-
Medical journals
- Career
Effect of ibrutinib on platelet aggregation
Authors: J. Sokol 1; J. Chudej 1; M. Hrncar 2; I. Skornova 1; J. Fedorova 3; P. Kubisz 1; J. Stasko 1
Authors‘ workplace: Department of Haematology and Transfusion Medicine, National Centre of Haemostasis and Thrombosis Jessenius Faculty of Medicine in Martin, Comenius University in Bratislava, Martin, Slovakia 1; Department of Oncology, F. D. Roosevelt Teaching Hospital with Policlinic Banska Bystrica Banska Bystrica, Slovakia 2; Haemostasis and Thrombosis Centre, Hemo Medika, Martin, Slovakia 3
Published in: Transfuze Hematol. dnes,22, 2016, No. 4, p. 264-267.
Category: Comprehensive Reports, Original Papers, Case Reports
Overview
Objective:
We aimed to evaluate the in vitro impact of ibrutinib on platelet aggregation.Methods:
Light transmission aggregometry was performed using the international protocol for the laboratory investigation of platelet function. Platelet aggregation was assessed using the PACKS-4 aggregometer. Each sample was tested with adenosine diphosphate (ADP) (2.5 μmol/L), epinephrine (EPI) (1.2 μmol/L), arachidonic acid (AA) (1.0 mmol/L), and collagen (1.25 μg/mL).Results:
We tested 7 relapsed/refractory CLL patients, median age 69 years (range 52–80 years), 5 of which were aged 65 years or older. All patients received 420 mg oral ibrutinib daily. As expected, patients on ibrutinib showed impairment of response to collagen. All patients showed reduced platelet aggregation after AA. In addition, the majority of patients also had a reduced response to EPI and ADP.Conclusion:
We suggest that ibrutinib inhibits in vitro collagen and AA-induced platelet aggregation in CLL patients without any other antiplatelet therapy. We also present certain evidence that ibrutinib affects in vitro ADP and EPI-induced aggregation in a small group of CLL patients without any other antiplatelet therapy. Our findings may have some important clinical implications given that we have shown ibrutinib to affect platelet activation and aggregation on many levels. These data are preliminary and will have to be confirmed in future studies.Key words:
aggregation – bleeding – ibrutinib – plateletINTRODUCTION
Ibrutinib is an inhibitor of Bruton’s tyrosine kinase (BTK). BTK is a cytoplasmic tyrosine kinase of the TEC family that is essential for B-cell receptor signalling. It demonstrates activity in chronic lymphocytic leukaemia (CLL). Ibrutinib is generally well tolerated but is associated with an increased risk of bleeding [1, 2]. Understanding the mechanism by which ibrutinib may contribute to bleeding is important for clinical management because the majority of CLL patients are elderly and many of them have comorbidities requiring anticoagulation and/or antiplatelet therapy. It is assumed that the bleeding events are caused by platelet dysfunction [3]. However, previous studies have yielded variable results. Farooqui et al. tested samples from ibrutinib-treated patients using the PFA-100 device with epinephrine (EPI) and adenosine diphosphate (ADP); no functional abnormalities were identified [4]. Until now, the best paper on this topic was published by Kamel et al. [3]. He showed (n = 23 patients) a specific and reversible effect on collagen-induced platelet aggregation. He claimed that he found no evidence of impaired ADP, EPI or arachidonic acid (AA) response. On the other hand, he showed in his graphs that a proportion of patients who had received ibrutinib had reduced aggregation after AA and EPI. Unfortunately, these findings were not discussed in the paper itself. We therefore aimed to evaluate the in vitro impact of ibrutinib on platelet aggregation.
MATERIAL AND METHODS
This study was approved by the local Ethics Committee. All study participants agreed to take part in the project and signed a written informed consent in accordance with the Declaration of Helsinki. Light transmission aggregometry (LTA) was performed using the international protocol for the laboratory investigation of platelet function [5]. Antecubital venous blood was collected into tubes containing 3.2% buffered sodium citrate (anticoagulant-blood ratio 1 : 9) to assess platelet aggregation. Platelet aggregability was tested with platelet-rich plasma using platelet aggregometry (PACKS-4 aggregometer, Helena Laboratories, USA). Each sample was tested with ADP (2.5 μmol/L), EPI (1.2 μmol/L), AA (1.0 mmol/L), and collagen (1.25 μg/mL). Based on our own laboratory assessment of normal subjects, we defined the upper and lower normal values of aggregometry (the reference range is 55–90%). Testing was performed on patients without any antiplatelet therapy (10–14 days before measurement) and when the platelet counts had improved sufficiently following ibrutinib therapy to permit reliable testing (≥ 100 × 109/L). All patients had normal basic coagulation tests (prothrombin time, thrombin time, activated partial thromboplastin time). Testing was performed within one hour of taking ibrutinib.
RESULTS
We tested 7 relapsed/refractory CLL patients, median age 69 years (range 52–80 years), 5 of which were aged 65 years or older. Five patients had received ≥ 2 previous lines of treatment. All patients received 420 mg oral ibrutinib daily. Median time from ibrutinib initiation to platelet aggregometry was 171 days (range 80–272 days). Median platelet count from whole blood was 117 x 109/L (range 103–195 x 109/L). None of these patients received antiplatelet or anticoagulation treatment. Clinical bleeding while taking ibrutinib did not occur.
The results of LTA for subjects on stable ibrutinib therapy are shown in Figure 1 and Table 1. As expected, patients on ibrutinib showed impairment of response to collagen. The maximum aggregation value was 44% and the minimum 3.86%. However, we did not expect the results after aggregation with AA, EPI and ADP. All patients showed reduce platelet aggregation after AA (maximum 52.28% and minimum 30%). In addition, the majority of patients also had a reduced response to EPI (maximum 61% and minimum 30%) and ADP (maximum 71.67% and minimum 20%).
Fig. 1. LTA in samples taken from patients on stable ibrutinib therapy The percentages of peak responses for each of the different platelet agonists are shown. 
1. LTA results from patients on stable ibrutinib therapy 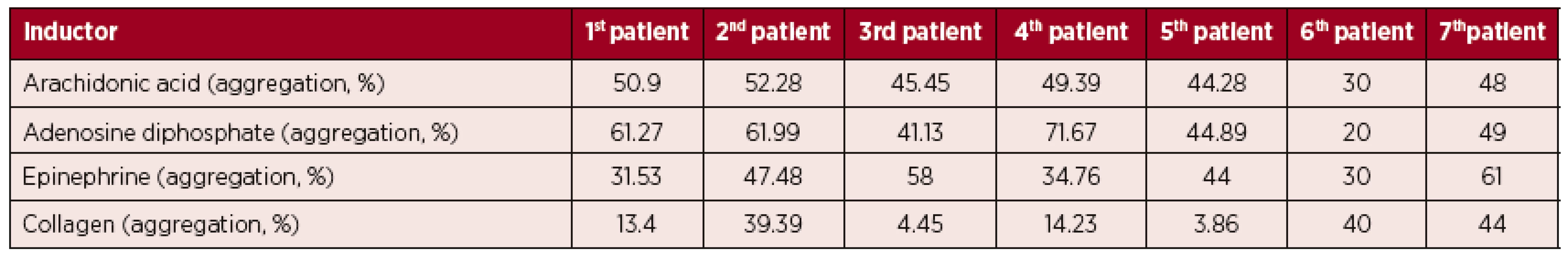
DISCUSSION
This study reports the use of the sensitive LTA method to assess platelet function in subjects treated with ibrutinib. We showed an effect on collagen and AA-induced platelet aggregation, and some evidence of an effect on ADP and EPI-induced aggregation. We asked ourselves, “Is there any connection between collagen, ADP, EPI and thromboxane A2 (TXA2) receptors?” In the next section, we will try to find the answer.
Activation of glycoprotein VI (GPVI) by collagen results in a complex cascade of signalling events [6]. The initial step is phosphorylation of the FcR -chain by Fyn and Lyn. Subsequent activation of the tyrosine kinase Syk leads to phosphorylation of multiple signalling proteins, including BTK. The activated BTK then phosphorylates phospholipase C (PLC), leading to its activation. The activated PLC converts phosphatidylinositol 4,5-bisphosphate (PIP2) into the second messenger inositol 1,4,5-trisphosphate (IP3), which in turn binds to IP3 receptors (IP3R) located on the endoplasmic reticulum (ER). Binding of IP3 to the IP3R is essential for triggering a calcium release from the ER [7].
Receptors for ADP, EPI and TXA2 belong to G protein-coupled receptors (GPCRs). GPCRs interact with the trimeric G-protein alpha-s/beta/gamma complex and trigger the exchange of GDP to GTP bound to G-protein alpha subunits leading to the dissociation of beta/gamma heterodimers. G-protein beta/gamma signalling also regulates phosphoinositide metabolism by increasing the kinase activity of BTK, a known activator of PLC or by direct activation of PLC [8].
Now, we see that the common activation site of PLC is BTK, see Figure 2. On the other hand, GPCRs can directly activate PLC. Therefore, we assume that the differences in results between studies are also affected by this fact. Our findings could have certain important clinical implications because platelet activation and aggregation is affected at many levels. Nevertheless, reduction or absence in collagen-induced platelet aggregation appears to be largely dispensable in normal life, presumably due to activation in other platelet pathways [3]. However, the situation changes dramatically during concomitant administration of antiplatelet or anticoagulant agents. Concomitant use of these drugs should be instituted with utmost care and only when really necessary.
Fig. 2. PLC activation via BTK 
Abbreviations: AA – arachidonic acid; α2AR - α2-adrenergic receptor; ADP – adenosine diphosphate; BTK – Bruton’s tyrosine kinase; Ca2+ – calcium ions; DAG – diacylglycerol; EPI – epinephrine; Gp – G protein; GPVI – glycoprotein VI; IP3 – inositol 1,4,5-trisphosphate; IP3R – inositol 1,4,5-trisphosphate receptor; PIP2 – phosphatidylinositol 4,5-bisphosphate; PLC – phospholipase C; TR – thromboxane receptor; TXA2 – thromboxane A2. There were several limitations in our study, including the small number of patients and platelet count during aggregometry. Currently, only 12 patients with CLL are treated with ibrutinib in Slovakia. Platelet aggregability is greatly affected by preanalytical issues and therefore interpretation of platelet aggregability may be potentially adversely affected. Therefore, we follow the international protocol [7].
CONCLUSION
We showed an effect on collagen and AA-induced platelet aggregation and some evidence of an effect on ADP and EPI-induced aggregation. Our findings may have some important clinical implications given that we have shown ibrutinib to affect platelet activation and aggregation on many levels.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgement
The study was supported by grant VEGA 1/0168/16 and the civic association HematologySK.
Author contribution
JS, JC and IS collected and analysed data. All authors reviewed the literature and wrote the manuscript.
Juraj Sokol, MD, PhD
Department of Haematology and Transfusion Medicine,
National Centre of Haemostasis and Thrombosis
Kollarova 2
036 59 Martin
Slovakia
E-mail: juraj.sokol@jfmed.uniba.sk
Sources
1. Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 2013; 369 : 32–42.
2. Shakeel F, Iqbal M, Ezzeldin E, et al. Bioavailability enhancement and pharmacokinetic profile of an anticancer drug ibrutinib by self-nanoemulsifying drug delivery system. J Pharm Pharmacol 2016; 68(6): 772–780.
3. Kamel S, Horton L, Ysebaert L, et al. Ibrutinib inhibits collagen-mediated but not ADP-mediated platelet aggregation. Leukemia 2015; 29 : 783–787.
4. Farooqui M, Lozier JN, Valdez J, et al. Ibrutinib (PCI 32765) rapidly improves platelet counts in chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) patients and has minimal effects on platelet aggregation. ASH Annual Meeting Abstracts 2012; 120 : 1789.
5. Harrison P, Mackie I, Mumford A, et al. British Committee for Standards in Haematology. Guidelines for the laboratory investigation of heritable disorders of platelet function. Br J Haematol 2011; 155 : 30–44.
6. Ezumi Y, Shindoh K, Tsuji M, Takayama H. Physical and functional association of the Src family kinases Fyn and Lyn with the collagen receptor glycoprotein VI-Fc receptor γ-chain complex on human platelets. J Exp Med 1998; 188 : 267–276.
7. Wonerow P, Pearce AC, Vaux DJ, Watson SP. A critical role for phospholipase Cγ2 in αIIbβ3-mediated platelet spreading. J Biol Chem 2003; 278 : 37520–37529.
8. Lowry WE, Huang X-Y. G Protein βγ subunits act on the catalytic domain to stimulate Bruton’s agammaglobulinemia tyrosine kinase. J Biol Chem 2002; 277 : 1488–1492.
Labels
Haematology Internal medicine Clinical oncology
Article was published inTransfusion and Haematology Today

2016 Issue 4-
All articles in this issue
- The monoclonal antibody daratumumab in the treatment of multiple myeloma
- Low-dose computed tomography of the skeleton in multiple myeloma staging
- Apheresis platelets versus pooled platelets – comparison of quality and safety
- Stability of complete blood count and microscopic differential count parameters
- Frozen platelets in clinical praxis: comparative study of native platelets
- Effect of ibrutinib on platelet aggregation
- Transfusion and Haematology Today
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Apheresis platelets versus pooled platelets – comparison of quality and safety
- Stability of complete blood count and microscopic differential count parameters
- Low-dose computed tomography of the skeleton in multiple myeloma staging
- Frozen platelets in clinical praxis: comparative study of native platelets
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

