich-Montiel F., Rodriguez AE. The role of ”eye platelet rich plasma” (E-PRP) for wound healing in ophthalmology. Curr Pharm Biotechnol. 2012, 13(7): 1257–1265.
16. Albanese A., Licata ME., Polizzi B., et al. Platelet-rich plasma (PRP) in dental and oral surgery: from the wound healing to bone regeneration. Immun Ageing. 2013, 10(1): 23.17. Carlson NE., Roach RB. Jr. Platelet-rich plasma: clinical applications in dentistry. J Am Dent Assoc. 2002, 133(10): 1383–1386.
18. Khatu SS., More YE., Gokhale NR., et al. Platelet-rich plasma in androgenic alopecia: myth or an effective tool. J Cutan Aesthet Surg. 2014, 7(2): 107–110.
19. Oryan A., Alidadi S., Moshiri A. Platelet-rich plasma for bone healing and regeneration. Expert Opin Biol Ther. 2016, 16(2): 213–232.
20. De Sousa A. Psychological issues in oral and maxillofacial reconstructive surgery. Br J Oral Maxillofac Surg. 2008, 46(8): 661–664.
21. De Sousa A. Psychological issues in acquired facial trauma. Indian J Plast Surg. 2010, 43(2): 200–205.
22. Gasparyan AY., Ayvazyan L., Pretorius E., et al. Platelets in rheumatic diseases: friend or foe? Curr Pharm Des. 2014, 20(4): 552–566.
23. Cole BJ., Seroyer ST., Filardo G., et al. Platelet-rich plasma: where are we now and where are we going? Sports Health. 2010, 2(3): 203–210.
24. Lacci KM., Dardik A. Platelet-rich plasma: support for its use in wound healing. Yale J Biol Med. 2010, 83(1): 1–9.
25. Chicharro-Alcántara D., Rubio-Zaragoza M., Damiá-Giménez E., et al. Platelet rich plasma: new insights for cutaneous wound healing management. J Funct Biomater. 2018, 9(1): 10.
26. Wang L., Gu Z., Gao C. Platelet-rich plasma for treating acute wounds: a meta-analysis. Zhonghua Yi Xue Za Zhi. 2014, 94(28): 2169–2174.
27. Prabhu R., Vijayakumar C., Bosco Chandra AA., et al. Efficacy of homologous, platelet-rich plasma dressing in chronic non-healing ulcers: an observational study. Cureus. 2018, 10(2): e2145.
28. Malay S., Chung KC. How to use outcomes questionnaires: pearls and pitfalls. Clin Plast Surg. 2013, 40(2): 261–269.
29. Rezaei F., Rezaei F., Abbasi H., et al. A comparison of doctor/patient satisfaction with aesthetic outcomes of rhinoplasty: a prospective study. J Med Life. 2019, 12(4): 374–380.
30. Fearmonti R., Bond J., Erdmann D., et al. A review of scar scales and scar measuring devices. Eplasty. 2010, 10: e43.
31. Commander SJ., Chamata E., Cox J., et al. Update on postsurgical scar management. Semin Plast Surg. 2016, 30(3): 122–128.
32. Marshall CD., Hu MS., Leavitt T., et al. Cutaneous scarring: basic science, current treatments, and future directions. Adv Wound Care (New Rochelle) 2018, 7(2): 29–45.
33. Garg S., Dahiya N., Gupta S. Surgical scar revision: an overview. J Cutan Aesthet Surg. 2014, 7(1): 3–13.
34. Jourdan M., Madfes DC., Lima E., et al. Skin care management for medical and aesthetic procedures to prevent scarring. Clin Cosmet Investig Dermatol. 2019, 12 : 799–804.
35. Moher D., Schulz KF., Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Ann Intern Med. 2001, 134(8): 657–662.
36. Breddin HK. Can platelet aggregometry be standardized? Platelets 2005, 16(3–4): 151–158.
37. Araki J., Jona M., Eto H., et al. Optimized preparation method of platelet-concentrated plasma and noncoagulating platelet-derived factor concentrates: maximization of platelet concentration and removal of fibrinogen. Tissue Eng Part C Methods. 2012, 18(3): 176–185.
38. Dugrillon A., Eichler H., Kern S., et al. Autologous concentrated platelet-rich plasma (cPRP) for local application in bone regeneration. Int J Oral Maxillofac Surg. 2002, 31(6): 615–619.
39. Franco D., Franco T., Schettino AM., et al. Protocol for obtaining platelet-rich plasma (PRP), platelet-poor plasma (PPP), and thrombin for autologous use. Aesth Plast Surg. 2012, 36(5): 1254–1259.
40. Weibrich G., Hansen T., Kleis W., et al. Effect of platelet concentration in platelet-rich plasma on periimplant bone regeneration. Bone. 2004, 34(4): 665–671.
41. Akhmerov RR., Korotkova OI., Ovechkina MV., et al. Use of the autoplazma containing thrombocytes in a dermatokosmetologiya and an odontology. Plasmolifting™ technology. Plastic surgery and cosmetology. Russian Journal (Plasticheskaya khirurgiya i kosmetologiya) 2013, 1 : 94.
42. Hom DB., Linzie BM., Huang TC. The healing effects of autologous platelet gel on acute human skin wounds. Arch Facial Plast Surg. 2007, 9(3): 174–183.
43. Khalafi RS., Bradford DW., Wilson MG. Topical application of autologous blood products during surgical closure following a coronary artery bypass graft. Eur J Cardiothorac Surg. 2008, 34(2): 360–364.
44. Kazakos K., Lyras DN., Verettas D., et al. The use of autologous PRP gel as an aid in the management of acute trauma wounds. Injury 2009, 40(8): 801–805.
45. Yoo J., Roth K., Hughes B., et al. Evaluation of postoperative drainage with application of platelet-rich and platelet-poor plasma following hemithyroidectomy: a randomized controlled clinical trial. Head Neck. 2008, 30(12): 1552–1558.
46. Guo Y., Qiu J., Zhang C. Follow-up study on platelet-rich plasma in repairing chronic wound nonunion of lower limbs in 47 cases. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2008, 22(11): 1301–1305.
47. Eichler C., Najafpour M., Sauerwald A., et al. Platelet-rich plasma in the treatment of subcutaneous venous access device scars: a head-to-head patient survey. Biomed Res Int. 2015, 2015 : 630601.
48. Willemsen JC., van der Lei B., Vermeulen KM., et al. The effects of platelet-rich plasma on recovery time and aesthetic outcome in facial rejuvenation: preliminary retrospective observations. Aesthetic Plastic Surg. 2014, 38(5): 1057–1063.
49. Majani U., Majani A. Correction of scars by autologous fat graft and platelet rich plasma (PRP). Acta Medica Mediterranea 2012, 28(2): 99–100.
50. Balbo R., Avonto I., Marenchino D., et al. Platelet gel for the treatment of traumatic loss of finger substance. Blood Transfus. 2010, 8(4): 255–259.
Labels
Plastic surgery Orthopaedics Burns medicine Traumatology
Article was published inActa chirurgiae plasticae

2021 Issue 3-
All articles in this issue
- Reconstructive and esthetic breast surgery after solid organ transplantation – a systematic review, proposal of a novel protocol and case presentations
- Characteristics of fingertip injuries and proposal of a treatment algorithm from a hand surgery referral center in Mexico City
- Platelet-rich plasma improves esthetic postoperative outcomes of maxillofacial surgical procedures
- Breast implant-associated anaplastic large-cell lymphoma – an evolution through the decades: citation analysis of the top fifty most cited articles
- Bone invasion by oral squamous cell carcinoma
- Three-dimensional navigation in maxillofacial surgery – the way to minimize surgical stress and improve accuracy in fibula free flap and Eagle’s syndrome surgical procedures
- Is it a second scrotum?
- Professor Radana Königová Prize awards
- Editorial
- Fournier’s gangrene secondary to male’s circumcision – a case report and review of the literature
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Bone invasion by oral squamous cell carcinoma
- Platelet-rich plasma improves esthetic postoperative outcomes of maxillofacial surgical procedures
- Breast implant-associated anaplastic large-cell lymphoma – an evolution through the decades: citation analysis of the top fifty most cited articles
- Characteristics of fingertip injuries and proposal of a treatment algorithm from a hand surgery referral center in Mexico City
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career


