-
Medical journals
- Career
Primary retroperitoneal Ewing’s sarcoma
Authors: J. Spacek 1; K. Kopeckova 2; J. Kosina 1; J. Pacovsky 1; J. Petera 3; L. Krbal 4; M. Mrhalová 5; P. Dvorak 6; M. Broďák 1
Authors‘ workplace: Department of Urology, Faculty of Medicine in Hradec Kralove and University Hospital, Hradec Kralove 1; Department of Oncology, 2nd Faculty of Medicine and Faculty Hospital Motol, Charles University, Prague 2; Department of Oncology and Radiotherapy, Faculty of Medicine in Hradec Kralove and University Hospital, Hradec Kralove 3; The Fingerland Department of Pathology, Faculty of Medicine in Hradec Kralove and University Hospital, Hradec Kralove 4; Department of Pathology and Molecular Medicine, 2nd Faculty of Medicine and Faculty Hospital Motol, Charles University, Prague 5; Department of Radiology, Faculty of Medicine in Hradec Kralove and University Hospital, Hradec Kralove 6
Published in: Rozhl. Chir., 2019, roč. 98, č. 3, s. 121-124.
Category:
Overview
The Ewing’s sarcoma (EWS) family tumors are small, round, cell tumors with different degrees of neuroectodermal differentiation with a peak incidence in children and young adults. About 10–20% of cases are extraskeletal EWS.
Keywords:
Ewing’s sarcoma – retroperitoneum – computed tomography – surgery
INTRODUCTION
Retroperitoneal EWS tumors including extraskeletal EWS are rare malignant tumors. However, because of the pattern of its presentation and the presence of nonspecific symptoms, the diagnosis of retroperitoneal EWS remains challenging. Systemic chemotherapy is the crucial therapeutic modality in the treatment of EWS family tumors. Surgery represents a part of a complex treatment, but it is not the initial step in majority of the cases. The management of therapy must be active and multidisciplinary, using all available methods of modern medicine.
CASE REPORT
A 27-year-old woman was admitted to the department of general medicine following uncorrected arterial hypertension, vision disorder because of hypertension retinopathy, and epigastric pain. It is worth mentioning that she had a two-year history of untreated hypertension. The patient has only taken hormonal contraceptives over the long term.
The high blood pressure was successfully corrected by the administration of parenteral antihypertensives (urapidil), with a rapid vision improvement at admission. Subsequent contrast computed tomography (CT) examination of the abdomen revealed extensive tumor expansion in the retroperitoneum, with close relation to the left kidney. However, the presence of fluid in the abdominal cavity and retroperitoneum increased the probability of a ruptured tumor (Fig. 1). Due to progressive anemia with the absence of gross hematuria, the patient was transferred to an intensive care unit. A urologist consultant examined the patient subsequently and advised the patient to undergo an acute surgery with the revision of the abdominal cavity and retroperitoneum. Because of the unclear origin of the tumor and the possibility of hypersecretion of catecholamines, free plasma metanephrine test was performed, the level of which eliminates their overproduction. The surgery was performed by a general surgeon using a lumbotomy approach. Because of the proximity between the tumor and the vascular structures of the left kidney, left-sided nephrectomy with adrenalectomy and para-aortic lymphadenectomy were performed along with tumor extirpation (Fig. 2, 3). The surgery took 170 minutes with a 500 mL perioperative blood loss. The surgeon confirmed a rupture of the tumor with perirenal hematoma and hemorrhagic fluid in the abdominal cavity. There were no complications during the postoperative period with early patient’s mobilization, and the patient was discharged a week after surgery. Because of the unexpected histological finding of retroperitoneal EWS, a special molecular pathology examination was performed. Molecular cytogenetic testing confirmed definitive diagnosis, and fluorescence in situ hybridization of interphase nuclei (I-FISH) revealed translocation t (11; 22) (q24; q12). Due to the diagnosis and presence of tumor cells in one of the removed lymph nodes, the urologist ordered a positron emission tomography/computed tomography (PET/CT) scan to be performed and the patient was referred to a specialized oncology center.
1. Computed tomography of an abdomen : a huge retroperitoneal tumor with a fluid in peritonel cavity 
2. Retroperitoneal tumor extirpation from lumbothomy approach 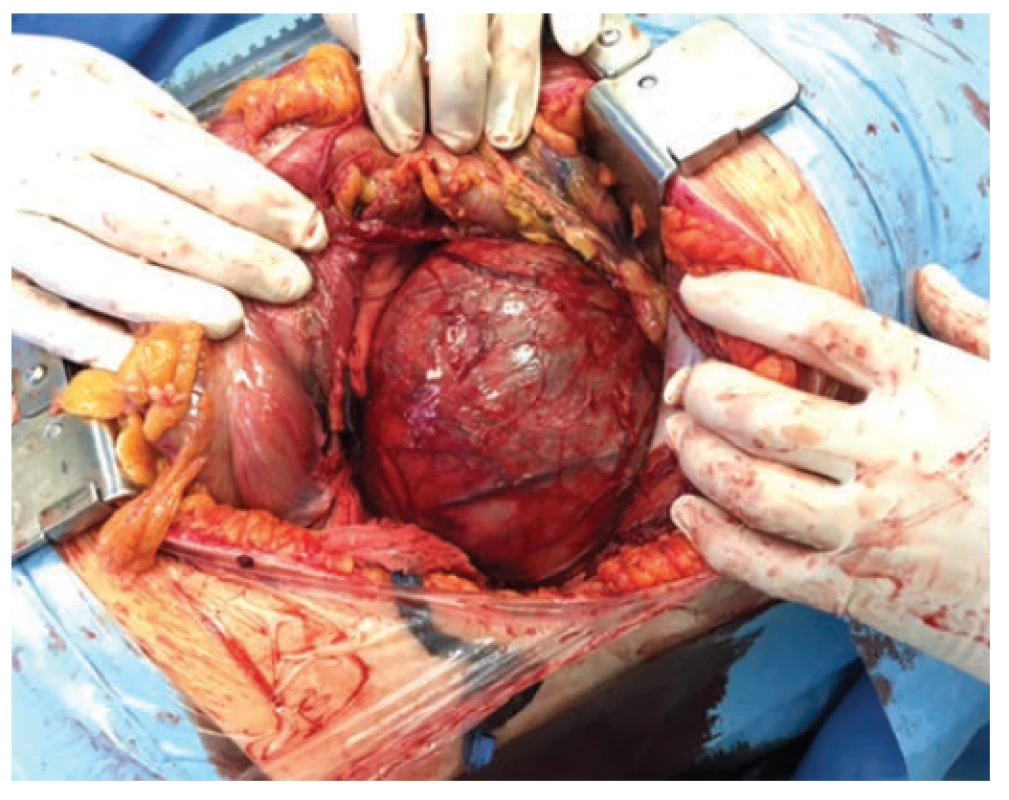
3. Cross section of a tumor and kidney with capsule rupture pointed by an arrow 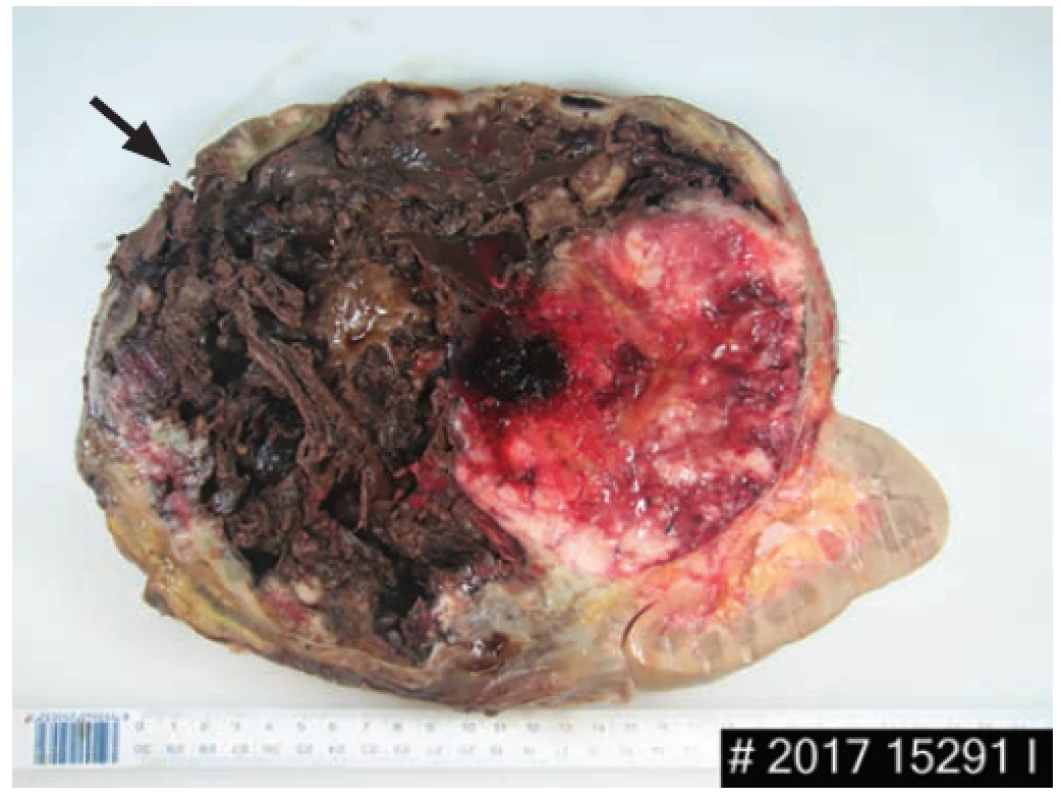
Based on the preliminary histology result, the urologist referred to an optimal diagnostic algorithm along with an oncologist from the specialized complex oncology center, where the patient was finally referred. The PET/CT imaging showed suspected hypermetabolic deposits in the liver and retroperitoneum, which was diagnosed as a metastatic disease.
The oncologist included the patient in an international, open-label study (Euro Ewing 2012, phase III), randomized to a vincristine, doxorubicin, cyclophosphamide/ifosfamide, etoposide (VDC/IE) chemotherapy arm, which started one month after surgery. The patient has currently finished her chemotherapy, and complete remission has been achieved. Another PET/CT control imaging will be performed in three months.
DISCUSSION
The EWS tumors are poorly differentiated neoplasms formed by small, round cells. These tumors have mesenchymal origin, with a median age of about 15 years [1]. They rarely occur in the elderly patients, and these are an immensely unusual finding after 40 years of age [2].
The diagnosis of this group of tumors remains clinically and diagnostically challenging. The development of new histochemical and immunohistochemical methods has deepened the understanding of this relatively heterogeneous group of tumors. The classification of EWS into bone and extraskeletal form of EWS, primitive neuroectodermal tumor (PNET), Askin’s tumor (EWS/PNET in the chest wall), and atypical EWS has been established for a long time [3]. The current World Health Organization classification of bone and soft tissue tumors from 2013 is based on the results of a molecular genetic examination, and the abovementioned division of the Ewing group sarcomas is perceived as a spectrum of similar tumors rather than individual distinctly different entities [4].
James Ewing first described the skeletal form of EWS in 1921 [5] and the extraosseous form of EWS that M. Tefft mentioned in his article in 1969 (6). Literary sources differ in the incidence of extraskeletal EWS/PNET, which fluctuates between 11 and 24% of the total number of EWS cases [2]. The retroperitoneum as the primary site of EWS/PNET is unusual and has been reported in individual case studies [7,8]. These types of tumors most commonly metastasize to the lungs, bones, and bone marrow and more rarely to the nodes, liver, and brain.
Retroperitoneal sarcomas, including the discussed EWS/PNET, are rare malignant tumors that may, due to their nonspecific symptoms (pain, weight loss), often be missed for a long time [9]. The skeletal forms of EWS are more often manifested by pain, swelling, or the development of the pathological fracture of the affected area. CT and magnetic resonance imaging have crucial roles in the diagnosis of retroperitoneal neoplasms. Final staging also requires hybrid PET/CT, bone scan, or bone marrow examination. However, histological examination of the biopsy using specific immunohistochemical and molecular biological methods remains the most crucial in the diagnosis of EWS. The most common histochemical and immunohistochemical phenotypes are glycogen-positive periodic acid-Schiff, FLI-1, CD117, Bcl-2, p53, S100, vimentin, and a typical CD99. In our case, the rich membrane activity of CD99 (Fig. 4) and the strongly positive reactivity of the keratin have been demonstrated. This property reflects the ability of cells for an epithelioid differentiation, although the epithelial tumor is indeed not identifiable. The diagnostic method with high specificity includes molecular cytogenetic examination (e.g., R-T PCR, I-FISH). Evidence of translocation and the development of fusion gene EWS from a family of E26 transformation-specific is currently a crucial step in molecular biology diagnostics. Specific translocations affecting chromosome 22 were found in EWS, and the most common structural reconstructions are translocations t(11; 22) (q24; q12) and t(21; 22) (q22; q12), which make up 85% of aberration. There may be sporadic minor translocations (9). The result of chromosomal aberration – translocation t(11; 22) (q24; q12) – is a fusion of the EWS gene on chromosome 22 and the FLI-1 gene on chromosome 11 (11). In our patient, a break in 22q12 (EWSR1) gene and the translocation t(11; 22) (q24; q12) FLI-1/EWSR1 (Fig. 5) were detected by molecular cytogenetic methods.
4. Immunohistochemical detection of high positive of membrane antigen CD99 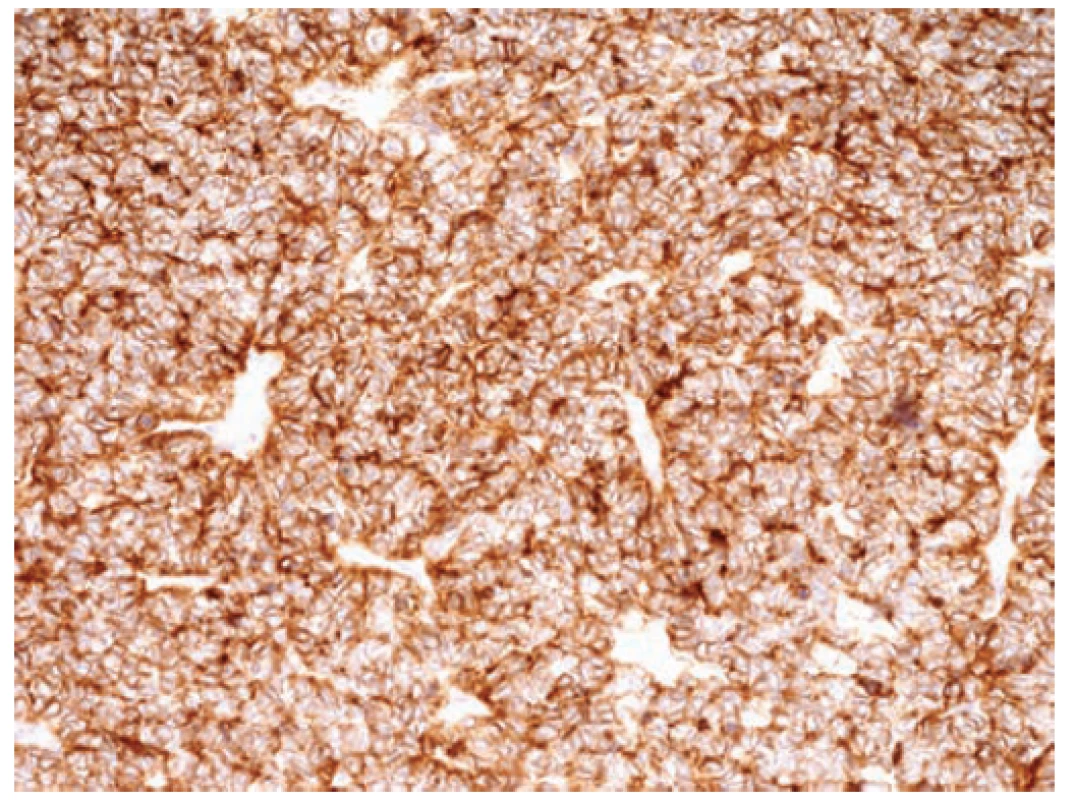
5. Interphase fluorescence in situ hybridization analysis 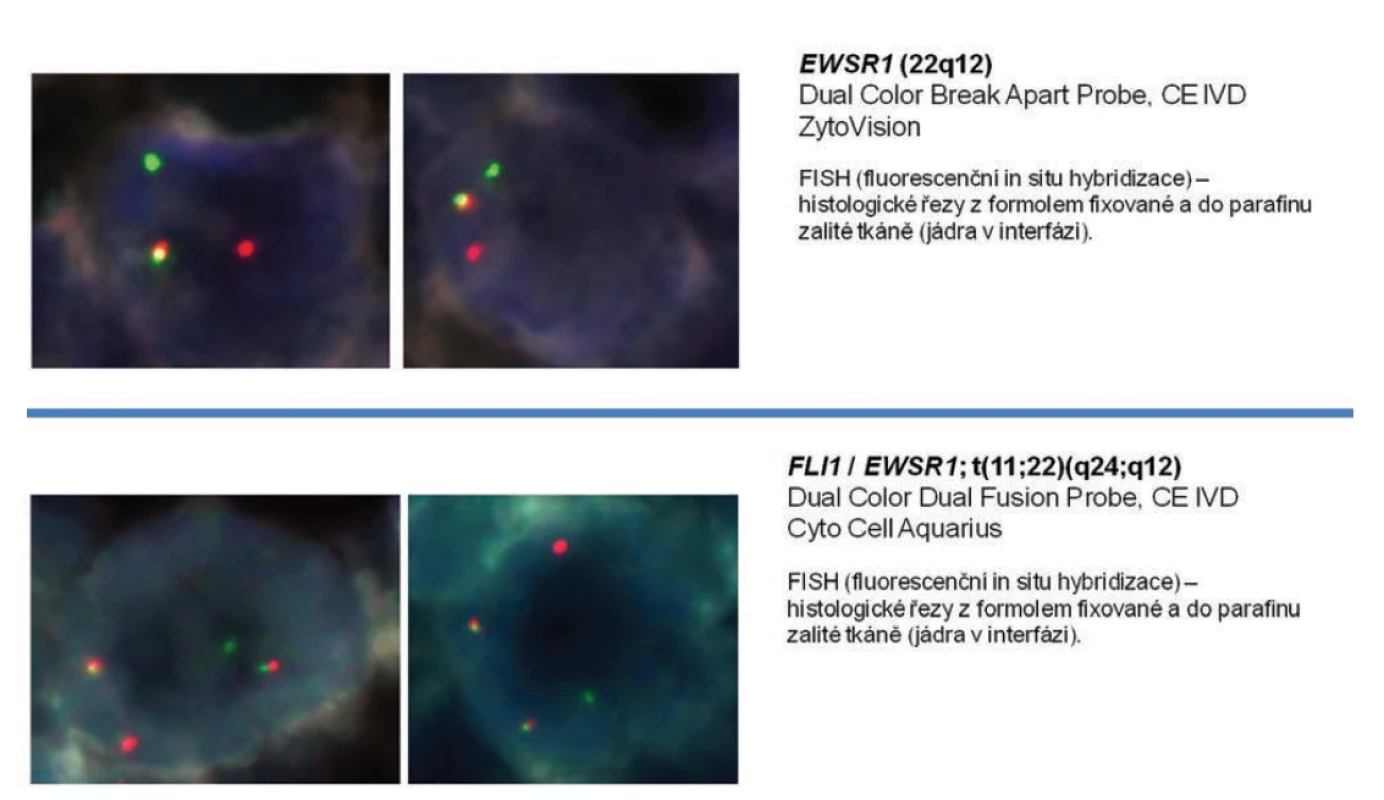
EWS/PNET is a group of chemosensitive and radiosensitive tumors. Systemic chemotherapy is the primary treatment modality. As a part of complex therapeutic management, surgery is also performed after chemoradiotherapy. However, in other retroperitoneal sarcomas, a critical step is the performance of radical surgery, ideally with a zero residual tumor [9,10]. In European centers, complex treatment of EWS/PNET is based on the Euro Ewing Working Group protocols [3,15,16]. In our case, the patient was included in the Euro Ewing 2012 clinical trial. Oncological treatment is applied according to risk stratification. The main negative prognostic factor is primarily metastatic disease except for isolated lung metastases, which have a better prognosis. Other negative factors are primary tumor volume (>200 ml), patient age (>14 years), bone bearing presence, and bone marrow involvement [16]. The individual protocols have varying degrees, but the primary treatment is induction chemotherapy, that is, VDC/IE or vincristine, ifosfamide, doxorubicin, and etoposide.
In some cases, local therapy is followed by surgery, which is being preferred over radiotherapy. Consolidation therapy involves chemotherapy and eventually irradiation. High-dose chemotherapy (megachemotherapy) followed by hematopoietic stem cell transplantation is performed in high-risk patients [3,16].
Targeted therapy in biological treatment still remains the domain of clinical studies. Their results have not yet shown sufficient efficiency and are not part of standard protocols [16,17,18]. Our case was unique with its initial symptoms the presence of fluid in the abdominal cavity, anemia, and hemodynamic instability, which required surgical intervention as an initial step of the treatment. It should be emphasized that this therapeutic approach goes beyond the recommendations mentioned above, which prefers tumor biopsy, histopathological examination, and chemotherapy. A preoperative histological examination with frozen sections was not performed, and it remains questionable whether it would have been beneficial considering it is time-consuming and involves specific difficulty in interpreting the histopathological findings and the urgency of the surgery.
CONCLUSION
We present an unusual case of retroperitoneal EWS. The systemic chemotherapy serves a crucial role in the treatment of this type of tumors. Surgical intervention remains indicated in case of presence of a residual tumor mass after previous systemic chemotherapy. The goal of surgery is tumor extirpation with survival benefit related to R0 resection. Treatment of these tumors should be performed in complex oncology centers that have experience with this kind of diagnosis and can provide a multidisciplinary approach.
List of abbreviations
EWS – Ewing’s sarcoma
ICU – intensive care unit
I-FISH – fluorescence in situ hybridization of interphase nuclei
MRI – magnetic resonance imaging
PET/CT – positron emission tomography/computed tomography
PNET – primitive neuroectodermal tumor
R-T PCR – real-time polymerase chain reaction
Supported by scientific programme Progres Q40/04.
Conflict of interests
The authors declare that they have not conflict of interest in connection with the emergence of and that the article was not published in any other journal.
Jiri Spacek M.D.
Department of Urology,
Faculty of Medicine in Hradec Kralove and University Hospital
Sokolovska 581
500 05 Hradec Kralove
e-mail: jiri.spacek@fnhk.cz
Sources
- Lin PP, Wang Y, Lozano G. Mesenchymal stem cells and the origin of Ewing’s sarcoma. Sarcoma 2011. doi:org/10.1155/2011/ 276463
- Bleyer WA, Barr RD. Cancer in adolescents and young adults, bone cancer. New York, Springer Verlag 2007 : 203– 215.
- Krákorová AD, Tuček Š, Tomášek J, et al. Léčba Ewingova sarkomu/periferního neuroektodermálního tumoru dospělých. Onkologie 2012;6 : 91−5.
- Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. World Health Organization classification of tumors of soft tissue and bone, 4th ed. Lyon, IARC Press 2013.
- Ewing J. Diffuse endothelioma of bone. Proc New York Path Soc 1921;21 : 17–24.
- Tefft M, Vawter GF, Mitus A. Paravertebral “round cell” tumors in children. Radiology 1969;92 : 1501–9.
- Rowe RG, Thomas DG, Schuetze SM, et al. Ewing sarcoma of the kidney: case series and literature review of an often overlooked entity in the diagnosis of primary renal tumors. Urology 2013;81 : 347−53.
- Raney RB, Asmar L, Newton WA Jr, et al. Ewing’s sarcoma of soft tissues in childhood: a report from the Intergroup Rhabdomyosarcoma Study, 1972 to 1991. J Clin Oncol 1997;15 : 574−82.
- Brodak M, Spacek J, Pacovsky J, et al. Multidisciplinary approach as the optimum for surgical treatment of retroperitoneal sarcomas in women. Eur J Gynaecol Oncol 2013;34 : 234−7.
- Bajčiová V, Štěrba J, Tomášek J, et al. Nádory adolescentů a mladých dospělých. Praha, Grada 2011 : 108−14.
- Procházka P, Vícha A, Kodet R, et al. Nádory ze skupiny Ewingova sarkomu – molekulární biologie a genetika. Klin Onkol 2007;20 : 205– 8.
- Berková A, Dundr P, Povýšil C, et al. Molekulární diagnostika Ewingova sarkomu: porovnání RT-PCR a FISH metodou pro tkáně zalité v parafínu. Čes-slov Patol 2008;44 : 67−70.
- Rud NP, Reiman HM, Pritchard DJ, et al. Extraosseous Ewing’s sar - coma. A study of 42 cases. Cancer 1989;64 : 1548−53.
- Covelli HD, Beekman JF, Kingry RL. Extraskeletal Ewing’s sarcoma: Prolonged survival with recurrence after operation. Southern Medical Journal 1980;73 : 1294−5.
- Thacker MM, Temple HT, Scully SP. Current treatment for Ewing‘s sarcoma. Expert Rev Anticancer Ther 2005;5 : 319−31.
- Gaspar N, Hawkins DS, Dirksen U, et al. Ewing Sarcoma: Current management and future approaches through collaboration. J Clin Oncol 2015;33;27 : 3036−46.
- Garofalo C, Mancarella C, Grilli A, et al. Identification of common and distinctive mechanisms of resistance to different anti-IGF-IR agents in Ewing’s sarcoma Mol Endocrinol 2012;26 : 1603−16.
- Brenner JC, Feng FY, Han S, et al. PARP-1 inhibition as a targeted strategy to treat Ewing’s sarcoma Cancer 2012;72 : 1608−13.
Labels
Surgery Orthopaedics Trauma surgery
Article was published inPerspectives in Surgery

2019 Issue 3-
All articles in this issue
- Všeobecná chirurgie?
- Current view on prostheses in herniology (hernia meshes) – classifications, indications, advantages and disadvantages of different implants, complications J. Skach, M. Slamborova, V. Blecher, P. Hromadka, R. Gurlich
- Animal models of liver diseases and their application in experimental surgery
- Assessment of anastomosis perfusion by fluorescent angiography in robotic low rectal resection: the results of a non-randomized study
- Distal intestinal obstruction syndrome in a patient with cystic fibrosis after lung transplantation
- Zemřel primář Michal Leško
- Pracovní dny Koloproktologické sekce České chirurgické společnosti ČLS JEP
- Dysphagia after anterior cervical discectomy and interbody fusion – prospective study with 1-year follow-up
- Primary retroperitoneal Ewing’s sarcoma
- Hip synovial cyst presenting as femoral hernia – case report
- Perspectives in Surgery
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Current view on prostheses in herniology (hernia meshes) – classifications, indications, advantages and disadvantages of different implants, complications J. Skach, M. Slamborova, V. Blecher, P. Hromadka, R. Gurlich
- Hip synovial cyst presenting as femoral hernia – case report
- Distal intestinal obstruction syndrome in a patient with cystic fibrosis after lung transplantation
- Zemřel primář Michal Leško
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

