-
Medical journals
- Career
Pedicled pectoralis major flap in head and neck reconstruction - technique and overview
Authors: Z. Dvořák 1,2,3; R. Pink 1,4; P. Michl 1,4; P. Heinz 1,4; P. Tvrdý 4,4
Authors‘ workplace: Department of Oral and Maxillofacial Surgery, University Hospital Olomouc, Czech Republic 1; Department of Plastic and Aesthetic Surgery, St. Anne`s University Hospital, Brno, Czech Republic 2; Faculty of Medicine, Masaryk University, Brno, Czech Republic 3; Faculty of Medicine, Palacky University, Olomouc, Czech Republic 4
Published in: ACTA CHIRURGIAE PLASTICAE, 60, 1, 2018, pp. 14-21
INTRODUCTION
For over 30 years, since the description by Ariyan (1,2), the pedicled pectoralis major (PPM) flap was the “workhorse” flap for head and neck cancer reconstruction (3). It was gradually replaced by versatile free-flaps with success rates of 94%–98%, fewer complications, better overall functional outcome (4–11) and minimal morbidity (12,13). Current trends in free-style perforator flaps have further improved cosmetic and functional results and decreased donor site morbidity (14–16). One disadvantage of this technique is however longer surgical procedure with higher risk of complications due to prolonged anesthesia (12,17). In developing countries, PPM flap is the flap of first choice (18,19) with large numbers of patients referred and with a success rate of 98%–100% (20–22). In developed countries, the PPM flap is not often the flap of first choice (23–25).
ADVANTAGES AND DISADVANTAGES
PPM flap technique advantages include relatively quick and easy elevation of the flap, reliability and versatility in terms of sufficient mass, span of transposed tissue and a relatively good reach to oropharynx (24,25,20,21,23). Compared to free flaps, the operation time using PPM flap is reduced because there is no need for microvascular anastomosis. A team of two surgeons can perform the procedure (as well as for free flap technique). The flap elevation itself is faster and the only prolongation may be construction of a cervical tunnel. It can be concluded, that for the team of surgeons trained in microsurgery, the overall reconstruction time using PPM flap will be shorter than the time of the same trained team using free flap technique (11,26–28). Shortening the procedure reduces the incidence of medical and surgical complications (29), and the absence of microvascular anastomosis decreases the risk of flap failure due to vascular problems. These factors should be taken into account especially for immunocompromised patients (30).
Based on empirical findings, the inpatient stay duration after PPM flap surgery is usually 14 days (31), which is the same as for free flap technique (28,32), though longer (26,33) and shorter (9,25) times are reported. Assuming an experienced microsurgical team and possibility of rapid surgical revision, the reliability of pedicled flaps and free flaps is comparable and ranges between 93% and 98%.
The greatest cos ts of free flap reconstruction technique are related to the creation of a microsurgical team and implementation of the new technique. The costs go beyond instruments and material costs such as purchase of surgical microscope, micro instrumentation, suturing material, clips and other equipment needed for intensive care units (28,34–38). They also include high initial microsurgery team training costs.
The disadvantages of the PPM flap technique results from the general characteristics of pedicled flaps: limited arc of flap rotation precluding use in reconstruction of soft palate and maxilla defects; the reach of the flap is externally to the level of the zygomatic arch and inside to the superior tonsillar field (9).
Other drawbacks of PPM flap include formation of a contacting band and tissue excess at the reconstruction site (39). This can be successfully mitigated (even after radiotherapy), however, by cutting the vascular pedicle without compromising flap viability (40) and muscle twitching can be eliminated using botulinum toxin (41).
Another drawback can be considered the greater mass of the PPM flap that cannot be compared to thin and pliable free flaps (26). For this reason, the PPM flap is unsuitable for reconstruction of superficial intraoral defects. It is suitable, however, for cases that require a large volume of tissue, e.g. major resection of tongue and floor of the oral cavity. In case of superficial defects, the use of PPM flap reduces the quality of speech and swallowing, compared to the thin and pliable free flap (8,42). Moreover, secondary thinning of the PPM flap is complicated by fibrotic changes and impaired tissue viability after radiotherapy, which frequently follows the surgical procedure.
Most PPM flap comparison studies published to date have been based on the Chinese flap (9). This is relatively hairless and the abundance of hair was often cited as one of the disadvantages of the PPM flap. Currently, the anterolateral thigh flap (ALTF) is considered the “workhorse” flap for oropharyngeal defects, especially in Asian publications (4,7,13). Measured by its “hairiness”, it is comparable to the PPM flap and the hairiness of the flap in the mouth diminishes after radiotherapy and can be further reduced using laser depilation.
PPM flap achieves similar results compared to the use of free tissue transfer of gracilis muscle (39). The extended vertical lower trapezius island myocutaneous flap (TIMF) in comparison to PPM flap has greater arc of rotation, larger skin island and fewer small defects but it is necessary to rotate the patient and it is also more bulky (43). Different texture and color of the skin is also considered a disadvantage of PPM flap, but these characteristics are similar for all types of flaps that are harvested from the body below the clavicle (including free flaps).
The most significant morbidity after the harvesting of PPM flap occurs at the donor site. In some cases the following complications may occur:
- disfigurement of the front of the chest with the disruption of typical contour of anterior axillary fold
- depression at the muscle harvesting site
- asymmetrical shift of nipple-areola complex
- extensive scars (endoscopically assisted elevation is used to reduce the incidence of scars of PPM flap) (44).
Limited range of neck motion may occur after radical neck dissection. “Frozen shoulder” and debilitating shoulder dysfunction may occur if the accesorius nerve was interrupted (45,46). These symptoms do not occur in cases of selective neck dissection where the PPM flap elevation increases the morbidity and dysfunction of the shoulder (47) – paradoxically, patients who undergo radical or modified radical neck dissection suffer less shoulder dysfunction, if PPM flap procedure has been used (48). Elevation of the PPM flap reduces shoulder and neck function in all cases (49). The shoulder movement restriction or reduced functional capability can be assessed only by prospective studies using indices such as shoulder strength and range of motion (ROM), neck ROM and subjective quality of life assessment using the shoulder pain and disability index (SPAD) and neck disability index (NDI) (50). PPM flap reconstruction has a small but significant negative effect on upper extremity dysfunction and also neck ROM limitations (48–50). In general, we can say that patients, who undergo primary reconstruction using PPM flap, often have significantly reduced working capacity, mainly due to preoperative polymorbidity.
Patients with oropharyngeal cancer who underwent radical surgical procedure followed by the reconstruction using PPM flap technique displayed comparable overall morbidity to patients using a free flap technique. However, patients who undergo free flap reconstruction show better results for speech and shoulder function. They also experience better “mood” than patients who undergo PPM flap reconstruction (51).
Advantages and disadvantages of PPM are summarized in Table 1.
1. Advantages, disadvantages, indications and contraindications of PPM fl ap 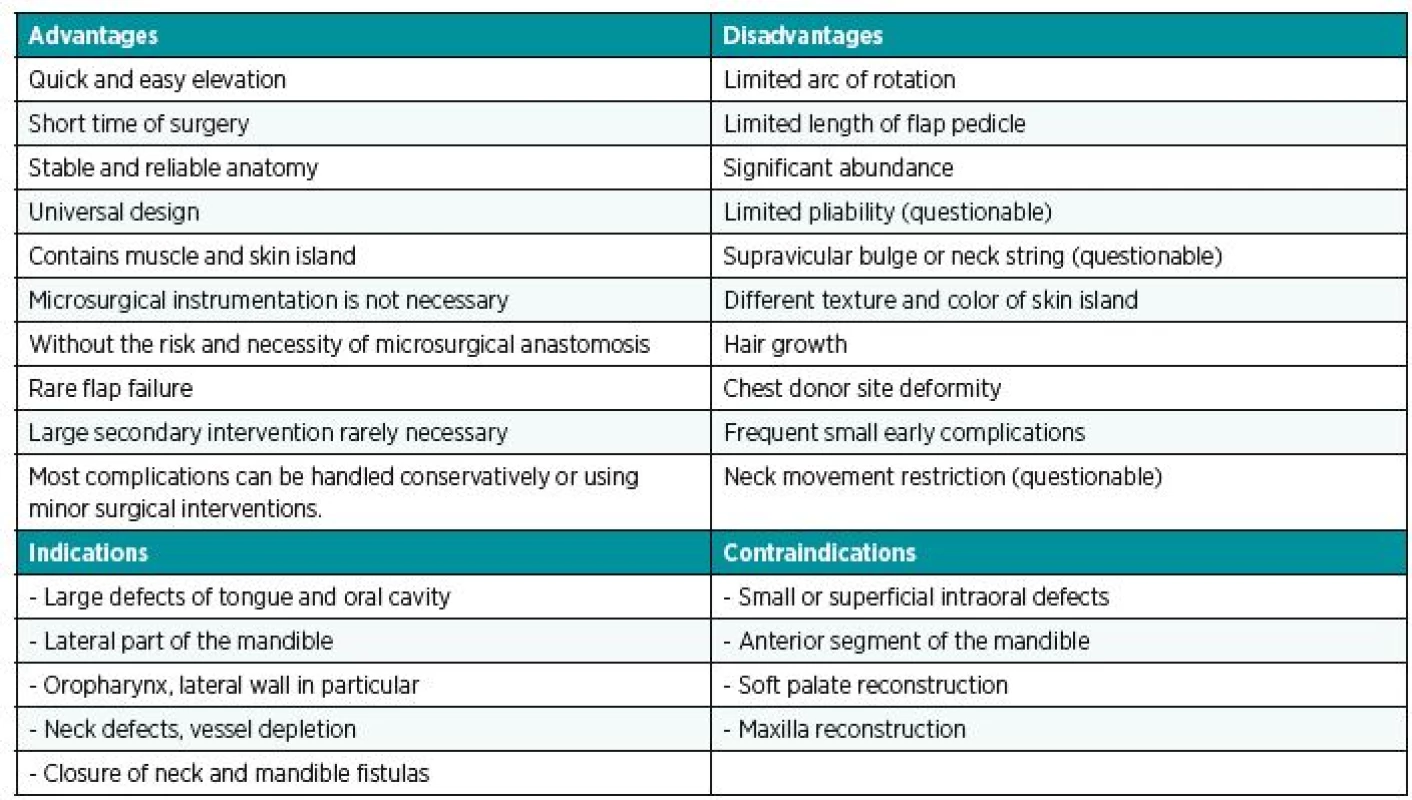
COMPLICATIONS
The number of complications depends primarily on:
- the type of procedure (primary or salvage procedure)
- location of reconstruction
- patient morbidity
- nutritional status
- previous radiotherapy
- duration of surgery
- experience of the surgeon
The rate of complications increases significantly if the flap is raised with a rib (52) or if two skin islands are harvested (22,34). From this point of view, these procedures can be considered obsolete and it is better to replace them with a combination of PPM flap and free flap, which is associated with lower complication rate.
According to the literature, the incidence of total flap failure ranges from 0% to 8% (34,37,38,51). In reports after 2009, flap losses are reported from 0% to 2.5% (18,19,24,53–56). The major partial skin flap loss varies from 1% to 7%, minor losses range from 13% to 29%, the incidence of orocutaneous fistulae ranges from 7%–29% and the incidence of infections is about 8% –39% (9,21,24,33,34,57–60). The overall incidence of any complication is fairly high and ranges from 16% to 68% (20–23,34,37,53,56–59,61,62). This is higher than for free flaps. However, most of the complications are minor (33) and handled using a conservative wound management technique. The need for a secondary surgical correction is 2%–12% (9,33,55,57), however, there are also studies reporting higher rates of major complications (53,61).
Complications at the donor sites are reported in approximately 5% to 8% (9,23,55,63–65). Because most patients are polymorbid, there is a higher risk of hematoma (23,34) that can be exacerbated by concomitant hepatic dysfunction.
CURRENT INDICATIONS FOR PPM
Avery (66) reported the frequency of PPM flap use for primary reconstruction in the range of 5%–62%. In the US, the frequency was reported at around 5% (25). However, a review of the US Academic Otolaryngology program revealed that the PPM flap was used two to three times more often than free flaps (67). PPM flap is always considered only as a second choice after free flaps and the reasons for their primary use varies. The most common indications for primary use of PPM are financial, associated comorbities, extended radical neck dissection, vessel depletion in the neck and previous different malignancies (9,17,32,36,37).
The other reasons for PPM flap are the area for reconstruction (lateral part of the mandible, parotidectomy) in order to reduce the donor site morbidity after bone harvesting and also the greater success in reconstructions in polymorbid patients (66,68).
Another preferred choice is the use of PPM in combination with a free flap (23,25,34), rather than using the combination of two free flaps that increases the risk of reconstruction failure. This is suitable only for lateral mandible defects and in patients after extensive neck dissection, especially if radiotherapy is planned.
More frequent use of PPM flap is reported in salvage reconstruction following complications (25), but in these scenarios it is necessary to take into account the increased risk of complications (compared to primary reconstruction). In this case, the main indications include pharyngeal fistula (69,70) and exposure of great vessels (71). Other indications may be orocutaneous fistula, obliteration of dead space in the neck (38), osteoradionecrosis, exposure of hardware or wound breakdown.
PPM flap is also employed as a salvage reconstruction following free flap failure (24), especially for large composite defects, in comorbidities, in malnutrition, wound breakdown, infection, lack of blood vessels in the neck and in psychological causes. On the other hand, Wei and other authors recommend another free flap for its similar risk of failure as PPM flap for salvage reconstruction after free flap failure (8,10,72). This procedure is performed at some centers in developed countries but in some cases the success of secondary free flap reconstruction has been reported as only 73% (73). Very high success rates of reconstruction using PPM flap is confirmed in other studies although partial skin flap loss may occur more frequently (4,34,59).
Last but not least, salvage reconstruction in prevailing or recurrent primary disease is also a typical indication for PPM flap (74). In these patients, the PPM remains twice as frequently used as free flap reconstruction (66). An overview of indications is summarised in Table 2.
2. Summary of PPM flap indications for head and neck reconstructions 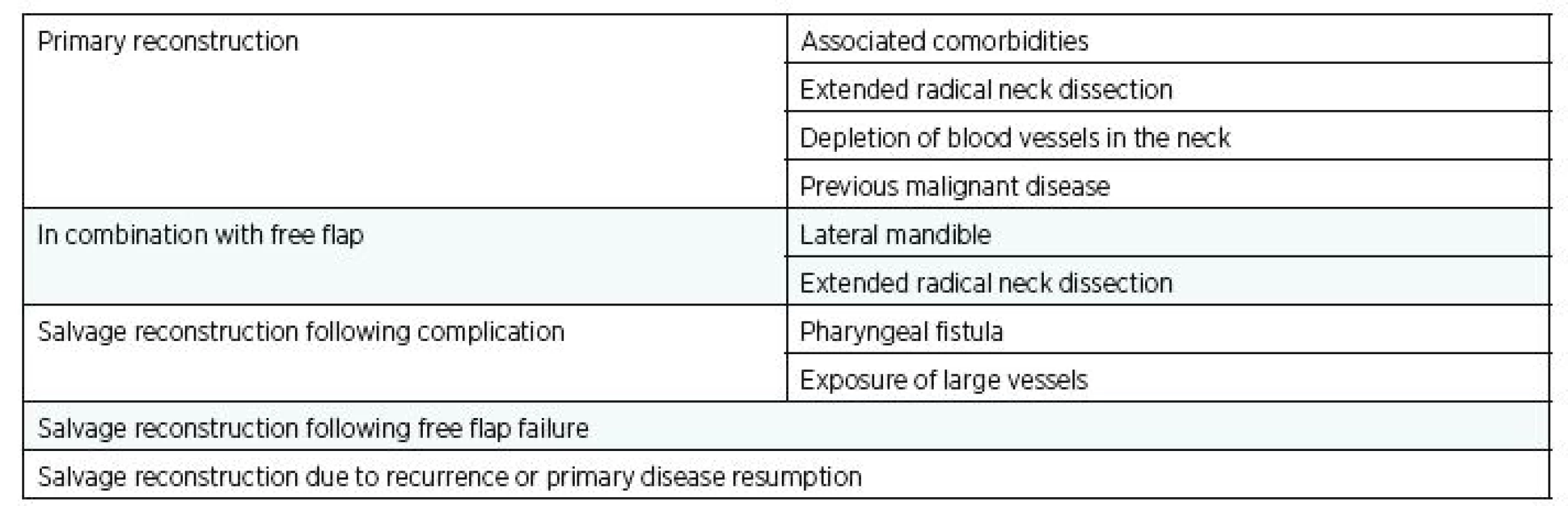
FLAP ELEVATION TECHNIQUES
The flap can be elevated as muscular, musculocutaneous or osteomyocutaneous (including the fifth rib into the flap) (52). Planning of the position and size of the flap is best done on a standing patient before surgery. The position of the lower border of the pectoralis major muscle can be marked when the patient is standing with hands on hips and muscles clenched. It is also advisable to mark the direction of the dominant vascular pedicle. The line from the shoulder tip to the xiphoid determines the direction of the lower part of the flap and position of the thoracoacromial artery (2). The upper part of the flap and start of the pedicle can be marked by plotting the perpendicular from the middle of the clavicle to xiphoid acromial-line (75). The lateral thoracic artery can be found using Doppler along a line extending from the apex of the axilla to the iliac crest (76).
The best way to design the skin island of the PPM flap for oropharyngeal localisation of defects is medially below the nipple-areola complex and above the pectoralis major muscle (22). Extension of the skin island over the rectus sheath should be avoided – especially in the case of female patients with ptotic breast and the skin island placed submammary (55). When only a small skin island is needed, it is better to harvest a large one and de-epithelise redundant areas (77). Preoperative planning is shown in Figure 1.
1. Preoperative planning: green color - the line from the shoulder tip to xiphoid and its perpendicular from middle of the clavicle showing expected position of pectoral branches of thoracoacromial artery; blue color – skin island design; dashed blue line – muscle dissection design 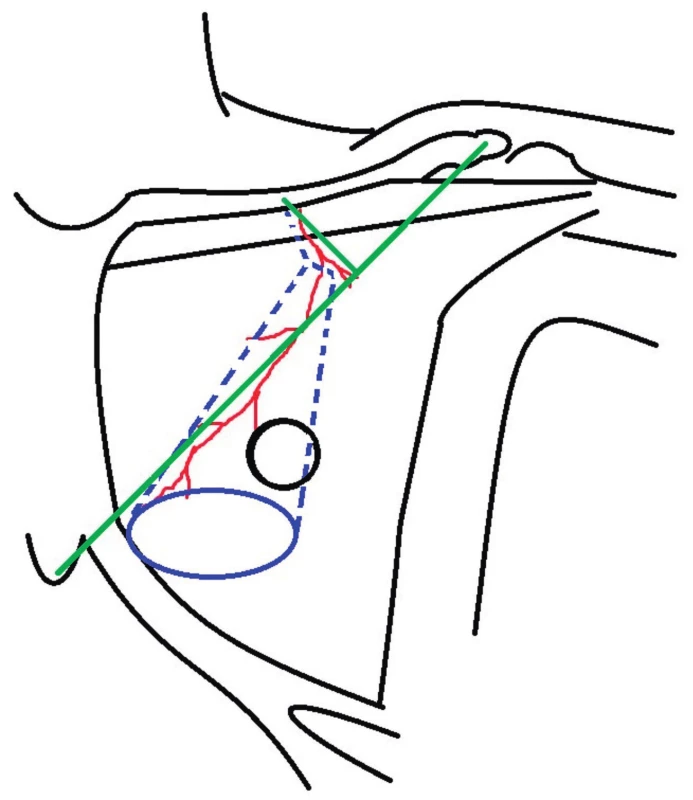
Raising the flap begins with incision of the lower portion of the skin paddle. The incision continues towards the projection of the beginning of the vascular bundle or towards the axilla (if it is planned to spare the deltopectoral flap) (78). After exposing the lateral part of the sternocostal portion of the major pectoral muscle, in a lateromedial direction, the lower portion of the major pectoral muscle is dissected from the chest wall. After the exposure of the medial portion of the muscle, we proceed with the elevation of a muscle strip with the skin island. Dissection from the thorax is done bluntly using fingers in the direction towards the clavicle. Underlying nutritional vessels may be visible on the upper half of the muscle. In case the lateral thoracic artery is visible, it is better to include it together with the pectoral minor muscle into the flap or dissect this muscle in the proximity of the acromion to enable release and transposition of the blood vessel (79,80), not to restrict the arc of flap rotation and disrupt the dominant nutrition of the laterocaudal part of the flap (81). When the pedicle is visible and with thoracoacromial vessels at the base of pectoral major muscle, we continue with the reduction of muscle tissue around the vascular pedicle until a thin muscle strip ending at the border of the clavicular and sternocostal portion of the muscle is left. In this part, the vascular pedicle is safely under the muscle tissue and clavicular portion of the muscle does not have to be included in the flap (Fig. 2, Fig. 3), (2,82). It is simply severed (34), not to limit the arc of the rotation of the pedicle around the clavicle. Afterwards, the pedicle is dissected, branches of lateral pectoral nerve are identified and tested through neurostimulation and cut off to a length of 3–4 cm to achieve denervation of the muscle. These two maneuvers (cut through the portion of the muscle and denervation) mitigate the occurrence of muscle abundance above the clavicle and contracture band on the lateral side of the neck. Following creation of a subcutaneous tunnel, blunt dissection above the clavicle towards the site of neck dissection, prepares the route for flap transportation to the site of the defect. Smaller flaps can be transposed subclaviculary without damaging the subclavian vessel (21,83). Flap donor site on the chest is closed directly (Fig. 4) or with a lateral thoracic flap. (76,84).
3. Elevated PPM flap with the pedicle reduced only to the vessels 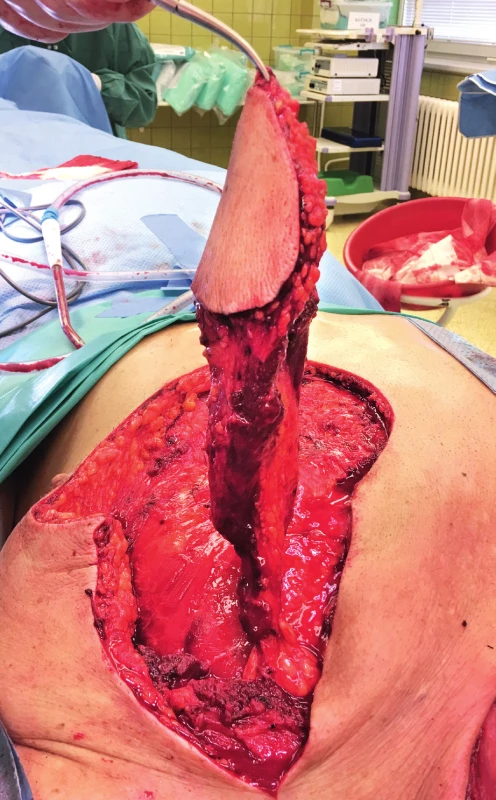
4. The immediate postoperative result 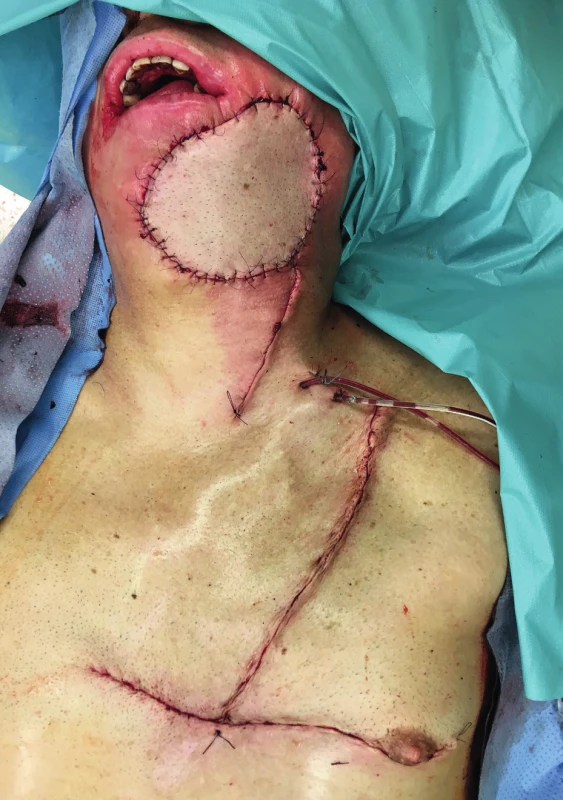
DENERVATION AND FLAP TRANSPOSITION
A number of surgeons perform transection of the lateral pectoral nerve, which has a similar course to the pectoral branches of the thoracoacromial trunk and contributes to the innervation of the pectoral muscle (20). The bulges or contracting folds in place of the vascular pedicle are often reported in the literature (based on assumptions that are best described for cases of transposition of the latissimus dorsi muscle for breast reconstruction). Flap volume changes have been observed in this field of surgery interventions thanks to complete trans-sectioning of the muscle tendon from its insertions and release of both ends of the muscle regardless of the denervation of the muscle (85). This finding is supported by Kääriäinen et al. and Szychta et al. who found no difference in volume regardless whether the thoracodorsal nerve was transected or not (86,87). Furthermore, in one prospective study, Szychta et al. showed that patients with latissimus dorsi muscle denervation achieved lower scores for pain, animation of reconstructed breast and also scored higher on overall satisfaction with their reconstructed breasts (87). Nerve resection is performed in a length of 3–4 cm based on a study by Schroegendorfer et al., who observed twitching in a sample of 74 patients undergoing latissimus dorsi breast reconstruction. At 12 and 24-month follow-up, all patients with an intact thoracodorsal nerve showed twitching of the muscle. However, only 50% (67.9% after 24 months) of the denervated patients showed twitching. No patient had twitching at 12 or 24 months postoperatively if more than 4 cm of the nerve was excised and the length of nerve resection was predictive of twitching. (88). Another factor that is fundamentally involved in any supraclavicular bulge is strict disconnection of the transferred muscle flap and rotation around the clavicle only on the vascular bundle (2,82).
Kanno et al. reported (79) that preservation of the lateral thoracic artery and use of the subclavian route are alternatives to ensure sufficient blood supply and an increased rotation arc of PPM flap. This approach enables harvesting of the flap that can reach the entire oral cavity, including the infraorbital region, palate, middle pterygopalatine fossa and nasopharynx, with no risk of vascular insufficiency, to the distal skin island. Greater reach of the flap has not been confirmed by anatomical studies (89). According to literature resources, most authors did not use the subclavian route.
CONCLUSION
PPM flap cannot be considered obsolete or a secondary choice for reconstruction of the head and neck in developed countries. Currently the “renaissance” of pedicled flaps with improvement in flap elevation techniques means that PPM flap may be optimal for: specific oropharyngeal defects, even primary resections, especially for less cooperative patients and patients with extensive neck dissection, extensive resection of cervical lymph nodes, free flap failure, depleted blood vessels in the neck, combined with free flap and in case of disease recurrence or progression. PPM flap remains a valid reconstruction tool that should be included in the armamentarium of surgeons involved in reconstruction of the head and neck, especially in the elderly and thus polymorbid population.
Acknowledgements: This research received no funding from public agencies, commercial, or non-profit sectors.
Conflict of interest statement: The authors state that there are no conflicts of interest regarding the publication of this article.
Corresponding author:
Zdeněk Dvořák, M.D., Ph.D.
Department of Plastic and Aesthetic Surgery,
St. Anne’s University Hospital,
Berkova 34, 612 00 Brno, Czech Republic
E-mail: zdenek.dvorak@fnusa.cz
Sources
1. Ariyan S. The pectoralis major myocutaneous flap. A versatile flap for reconstruction in the head and neck. Plast Reconstr Surg. 1979 Jan;63(1):73-81.
2. Ariyan S. Further experiences with the pectoralis major myocutaneous flap for the immediate repair of defects from excisions of head and neck cancers. Plast Reconstr Surg. 1979 Nov;64(5):605-12.
3. Ariyan S. The functional pectoralis major musculocutaneous island flap for head and neck reconstruction. Plast Reconstr Surg. 1990 Oct;86(4):807-8.
4. Kucur C, Durmus K, Uysal I, et al. Management of complications and compromised free flaps following major head and neck surgery. Eur Arch Otorhinolaryngol. 2016 Jan;273(1):209-13.
5. Costa H, Zenha H, Sequeira H, et al. Microsurgical reconstruction of the maxilla: Algorithm and concepts. J Plast Reconstr Aesthet Surg. 2015 May;68(5):e89-e104.
6. Nouraei SA, Middleton SE, Hudovsky A, et al. Role of reconstructive surgery in the management of head and neck cancer: a national outcomes analysis of 11,841 reconstructions. J Plast Reconstr Aesthet Surg. 2015 Apr;68(4):469-78.
7. Thiele OC, Seeberger R, Engel M, Freier K, Hoffmann J. Development of the clinical use of distant flaps for head and neck reconstruction. J Craniomaxillofac Surg. 2014 Jan;42(1):79-83.
8. Corbitt C, Skoracki RJ, Yu P, Hanasono MM. Free flap failure in head and neck reconstruction. Head Neck. 2014 Oct;36(10):1440–5.
9. O’Neill JP, Shine N, Eadie PA, Beausang E, Timon C. Free tissue transfer versus pedicled flap reconstruction of head and neck malignancy defects. Ir J Med Sci. 2010 Sep;179(3):337–43.
10. Germain MA, Hartl DM, Marandas P, Juliéron M, Demers G. Free flap reconstruction in the treatment of tumors involving the hard palate. Eur J Surg Oncol. 2006 Apr;32(3):335-9.
11. McCrory AL, Magnuson JS. Free tissue transfer versus pedicled flap in head and neck reconstruction. Laryngoscope. 2002 Dec;112(12):2161-5.
12. Eckardt A, Fokas K. Microsurgical reconstruction in the head and neck region: an 18-year experience with 500 consecutive cases. J Craniomaxillofac Surg. 2003 Aug;31(4):197-201.
13. Zhang C, Sun J, Zhu H, et al. Microsurgical free flap reconstructions of the head and neck region: Shanghai experience of 34 years and 4640 flaps. Int J Oral Maxillofac Surg. 2015 Jun;44(6):675-84.
14. Cho A, Hall FT. Review of perforator flaps in head and neck cancer surgery. Curr Opin Otolaryngol Head Neck Surg. 2016 Oct;24(5):440-6.
15. Lyons AJ. Perforator flaps in head and neck surgery. Int J Oral Maxillofac Surg. 2006 Mar;35(3):199-207.
16. Bravo FG, Schwarze HP. Free-style local perforator flaps: concept and classification system. J Plast Reconstr Aesthet Surg. 2009 May;62(5):602-8; discussion 609.
17. Singh B, Cordeiro PG, Santamaria E, Shaha AR, Pfister DG, Shah JP. Factors associated with complications in microvascular reconstruction of head and neck defects. Plast Reconstr Surg. 1999 Feb;103(2):403-11.
18. Gadre KS, Gadre P, Sane VD, Halli R, Doshi P, Modi S. Pectoralis major myocutaneous flap--still a workhorse for maxillofacial reconstruction in developing countries. J Oral Maxillofac Surg. 2013 Nov;71(11):2005.e1-2005.e10.
19. Metgudmath RB, Metgudmath AR, Metgudmath VV, Roy B, Das AT. Versatility of pectoralis major myocutaneous flap in oncosurgery and its role in developing countries. Indian J Otolaryngol Head Neck Surg. 2013 Jul;65(Suppl 1):80-4.
20. Milenović A, Virag M, Uglesić V, Aljinović-Ratković N. The pectoralis major flap in head and neck reconstruction: first 500 patients. J Craniomaxillofac Surg. 2006 Sep;34(6):340-3.
21. Vartanian JG, Carvalho AL, Carvalho SM, Mizobe L, Magrin J, Kowalski LP. Pectoralis major and other myofascial/myocutaneous flaps in head and neck cancer reconstruction: experience with 437 cases at a single institution. Head Neck. 2004 Dec;26(12):1018-23.
22. Bhola N, Jadhav A, Borle R, Khemka G, Kumar S, Shrivastava H. Is there still a role for bilobed/bipaddled pectoralis major myocutaneous flap for single-stage immediate reconstruction of post ablative oncologic full-thickness defects of the cheek? Oral Maxillofac Surg. 2015 Jun;19(2):125-31.
23. Liu R, Gullane P, Brown D, Irish J. Pectoralis major myocutaneous pedicled flap in head and neck reconstruction: retrospective review of indications and results in 244 consecutive cases at the Toronto General Hospital. J Otolaryngol. 2001 Feb;30(1):34-40.
24. Avery CM, Gandhi N, Peel D, Neal CP. Indications and outcomes for 100 patients managed with a pectoralis major flap within a UK maxillofacial unit. Int J Oral Maxillofac Surg. 2014 May;43(5):546-54.
25. Schneider DS, Wu V, Wax MK. Indications for pedicled pectoralis major flap in a free tissue transfer practice. Head Neck. 2012 Aug;34(8):1106-10.
26. Mallet Y, El Bedoui S, Penel N, Ton Van J, Fournier C, Lefebvre JL. The free vascularized flap and the pectoralis major pedicled flap options: comparative results of reconstruction of the tongue. Oral Oncol. 2009 Dec;45(12):1028-31.
27. Smeele LE, Goldstein D, Tsai V, et al. Morbidity and cost differences between free flap reconstruction and pedicled flap reconstruction in oral and oropharyngeal cancer: Matched control study. J Otolaryngol. 2006 Apr;35(2):102-7.
28. Tsue TT, Desyatnikova SS, Deleyiannis FW, et al. Comparison of cost and function in reconstruction of the posterior oral cavity and oropharynx. Free vs pedicled soft tissue transfer. Arch Otolaryngol Head Neck Surg. 1997 Jul;123(7):731-7.
29. Ferrier MB, Spuesens EB, Le Cessie S, Baatenburg de Jong RJ. Comorbidity as a major risk factor for mortality and complications in head and neck surgery. Arch Otolaryngol Head Neck Surg. 2005 Jan;131(1):27–32.
30. Gerude MF, Dias FL, de Farias TP, Albuquerque Sousa B, Thuler LC. Predictors of postoperative complications, prolonged length of hospital stay, and short-term mortality in elderly patients with malignant head and neck neoplasm. ORL J Otorhinolaryngol Relat Spec. 2014;76(3):153-64.
31. Kekatpure VD, Trivedi NP, Manjula BV, Mathan Mohan A, Shetkar G, Kuriakose MA. Pectoralis major flap for head and neck reconstruction in era of free flaps. Int J Oral Maxillofac Surg. 2012 Apr;41(4):453-7.
32. Schusterman MA, Horndeski G. Analysis of the morbidity associated with immediate microvascular reconstruction in head and neck cancer patients. Head Neck. 1991 Jan-Feb;13(1):51-5.
33. Chepeha DB, Annich G, Pynnonen MA, et al. Pectoralis major myocutaneous flap vs revascularized free tissue transfer: complications, gastrostomy tube dependence, and hospitalization. Arch Otolaryngol Head Neck Surg. 2004 Feb;130(2):181-6.
34. Mehta S, Sarkar S, Kavarana N, Bhathena H, Mehta A. Complications of the pectoralis major myocutaneous flap in the oral cavity: a prospective evaluation of 220 cases. Plast Reconstr Surg. 1996 Jul;98(1):31–7.
35. Ord RA. The pectoralis major myocutaneous flap in oral and maxillofacial reconstruction: a retrospective analysis of 50 cases. J Oral Maxillofac Surg. 1996 Nov;54(11):1292-5; discussion 1295-6.
36. Talesnik A, Markowitz B, Calcaterra T, Ahn C, Shaw W. Cost and outcome of osteocutaneous free-tissue transfer versus pedicled soft-tissue reconstruction for composite mandibular defects. Plast Reconstr Surg. 1996 May;97(6):1167-78.
37. Kroll SS, Evans GR, Goldberg D, et al. A comparison of resource costs for head and neck reconstruction with free and pectoralis major flaps. Plast Reconstr Surg. 1997 Apr;99(5):1282–6.
38. Shektman A, Silver C, Strauch B. A re-evaluation of hypopharyngeal reconstruction: pedicled flaps versus microvascular free flaps. Plast Reconstr Surg. 1997 Dec;100(7):1691–6.
39. Jing SS, O’Neill T, Clibbon JJ. A comparison between free gracilis muscle flap and pedicled pectoralis major flap reconstructions following salvage laryngectomy. J Plast Reconstr Aesthet Surg. 2014 Jan;67(1):17-22.
40. van Rossen ME, Verduijn PV, Mureau MA. Survival of pedicled pectoralis major flap after secondary myectomy of muscle pedicle including transection of thoracoacromial vessels: does the flap remain dependent on its dominant pedicle? J Plast Reconstr Aesthet Surg. 2011 Mar;64(3):323-8.
41. Trignano E, Dessy LA, Fallico N, et al. Treatment of pectoralis major flap myospasms with botulinum toxin type A in head and neck reconstruction. J Plast Reconstr Aesthet Surg. 2012 Feb;65(2):e23-8.
42. Su WF, Hsia YJ, Chang YC, Chen SG, Sheng H. Functional comparison after reconstruction with a radial forearm free flap or a pectoralis major flap for cancer of the tongue. Otolaryngol Head Neck Surg. 2003 Mar;128(3):412-8.
43. Chen WL, Wang YY, Zhang DM, Fan S, Lin ZY. Extended vertical lower trapezius island myocutaneous flap versus pectoralis major myocutaneous flap for reconstruction in recurrent oral and oropharyngeal cancer. Head Neck. 2016 Apr;38 Suppl 1:E159-64.
44. Turkmen A, Perks AG. Endoscopic assisted harvest of the pedicled pectoralis major muscle flap. Br J Plast Surg. 2005 Mar;58(2):170–4.
45. Kuntz AL, Weymuller EA Jr. Impact of neck dissection on quality of life. Laryngoscope. 1999 Aug;109(8):1334-8.
46. Rogers SN, Ferlito A, Pellitteri PK, Shaha AR, Rinaldo A. Quality of life following neck dissections. Acta Otolaryngol. 2004 Apr;124(3):231-6.
47. Cappiello J, Piazza C, Giudice M, De Maria G, Nicolai P. Shoulder disability after different selective neck dissections (levels II-IV versus levels II-V): a comparative study. Laryngoscope. 2005 Feb;115(2):259-63.
48. Refos JW, Witte BI, de Goede CJ, de Bree R. Shoulder morbidity after pectoralis major flap reconstruction. Head Neck. 2016 Aug;38(8):1221-8.
49. Sun Q, Guo S, Wang D, Xu N, Jin SF, Wang CC. Does pectoralis major flap harvesting induce upper extremity dysfunction? J Int Med Res. 2015 Aug;43(4):555-9.
50. Moukarbel RV, Fung K, Franklin JH, et al. Neck and shoulder disability following reconstruction with the pectoralis major pedicled flap. Laryngoscope. 2010 Jun;120(6):1129-34.
51. Hsing CY, Wong YK, Wang CP, et al. Comparison between free flap and pectoralis major pedicled flap for reconstruction in oral cavity cancer patients--a quality of life analysis. Oral Oncol. 2011 Jun;47(6):522-7.
52. Shunyu NB, Medhi J, Laskar HA, Lyngdoh N, Syiemlieh J, Goyal A. 5th Rib Osteo-pectoralis Major Myocutaneous Flap-Still a Viable Option for Mandibular Defect Reconstruction. Indian J Otolaryngol Head Neck Surg. 2014 Dec;66(4):414-7.
53. Ribeiro Salles Vanni CM, de Matos LL, Faro Junior MP, et al. Enhanced morbidity of pectoralis major myocutaneous flap used for salvage after previously failed oncological treatment and unsuccessful reconstructive head and neck surgery. ScientificWorld Journal. 2012;2012 : 384179.
54. McLean JN, Carlson GW, Losken A. The pectoralis major myocutaneous flap revisited: a reliable technique for head and neck reconstruction. Ann Plast Surg. 2010 May;64(5):570–3.
55. Ramakrishnan VR, Yao W, Campana JP. Improved skin paddle survival in pectoralis major myocutaneous flap reconstruction of head and neck defects. Arch Facial Plast Surg. 2009 Sep-Oct;11(5):306–10.
56. Pinto FR, Malena CR, Vanni CM, Capelli Fde A, Matos LL, Kanda JL. Pectoralis major myocutaneous flaps for head and neck reconstruction: factors influencing occurrences of complications and the final outcome. Sao Paulo Med J. 2010 Dec;128(6):336-41.
57. IJsselstein CB, Hovius SE, ten Have BL, Wijthoff SJ, Sonneveld GJ, Meeuwis CA, et al. Is the pectoralis myocutaneous flap in intraoral and oropharyngeal reconstruction outdated? Am J Surg. 1996 Sep;172(3):259–62.
58. Wilson JS, Yiacoumettis AM, O’Neill T. Some observations on 112 pectoralis major myocutaneous flaps. Am J Surg. 1984 Feb;147(2):273–9.
59. Shah JP, Haribhakti V, Loree TR, Sutaria P. Complications of the pectoralis major myocutaneous flap in head and neck reconstruction. Am J Surg. 1990 Oct;160(4):352–5.
60. Wang CH, Wong YK, Wang CP, et al. Risk factors of recipient site infection in head and neck cancer patients undergoing pectoralis major myocutaneous flap reconstruction. Eur Arch Otorhinolaryngol. 2015 Nov;272(11):3475-82.
61. Khan F, Hameed H, Khan NU, Shah SA. Head and neck reconstruction; our experience of pectoralis major myocutaneous pedicled flap. Professional Med J. 2011 Jun;18(2):310-316.
62. Rudes M, Bilić M, Jurlina M, Prgomet D. Pectoralis major myocutaneous flap in the reconstructive surgery of the head and neck--our experience. Coll Antropol. 2012 Nov;36 Suppl 2 : 137-42.
63. Biller HF, Baek SM, Lawson W, Krespi YP, Blaugrund SM. Pectoralis major myocutaneous island flap in head and neck surgery: Analysis of complications in 42 cases. Arch Otolaryngol. 1981 Jan;107(1):23-6.
64. Baek SM, Lawson W, Biller HF. An analysis of 133 pectoralis major myocutaneous flaps. Plast Reconstr Surg. 1982 Mar;69(3):460–9.
65. Ossoff RH, Wurster CF, Berktold RE, Krespi YP, Sisson GA. Complications after pectoralis major myocutaneous flap reconstruction of head and neck defects. Arch Otolaryngol. 1983 Dec;109(12):812-4.
66. Avery C. A perspective on the role of the pectoralis major flap in oral and maxillofacial oncology surgery. Oral Surg. 2014;7(3):130–142.
67. Bhaya MH, Har-el G. Resident training in head and neck flap reconstruction in U.S. academic otolaryngology programmes. J Laryngol Otol. 2001 Feb;115(2):119–21.
68. Avery CM, Crank ST, Neal CP, Hayter JP, Elton C. The use of the pectoralis major flap for advanced and recurrent head and neck malignancy in the medically compromised patient. Oral Oncol. 2010 Nov;46(11):829-33.
69. Kroll SS, Goepfert H, Jones M, Guillamondegui O, Schusterman M. Analysis of complications in 168 pectoralis major myocutaneous flaps used for head and neck reconstruction. Ann Plast Surg. 1990 Aug;25(2):93–7.
70. Virós Porcuna D1, León Vintró X, López Vilas M, Pujol Olmo A, Masià Ayala J, Quer Agustí M. Pectoralis major flaps. Evolution of their use in the age of microvascularized flaps. Acta Otorrinolaringol Esp. 2008 Jun-Jul;59(6):263-8.
71. Zbar RI, Funk GF, McCulloch TM, Graham SM, Hoffman HT. Pectoralis major myofascial flap: a valuable tool in contemporary head and neck reconstruction. Head Neck. 1997 Aug;19(5):412–8.
72. Wei FC, Celik N, Yang WG, Chen IH, Chang YM, Chen HC. Complications after reconstruction by plate and soft-tissue free flap in composite mandibular defects and secondary salvage reconstruction with osteocutaneous flap. Plast Reconstr Surg. 2003 Jul;112(1):37-42.
73. Ross G, Yla-Kotola TM, Goldstein D, et al. Second free flaps in head and neck reconstruction. J Plast Reconstr Aesthet Surg. 2012 Sep;65(9):1165-8.
74. Liu HL, Chan JY, Wei WI. The changing role of pectoralis major flap in head and neck reconstruction. Eur Arch Otorhinolaryngol. 2010 Nov;267(11):1759-63.
75. Serafin, D. The gracilis muscle-musculocutaneous flap. In: Atlas of Microsurgical Composite Tissue Transplantation. Philadelphia: WB Saunders Company; c1996.p.293-299.
76. Chan YW, Ng RW, Yuen AP. Lateral thoracic flap for donor site repair of pectoralis major myocutaneous flap. J Plast Reconstr Aesthet Surg. 2009 Aug;62(8):1004-7.
77. Kim EK, Yang SJ, Choi SH. Method to help ensure survival of a very small skin paddle of pectoralis major musculocutaneous flap in head and neck reconstruction. Head Neck. 2013 Aug;35(8):E237-9.
78. Nayak BB, Nilamani M. Single stage reconstructions in head and neck surgery using deltopectoral and pectoralis major myocutaneous flaps. Indian J Plast Surg. 2012 Jan;45(1):151-3.
79. Kanno T, Nariai Y, Tatsumi H, Karino M, Yoshino A, Sekine J. A modified pectoralis major myocutaneous flap technique with improved vascular supply and an extended rotation arc for oral defects: A case report. Oncol Lett. 2015 Nov;10(5):2739-2742.
80. Po-Wing Yuen A. Preservation of lateral thoracic artery to improve vascular supply of distal skin without compromising pedicle length in harvesting pectoralis major myocutaneous flap. J Plast Reconstr Aesthet Surg. 2006;59(12):1433-5.
81. Kim DM, Jeon A, Kim KY, et al. Neurovascular distribution within the abdominal head of the pectoralis major muscle: Application to breast and flap surgery. Clin Anat. 2015 May;28(4):520-6.
82. Temiz G, Şirinoğlu H, Yeşiloğlu N, et al. A salvage maneuver for the caudal part of the pectoralis major muscle in the reconstruction of superior thoracic wall defects: The pectoralis kite flap. J Plast Reconstr Aesthet Surg. 2015 May;68(5):698-704.
83. de Azevedo JF. Modified pectoralis major myocutaneous flap with partial preservation of the muscle: a study of 55 cases. Head Neck Surg. 1986 May-Jun;8(5):327–31.
84. Tashiro K, Harima M, Mito D, et al. Preoperative color Doppler ultrasound assessment of the lateral thoracic artery perforator flap and its branching pattern. J Plast Reconstr Aesthet Surg. 2015 Jun;68(6):e120-5.
85. Bonomi S, Salval A, Sorbi F, Settembrini F. To sever or not the thoracodorsal nerve in latissimus dorsi flap breast reconstruction. Plast Reconstr Surg. 2012 Aug;130(2):367e-368e; author reply 368e-369e.
86. Kääriäinen M, Giordano S, Kauhanen S, Helminen M, Kuokkanen H. No need to cut the nerve in LD reconstruction to avoid jumping of the breast: a prospective randomized study. J Plast Reconstr Aesthet Surg. 2014 Aug;67(8):1106-10.
87. Szychta P, Butterworth M, Dixon M, Kulkarni D, Stewart K, Raine C. Breast reconstruction with the denervated latissimus dorsi musculocutaneous flap. Breast. 2013 Oct;22(5):667-72.
88. Schroegendorfer KF, Hacker S, Nickl S, Vierhapper M, Nedomansky J, Haslik W. Latissimus dorsi breast reconstruction: how much nerve resection is necessary to prevent postoperative muscle twitching? Plast Reconstr Surg. 2014 Dec;134(6):1125–9.
89. Vanni CM, Pinto FR, de Matos LL, de Matos MG, Kanda JL. The subclavicular versus the supraclavicular route for pectoralis major myocutaneous flap: a cadaveric anatomic study. Eur Arch Otorhinolaryngol. 2010 Jul;267(7):1141-6.
Labels
Plastic surgery Orthopaedics Burns medicine Traumatology
Article was published inActa chirurgiae plasticae

2018 Issue 1-
All articles in this issue
- Pedicled pectoralis major flap in head and neck reconstruction - technique and overview
- Securing intraoral skin grafts to the floor of the mouth: case report and technique desription
- Intraosseous haemangioma od the zygoma - case report and literature review
- Editorial
- Pedicled pectoralis major flap in head and neck reconstruction - our experience
- In memoriam: Professor Rajko Doleček
- Commemoration of professor Miroslav Fára, M.D., DSc.
- Use of licap and ltap flaps for breast reconstruction
- Image Possibilities of the Peripheral Nerve Tumor Using Magnetic Resonance Imaging - Case Report
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Pedicled pectoralis major flap in head and neck reconstruction - technique and overview
- Use of licap and ltap flaps for breast reconstruction
- Editorial
- Intraosseous haemangioma od the zygoma - case report and literature review
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career