-
Medical journals
- Career
DESIGN, MANUFACTURE AND TESTING OF RABBIT IMPLANTATION STRUCTURES FOR PRECLINICAL EXAMINATIONS
Authors: Lukáš Mitrík; Marianna Trebuňová; Radovan Hudák; Marek Schnitzer; Jozef Živčák
Published in: Lékař a technika - Clinician and Technology No. 1, 2019, 49, 11-15
Category:
Overview
The aim of this study was to develop a methodology of preclinical animal testing in case studies for implants with PEEK material and its derivatives with possible use for other materials. In the theoretical part, the study deals with the analysis of the current situation in the area of preclinical animal testing, especially in the field of implantology. Based on this analysis, the test implant structures were prepared at the workplace, which were subsequently inserted into appropriate rabbit individuals by surgical intervention in case studies. These case studies serve to create a comprehensive test methodology for animal preclinical tests within a statistically relevant animal sample for the needs of subsequent clinical testing and certification of the biomaterial used.
Keywords:
PEEK – animal – implants – osteointegration
Introduction
Polyetheretherketone (PEEK) material is very often used today as a biomaterial in various medical operations due to excellent mechanical and chemical properties. However, the untreated PEEK surface is biointerptive and hydrophobic and does not integrate in its pure form. PEEK material is a compound that is chemically inert, insoluble at room temperature and in all conventional solvents except 98% sulfuric acid. At room temperature, water solubility is 0.5% by weight. The modulus of elasticity is in the range of 3–4 GPa, whereas the human cortical bone elastic modulus is in the range of 7–30 GPa. Pure PEEK material melts at 334 °C, crystallizing PEEK material at 343 °C. Due to the relatively high melting point, it is a very suitable implant candidate because of its stability in the human body. It also has high resistance to gamma and electron beam radiation. Clinical classification of PEEK implants [1, 2]:
- Bone replacement implants—maxillofacial implants;
- Implants for spinal surgery—spinal cages;
- Implants for orthopedic surgery
- a) lumbar replacements and joint implants,
- b) fixing devices and screws;
- Implants for dental surgery—dental prostheses;
- Implants for cardiac surgery—heart valves, intra-cardiac pumps.
Utilization of a nano-modified surface on a PEEK implant
Pär Johansson et al. carried out a test using the nano-modified PEEK surface with aim to osteointegration. This experiment was subjected to 24 rabbits, which were embedded into the tibia of 48 threaded implants of PEEK material.
Half of the implants were coated with nanocrystalline hydroxyapatite while remaining implants were left without any coating. One part of the animals was euthanized after 3 weeks of healing and the remaining part after 12 weeks.
Micrometer-level surface topography characterized by an interferometer (MicroXAM, ADE Phase Shift, Tucson, AZ, USA) was used for measurement. The chemical evaluation of the surface was performed by XPS (PHI 5000C ESCA system, PerkinElmer Inc., Waltham, MA, USA).
Osteotomy was performed by a series of several boreholes, in addition to continuous humidification, until a final diameter of 3.2 mm was reached. The implants were implanted manually until they were fully embedded in the bone.
After 3 and 12 weeks of healing, animals were euthanized by overdose with sodium pentobarbital. The tibias were then dissected, removing the soft tissue. No signs of infection were observed during or after healing at surgically operated sites for all test subjects. All of the implants were successfully cured; one of the rabbits died of unknown causes during surgery. [3]
Hydroxyapatite coating on PEEK implants—biomechanical and histological study on rabbit model
Durham III et al. used PEEK-PETI-OPTIMA, for the painting of sticks with a diameter of 5 mm and a length of 9 mm. The substrates were subsequently abraded with silicon carbide paper, using an automatic grinding system. Subsequently, substrate surfaces were rinsed with deionized water to prevent particle contamination. The bars were then ultrasonically cleaned in acetone, isopropanol and deionized water for 10 minutes. Drying of the substrates was carried out with compressed air. The substrates were stored in sterile tissue cultures immediately after drying.
The application of HA / YSZ coatings was achieved by using a rotational base formulation and an IBAD system.
The test was subjected to 18 skeletal adult male rabbits weighing between 3.5–4.5 kg. The rabbits were ran-domly divided into two time groups, followed by 6 weeks (N = 9 animals) and 18 weeks (N = 9 animals) of observation. In each group, 18 implants were inserted into the rabbits.
Bone fragments as well as bone marrow were rinsed from the surgical site, allowing the implant to be inserted into the femur. The wounds were closed using resorbable sutures for subcutaneous tissues. After the surgery, radiograms (lateral and cranio-caudal) were performed to accurately document the location of the implant.
The EDS method has been used along with STEM technology to percentage the quantification of the atomic elements present in the HA coating layer. This quantification is useful in determining the Ca/P ratio, the ideal ratio for stoichiometric HA is 1.67. Table 1 shows the percentage of O, P and Ca atoms in the HA layer for coated samples which were subjected to microwaves and microwaves with autoclaving. The results showed that both coatings had a Ca/P ratio slightly above the stoichiometric HA ratio.
1. Atomic composition of the HA coating layer (the values given are in %) [4]. ![Atomic composition of the HA coating layer (the values given are in %) [4].](https://www.prolekare.cz/media/cache/resolve/media_object_image_small/media/image/3a6d2bbc2626f077e39c03684254581d.jpeg)
Images made by using the micro-CT device showed that the implants coated with the layer were characterized by the bone growth trend in the central canal after 6 and 18 weeks after implantation, in particular the AD + MW group. Bone volume regeneration analysis was performed in two different areas of interest. A 9×5-sized region was primarily responsible for bone growth, which ran along the implant axis. The volume of bones that regenerated in this area was significantly higher in implants coated AD + MW + AC, compared to implants from PEEK. It was also found that the volume of bone that regenerated on implants coated with AD + MW + AC increased significantly between 6 and 18 weeks, indicating strong and ongoing osteointegration. [4]
Based on the information obtained from the research, the study focused on describing different implant structures from the PEEK biomaterial, the methodology of testing the preclinical exams and designing its own implant structure and testing it on rabbits with an emphasis on the course of osteointegration.
Overview of PEEK implant structures in preclinical exams
Clinical classification of PEEK implants
Implants for bone replacement (maxillofacial implants), implants for spinal surgery (spinal cages), implants for dental surgery (dentures), implants for cardiac surgery (heart valves, intracardiac pumps), implants for orthopedic surgery (lumbar replacements and joint implants, fixing devices and bolts).
Different types of PEEK composites
CFR-PEEK—carbon fiber is a linear material with a specific effect of magnitude from several microns to tens of microns. Since it is a reinforcing material, carbon fibers have a wide range of uses in many areas. The main advantage of CFR-PEEK is its high strength and low modulus of elasticity, similarity to the bones found in the human body. Thus, this property gives it a great potential for using implants in bone tissue in the form of various implants.
GFR-PEEK—glass fiber PEEK is characterized by high modulus of elasticity, high strength, good thermal stability and stable coefficient of extensibility. The composition of this material consists of PEEK and 10% glass fibers whose diameter is in the range of several microns to ten microns. Like CFR-PEEK, GFR-PEEK has a bony-like elastic modulus.
HA-PEEK—Hydroxylapatite is a type of inorganic material that is the main component of an inorganic component found in bone tissue.
Sr-HA-PEEK—Strontium is a biologically active element, including its ability to promote adhesion and mineralization of osteoblasts, the ability to induce bone formation and reduce the risk of fracture. The Sr-HA-PEEK composites were formed with a 15% to 30% by volume Sr-HA filling by compression molding, the particle size ranging from 43.34 ± 0.08 microns.
n-TiO2-PEEK—n-TiO2 has a very good biological compatibility, biological activity, and is hydrophilic. The integration of n-TiO2 with PEEK material can lead to a significant improvement in biological activity. [1, 5–7]
Methodology of preclinical testing
The implant structure began to be proposed after a successful surgical procedure in removing the segment of the rabbit femur (Fig. 1). The prepared segment was scanned using the IDENTICA (Medit, Seoul, Korea) scanner (Fig. 2).
2. Scan of the femoral segment. 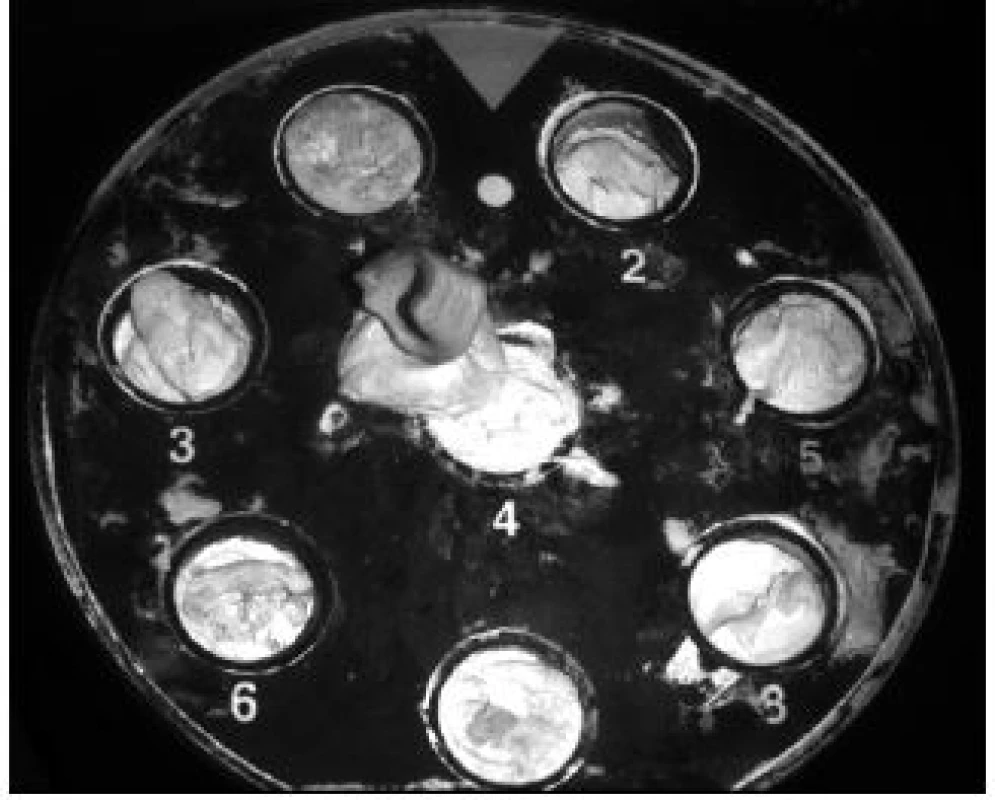
The implant was printed on a VSHAPER PRO 3D (Rzesow, Poland). The device is characterized by increased mechanical and thermal resistance. Areas of use are in the medical, automotive and aerospace industries. The patented head of the extruder can reach up to 430 °C. The resulting prints are characterized by high mechanical resistance in handling (Fig. 3).
Operational performance
The two case studies used in this work, which examined the effect of PEEK on osteointegration. Two adult rabbits with a femur fracture, weighing 4.5 kg, were subjected to the case study. Continuous exami-nations were performed on weeks 2 and 10. The individual case studies differ in that the fractured segment of the femur was removed from the rabbit on the first surgical procedure and replaced with a personalized PEEK implant made by the additive FFF technology. In the second surgical procedure, four holes were perforated into the fractured rabbit femur, with four different types of extruded fibers inserted into each opening.
Case study 1
The preparation of the rabbit began at pm by using a 1 ml (1 mg/ml, CP-Pharma Handelsgesellschaft mbH, Burgdorf, Germany) Cepetor anesthetic, representing a half dose due to a surgical surgeon's recommendation. Subsequently, he was given 1 mg of Butorfanol (1 mg/ml, Torbugesic®, Torbutrol®, Dolorex®, Labo-ratories Pharmavet SA, Santa Fe, Argentina), Ketamidor 1.2 mg (100 mg/ml, Richter Pharma Veterinär, Wels).
The incision was performed along the proximal tibial epiphysis and marigold cranialis was exposed. The osteotomy was performed with an oscillating coil with a 20 mm diameter disc. The removed femoral segment was placed in a tube, ready for cleansing and steril-ization. The resulting defect was provided with a thigh in fixation due to the correct anchorage of the bone and at the same time to avoid premature aging until the implanted segment was made using additive technology. Subsequently, the site was sewn.
Surgical insertion of the implant
The extruded implant was sterilized with the surgical instruments prior to surgery. Sterilization was carried out in accordance with and in accordance with STN EN ISO 11140-1 Sterilization of health care products.
Rabbit preparation for surgery (Fig. 4) was identical to that of the first operation. The incision was performed along the proximal tibial epiphysis and marigold cranialis was exposed. Subsequently, a personalized PEEK implant was inserted into the defect site and the thigh in fixation was reintroduced. Immediately following surgery, the rabbit received Metamizol 50 mg/kg (500 mg/ml, Sofarimex, Portugal), Tramadol 5 mg/kg (100 mg/ml, FAL Duiven BV, The Netherlands) and Betamox 1 ml, Norbrook® Laboratories Ltd., Newry, Northern Ireland, UK).
4. Surgical insertion of the implant. 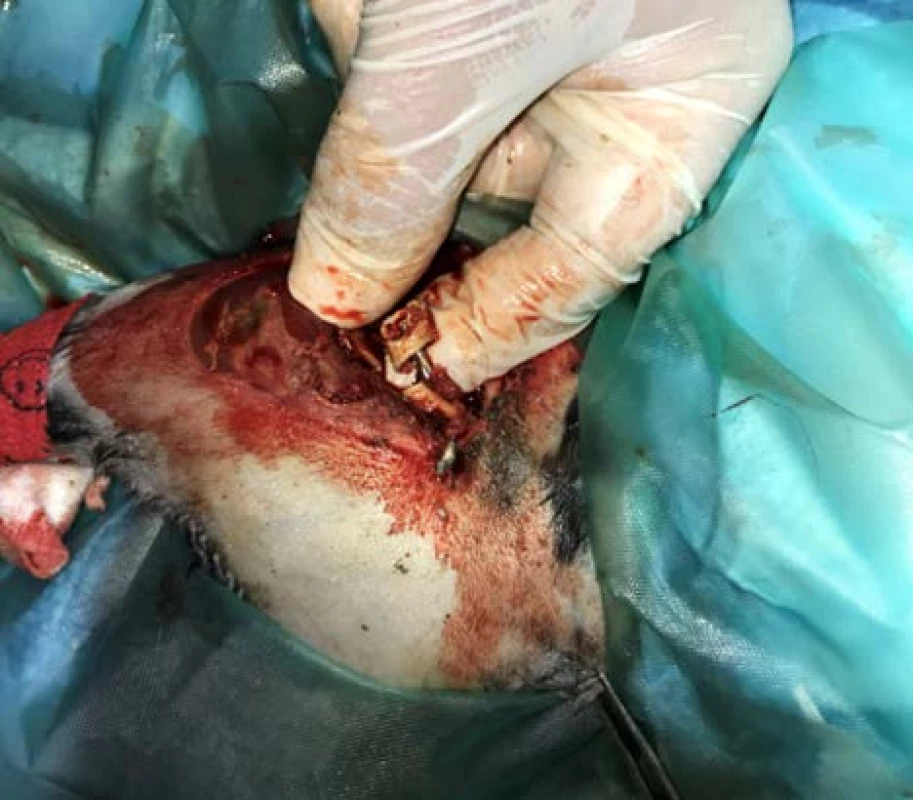
Results
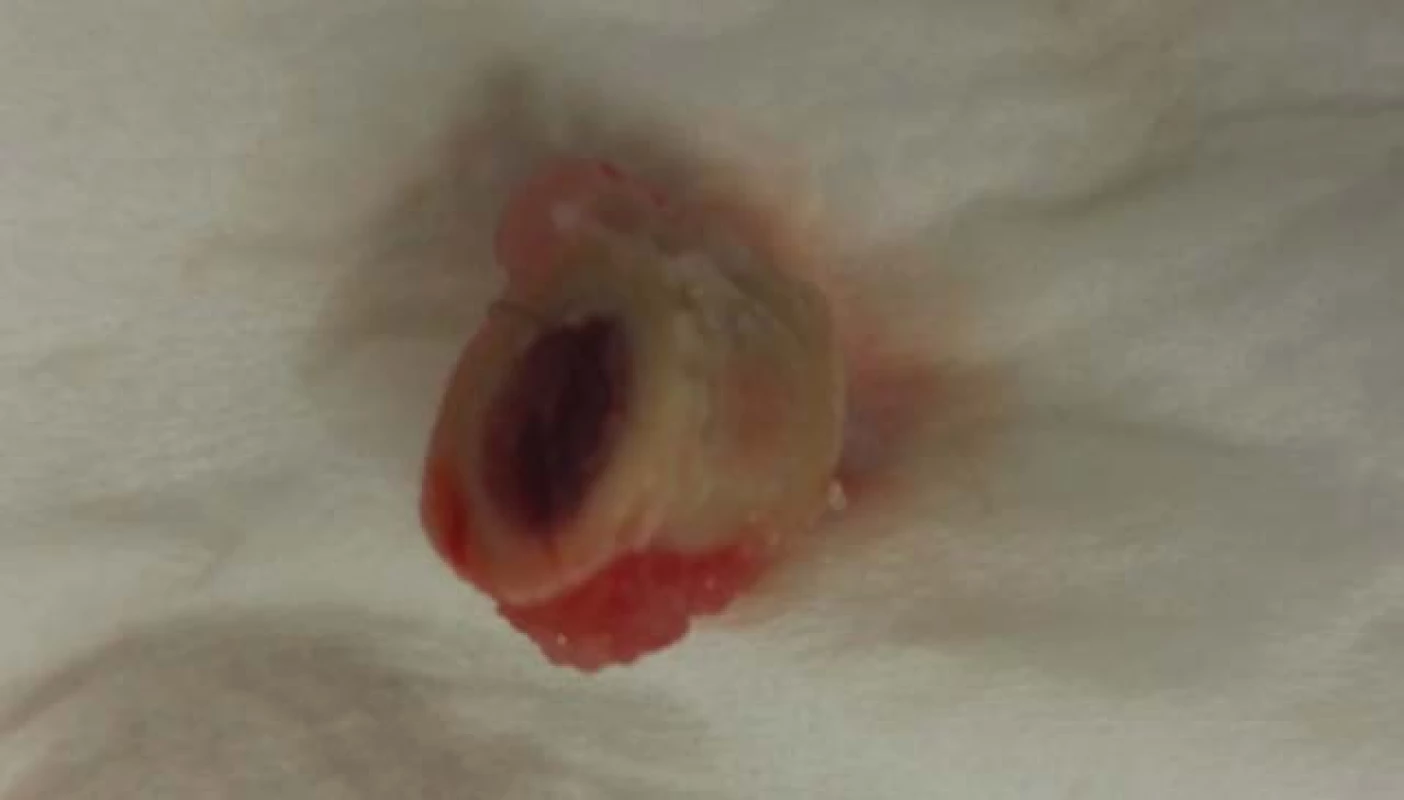
The rabbit was observed for 12 weeks, with ongoing examination at week 2 and 10. During the whole healing period, no changes in the health status of the test subject were observed. At the week 12, the thigh in fixation was eliminated and the femur was removed with owner’s permission for studying purposes (Fig. 5).
5. Prepared femur of subject with PEEK implant. 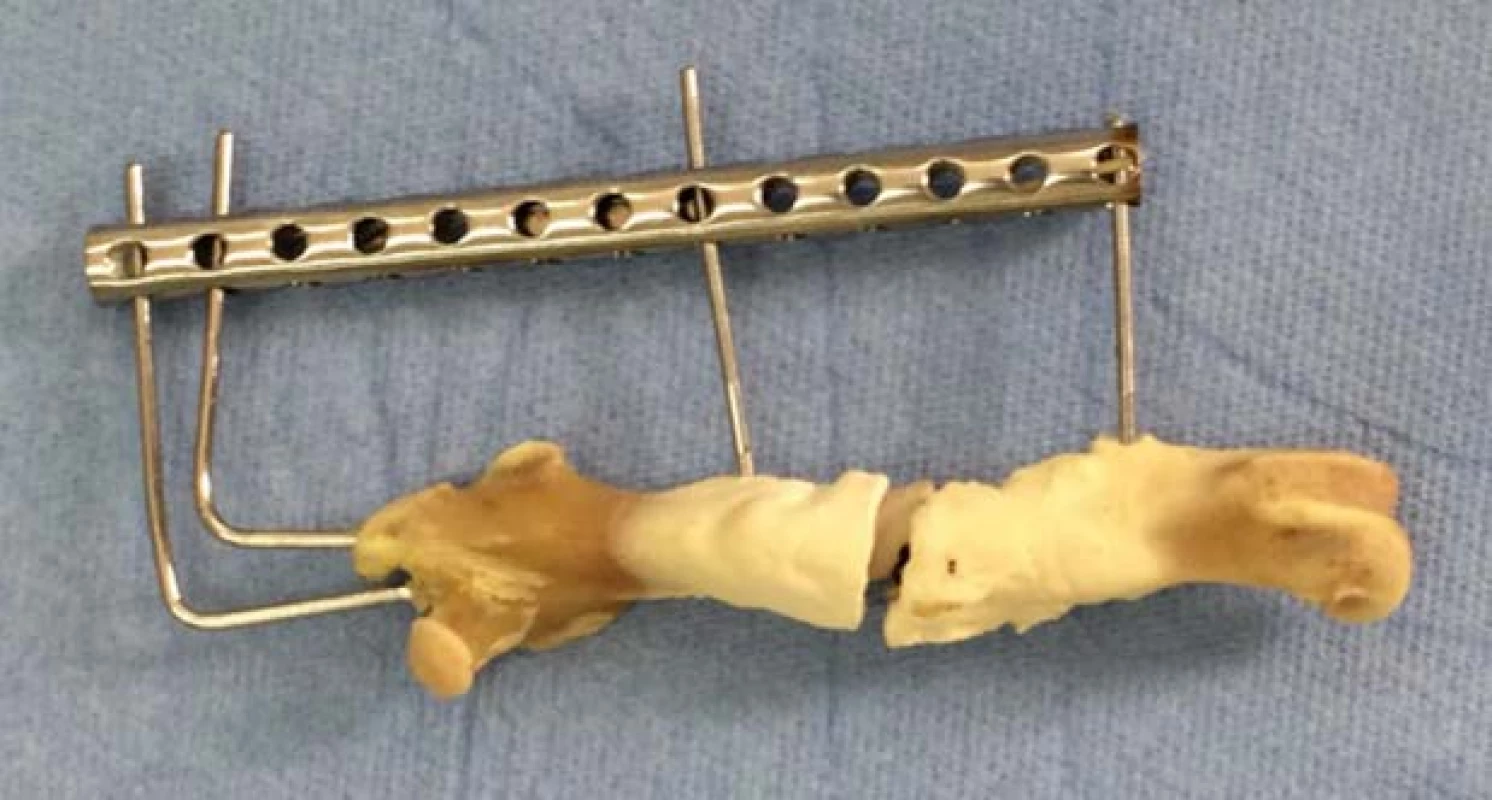
PEEK materials are known to be totally bio-inert. We have confirmed this fact when implanting a PEEK segment produced by additive technology. It is clear from the picture that the body did not take the segment, so there was no osteointegration between the implant and the bone tissue. Instead, a completely different situation arose, the bone tissue began to grow on implant’s surface and calus creation was observed.
Case study 2
The preparation of the rabbit for the treatment began at 11 pm, when the same medications were administered and the same preoperative methods were used as with the first rabbit.
The incision was performed along the proximal tibial epiphysis and marigold cranialis was exposed. Gradually, using a drill with a drill bit diameter of 2 mm, 4 holes were drilled into the bone of the femur. Each of the holes was subsequently implanted with PEEK filaments with various admixtures (Fig. 6).
6. Inserting the filament into a bone defect. 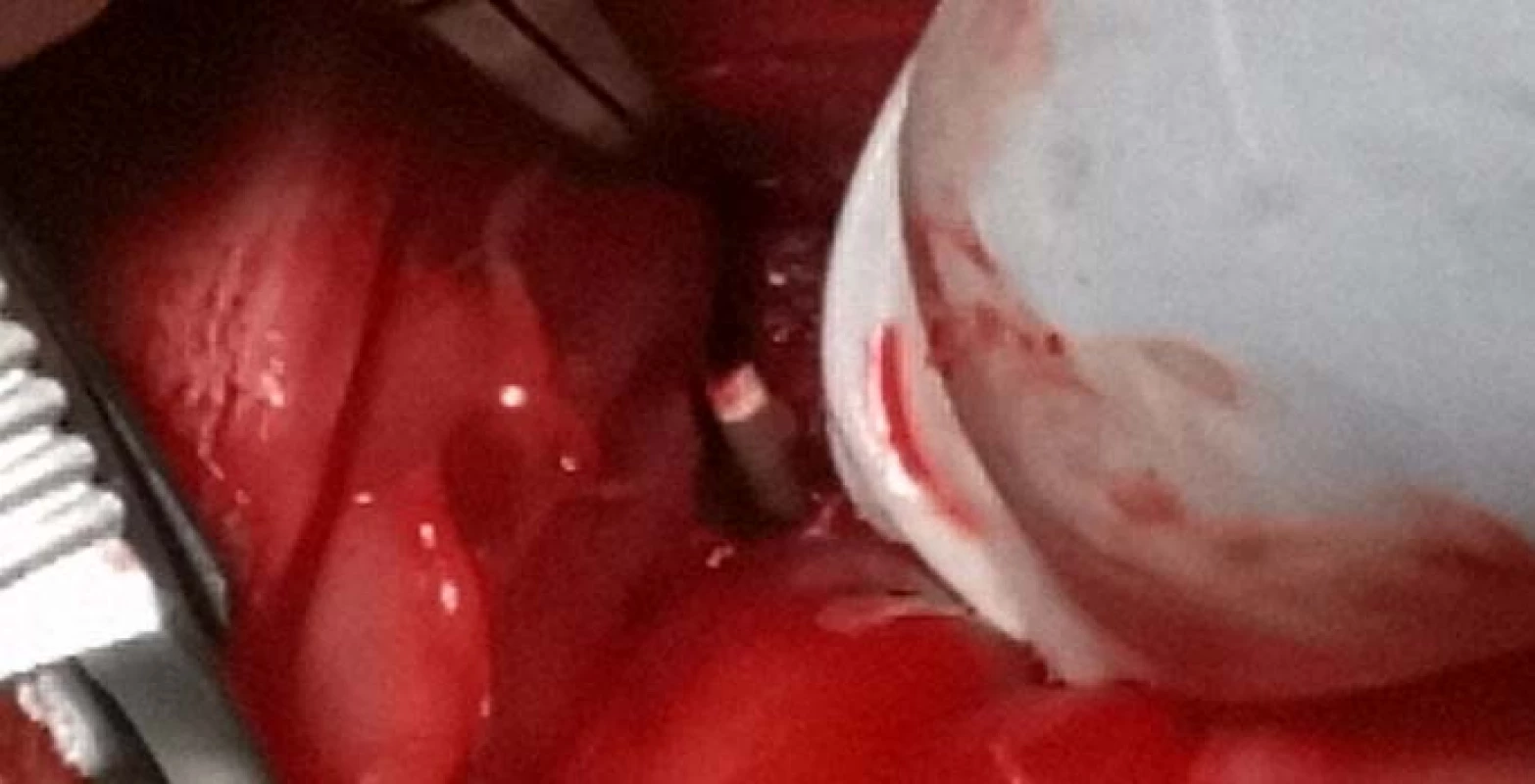
A first sample contained 100% PEEK material, the second sample contained 85% PEEK material and 15% TCP material, the third sample contained 85% PEEK material and 15% HA and the fourth sample contained 80% PEEK, 15% TCP, and 5% HA.
All samples were thoroughly sterilized according to STN EN ISO 11140-1 Sterilization of healthcare products in order to avoid any side effects and infections.
As with the first rabbit, the observation lasted 12 weeks, with ongoing controls at week 2 and week 10. During the whole healing period, no changes in the health status of the test subject were observed. Subject is still alive and prepared for advanced histological and CT examination of bone osteointegration.
Results
RTG image of the 2nd rabbit tested, showing 4 holes into which PEEK filaments with different admixtures were implanted (Fig. 7). As with the first subject tested, PEEK filaments are not captured on the X-ray image because of the inertness of the material to X-rays. The only thing that has been captured is the remnant of the artifact of one of the filaments.
7. RTG image of the subject after 2 weeks. 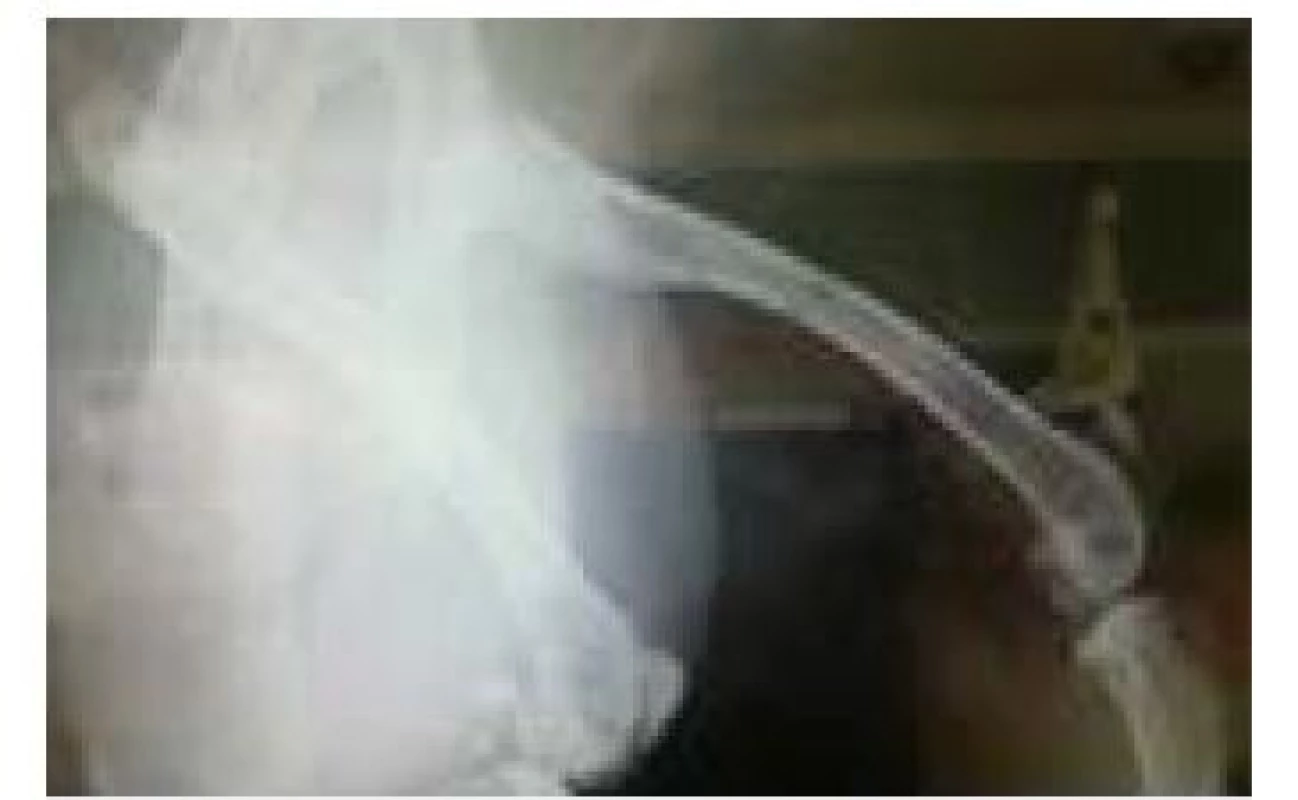
This image, therefore, discourages our assumption that visibility will be at least partially improved upon addition of various impurities to PEEK material or device setting was not properly set. For this reason, it is necessary to carry out further x-ray examinations on more powerful devices where it would be absolutely accurate to confirm whether the PEEK material is visible or not.
Necessary additional examinations
Histological examination is an examination in which osteointegration of implanted bone implants is ob-served.
Another necessary test to monitor the course of osteo-integration between the PEEK implant and the bone is to use the micro CT device. The micro CT differs from a conventional CT device in ability to scan only small objects (such as mice, rats, rabbits, etc.) but with much greater precision than conventional CT devices.
The last step in monitoring osteointegration between implants with PEEK material with various admixtures is the use of electron microscopy.
Conclusion
This paper provides basic information about the PEEK material and its used composites. There is also defined clinical distribution of implants with specific examples. The case studies were focused on the observation of osteointegration of individual implants, and completely recording the whole methodology of the implantation process. The end of the study is dedicated to recommendation of the necessary examinations to obtain the most relevant observation results.
Acknowledgement
This work was supported by research grant Inten-sification of methods molecular-proteomic biology in the field of study 05/02/47 Biomedical Engineering, doc. RNDr. Marianna Trebuňová, PhD., KEGA 069TU KE-4/2017, 01/2017–12/2019, Polymer PEEK analysis and possibilities of its additive production, Dr.h.c. prof. Ing. Jozef Živčák, PhD. MPH, APVV-15-0356, 07/2016 –06/2019; Manufacture and testing of tailored hard tissue replacements from hydroxyapatite (HA) with 3D printing technology, doc. Ing. Radovan Hudák, PhD., APVV-14-0294, 07/2015–06/2018.
Ing. Lukáš Mitrík
Department of Biomedical Technology
Faculty of Mechanical Engineering
Technical University in Košice
Letná 9, SK – 04200 Košice
E-mail: lukas.mitrik@tuke.sk
Phone: +421 908 238 351
Sources
- Panayotov IV, Orti V, Cuisinier F, Yachouh J. Polyether-etherketone (PEEK) for medical applications. Journal of Materials Science: Materials in Medicine. 2016 Jul 1;27(7):118.
- Shah AM, Jung H, Skirboll S. Materials used in cranioplasty: a history and analysis. Neurosurgical focus. 2014 Apr 1;36(4): E19.
- Johansson P, Jimbo R, Kjellin P, Currie F, Chrcanovic BR, Wennerberg A. Biomechanical evaluation and surface character-ization of a nano-modified surface on PEEK implants: a study in the rabbit tibia. International journal of nanomedicine. 2014;9 : 3903.
- Durham III JW, Montelongo SA, Ong JL, Guda T, Allen MJ, Rabiei A. Hydroxyapatite coating on PEEK implants: Bio-mechanical and histological study in a rabbit model. Materials Science and Engineering: C. 2016 Nov 1;68 : 723-31.
- Walsh WR, Pelletier MH, Bertollo N, Christou C, Tan C. Does PEEK/HA enhance bone formation compared with PEEK in a sheep cervical fusion model? Clinical Orthopaedics and Related Research. 2016 Nov 1;474(11):2364-72.
- Deng QX, Ou YS, Zhu Y, Zhao ZH, Liu B, Huang Q, Du X, Jiang DM. Clinical outcomes of two types of cages used in transforaminal lumbar interbody fusion for the treatment of degenerative lumbar diseases: n-HA/PA66 cages versus PEEK cages. Journal of Materials Science: Materials in Medicine. 2016 Jun 1;27(6):102.
- Kurtz SM. Applications of polyaryletheretherketone in spinal implants: fusion and motion preservation. In PEEK Biomaterials Handbook 2012 Jan 1 (pp. 201-220). William Andrew Publish-ing.
Labels
Biomedicine
Article was published inThe Clinician and Technology Journal

2019 Issue 1-
All articles in this issue
- DESIGN, MANUFACTURE AND TESTING OF RABBIT IMPLANTATION STRUCTURES FOR PRECLINICAL EXAMINATIONS
- APPLICATION OF SENSORY SYSTEMS TO MOVE DOGS WITH VISUAL IMPAIRMENT
- COST ANALYSIS OF TWO TYPES OF THE LUMBAR SPINE STABILIZING SURGERY
- CERAMIC ARCHITECTURES AS MODELS FOR 3D PRINTED TISSUE ENGINEERING APPLICATIONS
- OPTIMIZATION OF ZIRCONIA INKS TO FABRICATE 3D POROUS SCAFFOLDS BY ROBOCASTING
- The Clinician and Technology Journal
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- OPTIMIZATION OF ZIRCONIA INKS TO FABRICATE 3D POROUS SCAFFOLDS BY ROBOCASTING
- COST ANALYSIS OF TWO TYPES OF THE LUMBAR SPINE STABILIZING SURGERY
- CERAMIC ARCHITECTURES AS MODELS FOR 3D PRINTED TISSUE ENGINEERING APPLICATIONS
- DESIGN, MANUFACTURE AND TESTING OF RABBIT IMPLANTATION STRUCTURES FOR PRECLINICAL EXAMINATIONS
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career