-
Medical journals
- Career
Neonatal pneumonia caused by Trichomonas vaginalis
Authors: M. Pokrývková 1; P. Zárubová 2; H. Wiedermannová 1,2; H. Burčková 1; J. Mrázek 3; J. Pavlíček 4,5
Authors‘ workplace: Department of Neonatology, University Hospital Ostrava, Ostrava, Czech Republic 1; Faculty of Medicine, University of Ostrava, Ostrava, Czech Republic 2; Public Health Institute Ostrava, Ostrava, Czech Republic 3; Department of Pediatrics, University Hospital, Ostrava, Ostrava, Czech Republic 4; Biomedical Research Center, University Hospital Hradec Kralove, Hradec Kralove, Czech Republic 5
Published in: Epidemiol. Mikrobiol. Imunol. 69, 2020, č. 2, s. 96-99
Category:
Overview
Neonatal pneumonia is mostly bacterial and other etiology is considered less frequently. We report a case of newborn whose neonatal pneumonia has not improved, despite the aggressive ventilation regime and empiric antibiotic therapy. A special sample from the respiratory tract was collected for PCR examination. The test confirmed the presence of Trichomonas vaginalis. Antibiotic therapy was extended to include metronidazole. Targeted antibiotic therapy, which lasted for 28 days, improved the condition and the patient was discharged in a stabilized condition to home care on the 44th day of life. We demonstrate the need to consider atypical pathogens in the case of infections that do not respond to conventional therapy. The multiplex real-time PCR technique was used to detect the DNA of the pathogen. Targeted antibiotic therapy is the result of pathogen identification.
Keywords:
Trichomonas vaginalis – newborn – neonatal pneumonia
INTRODUCTION
In newborns neonatal pneumonia is a relatively frequent cause of postpartum adaptation disorders or respiratory distress, which require respiratory support of various degrees, oxygen therapy, and antibiotic treatment. The etiologic agent is typically bacterial, but less frequently, mycotic or viral. Pneumonias caused by protozoa are rare in humans, which is why they are only exceptionally considered. Atypical pathogen should be considered in cases where the clinical condition does not respond to conventional therapy, in newborns including pathogens of sexually transmitted diseases (Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium, Mycoplasma hominis, Ureaplasma sp., Trichomonas vaginalis).
Trichomonas vaginalis is a motile anaerobic protozoon with a cosmopolitan prevalence. Infections of Trichomonas vaginalis are most frequently transmitted during sexual intercourse, and less frequently through contaminated items. The infection may provoke an early birth in pregnant women, premature rupture of membranes, and it is associated with a low birth-weight baby [1, 2]. Nevertheless, perinatal mother-to-child transmission is possible and the infection may have a severe clinical course.
CASE
The patient was a child of a first pregnancy. The mother was a smoker, with a history of methamphetamine and heroin abuse and chronic hepatitis C. She attended pregnancy counselling only once, in the 23rd week of gestation. All pregnancy-screening tests were performed only after the mother was admitted to the hospital; thus, a sample was not collected for a Streptococcus agalactiae culture.
The baby was delivered at the estimated 37th week of gestation. Approximately 6 h prior to delivery, the amniotic membranes were ruptured, and turbid amniotic fluid was released. Therefore, antibiotic prophylaxis was administered. A male infant was spontaneously delivered, with normal immediate postpartum adaptation, an Apgar score of 9-10-10 points, and the umbilical pH was 7.22. Hyposaturation was diagnosed at approximately 20 min. Cardiopulmonary resuscitation was initiated, the newborn was intubated, and subsequently, received conventional artificial respiration. An aggressive respiratory regime, with a maximum fraction of oxygen, was required to maintain oxygen saturation within the normal range. A smear from the endotracheal cannula was collected for analysis, due to suspicion of pneumonia. The sample was outsourced for culturing and PCR testing to identify respiratory agents.
The patient was admitted to the intensive care unit. Aggressive conventional ventilation was continued. Auscultation revealed rales bilaterally, with more prominent rales on the right side. Large volumes of green-brown sputum were suctioned from the lower respiratory tract. A radiology examination of the lungs revealed extensive bilateral shading, which indicated inflammatory infiltration (Figure 1). The laboratory analyses showed metabolic acidosis, anaemia, based on the complete blood count (haemoglobin 127 g/L, haematocrit 0.37), and normal levels of leukocytes and thrombocytes. Inflammatory parameters (C-reactive protein, interleukin-6) indicated no inflammation. Samples were collected for bacteriological culture screening (haemoculture, throat culture, and a smear from the lower respiratory tract). Despite the negative signs of inflammation, due to the clinical condition of the patient and the findings on radiography, a combined antibiotic therapy (ampicillin-sulbactam, gentamicin) was introduced empirically. The clinical condition of the patient did not improve, despite aggressive ventilation. Due to the patient’s condition, a surfactant was administered endotracheally; however, it had practically no effect.
1. Inflammatory infiltration, neonatal pneumonia 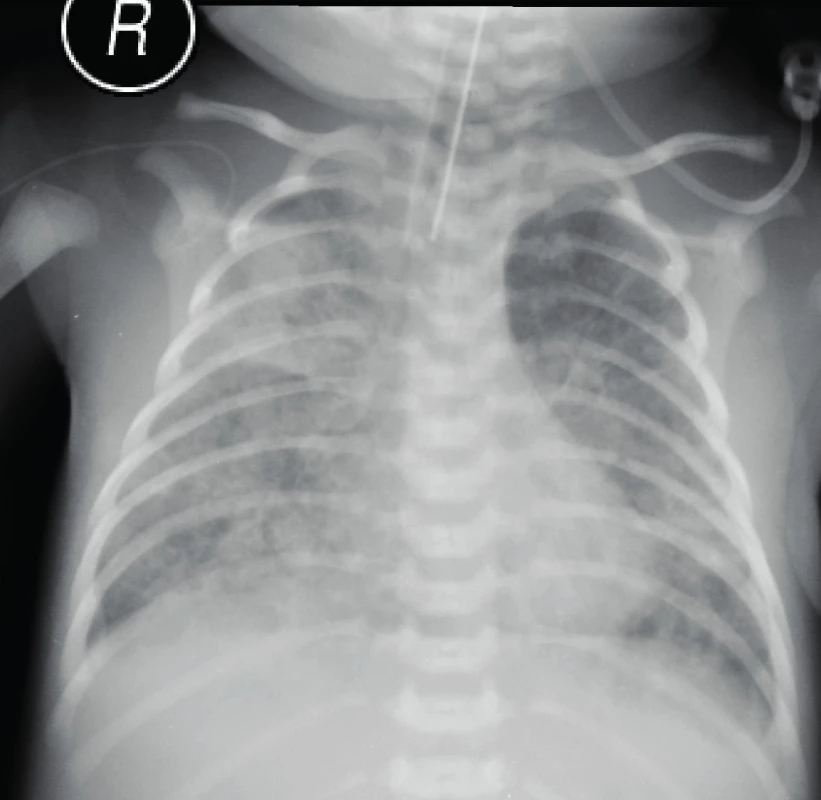
The microbiology test results were available on the third day. The culture results were negative; however, the PCR test confirmed the presence of Trichomonas vaginalis in the smear from the respiratory tract. Antibiotic therapy was extended to include metronidazole. Additional samples were sent for PCR examination. They confirmed the presence of Trichomonas vaginalis DNA. The clinical condition deteriorated, the patient exhibited hyposaturation, hypotension, and was transferred to high-frequency oscillation ventilation. The circulation required pharmacological support with catecholamines. Erythrocytes were transfused, due to anaemia. Subsequent echocardiography again excluded pulmonary hypertension. We consulted with the ECMO (extracorporeal membrane oxygenation) centre about the child’s condition. The criteria for ECMO were not fulfilled. A highly aggressive regime of oscillation ventilation was required (Paw 18; ΔP 31). After administering this regime, the patient’s condition gradually improved on the eighth day of life. The inspired fraction of oxygen and the ventilation regime were slowly reduced, according to the measured oxygen saturation and oxaemia. Radiography findings also improved during the course of treatment. A follow-up examination (PCR test in the smear from the respiratory tract) showed the absence of Trichomonas vaginalis with the targeted therapy.
After two weeks of high-frequency oscillation ventilation, we transferred the patient back to conventional ventilation, and 35% oxygen was sufficient to maintain the desired oxygen saturation. The patient was extubated on the 21st day of life, transferred to distension respiratory support, and subsequently, transferred to a high-flow nasal cannula with 25% oxygen. The intravenous therapy targeted for neonatal pneumonia was discontinued after 28 days, based on a consultation with colleagues specialized in infectious diseases. The child was transferred to an intermediary centre for further treatment. Subsequently, respiratory support and oxygen therapy were discontinued. Additionally, the chest Xray findings significantly improved prior to patient discharge. The patient was discharged in a stabilized condition to home care on the 44th day of life, after the mother underwent social investigation. Finally, the patient was followed in specialized outpatient departments.
DISCUSSION
Trichomonas vaginalis is a primitive eukaryote. Due to its body composition, Trichomonas vaginalis displays locomotion characterized by undulatory movements. Considering its genetic equipment, it cannot synthesize a number of vital molecules (purines, pyrimidines, lipids); accordingly, it is classified as a parasitic organism. Trichomonas vaginalis cannot survive very long without a host, and thus, it has little resistance against the environment. To ensure successful growth and development, it requires acidic pH and an adequate source of essential nutrients. These are obtained from the vaginal discharge or sperm. It can survive for 24 h in body secretions (urine, sperm, vaginal discharge) and for 1–2 h on contaminated items. In men, the infection might manifest as mild urethritis, and rarely, prostatitis. In women, the infection evokes vulvovaginitis and cervicitis. A completely asymptomatic course of infection is also possible. Perinatal transmission has also been described. In the newborn child, the infection may manifest as urethritis or vulvovaginitis; in this case, the agent may be detected in the urine [3, 4, 5]. The presence of the agent has also been observed in aspirates from the respiratory tracts and stomachs of newborns; subsequently, it manifests in the form of neonatal pneumonia and respiratory failure after birth [6, 7, 8]. Neonatal pneumonias caused by Trichomonas vaginalis are uncommon diseases. Incidence of vertical transmission, during vaginal delivery, to neonates is about 2 to 17%, girls are more affected. Many newborns can be asymptomatic [9, 10]. In cases of neonatal infection, identification of the cause may be difficult. Even a confirmation of Trichomonas vaginalis in a PCR analysis of smear samples might not confirm that the infection was caused by this pathogen. The suspicion of infection might be based on the presence of leukocyturia, with negative growth in aerobic culture conditions [5]. Our patient initially exhibited negative cultures, low levels of inflammatory markers, and zero response to empiric antibiotic therapy; therefore, it was reasonable to assume that the cause of the condition was Trichomonas vaginalis. The pathogen can be transferred to the respiratory tract of newborns in utero, by aspirating infected amniotic fluid, or intrapartially, by aspirating infected vaginal secretion [7, 8].
The diagnostic procedures include clinical and radiological examinations. In addition, microbiology testing is crucial for identifying the responsible microbial agent. Motile protozoa may be observed in the sample with microscopy. The microbiological examination includes a microscopic assessment of the native or stained sample, culture assays, immunohistochemistry, and a PCR assay [11]. The PCR technique can be performed with specific primers. Microbial DNA can be identified from both viable and non-viable trichomonads. At our centre, the standard of care for intubated children is to collect samples for the initial microbiology tests (apart from a haemoculture) and a smear from the lower respiratory tract for culture and microscopy examinations. In children supported with non-invasive ventilation, a smear from the throat is collected. Examinations of secretions from the upper or lower respiratory tract with PCR are not included in the standard panel of tests. We outsource special smears for testing only in indicated cases (e.g., significant findings on radiography; no improvement, despite standard antibiotic therapy; negative findings in common culture examinations and persistent clinical symptoms; or positive findings on an X-ray). In our patient, the diagnostics included the multiplex real-time PCR technique, which was designed to detect DNA of the most commonly observed sexually-transmitted pathogens (Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium, Mycoplasma hominis, Ureaplasma sp., Trichomonas vaginalis). This technique has a high specificity and sensitivity [11, 12].
Positive chest X-ray findings include flowing or striped infiltrations, bright cystic areas, or nidi with hyperinflation and atelectasis. However, these signs cannot distinguish neonatal pneumonia from other neonatal pneumopathies. Additionally, chronic long-term consequences cannot be ruled out in newborns with a history of pulmonary infection.
In diagnosing infections caused by Trichomonas vaginalis, the mother’s gynaecology and infectious anamnesis is highly important. Indeed, it is possible to prevent transferring the infection to the child by providing adequate therapy to the infected mother. Information regarding a vaginal discharge and discomfort could indicate the presence of a sexually transmitted disease in the mother. However, the mother of our patient did not attend pregnancy counselling regularly, and communication with the mother was sparse after delivery. Consequently, this information was missing in the gynaecologic history of the mother.
CONCLUSION
Due to the low frequency of infections caused by trichomonads in newborns, these infections are only seldom considered. In cases where newborn with neonatal pneumonia does not respond to empiric antibiotic therapy, it is appropriate to search for atypical causes of infection. A special sample from the respiratory tract, throat, or gastric aspirate should be collected for PCR examination. Then, based on the results, a targeted antibiotic therapy (with metronidazole) should be introduced in the child. It is not possible to rule out chronic long-term respiratory difficulties. Subsequent follow-up at a specialized pulmonary outpatient clinic is recommended after treating pneumonia caused by Trichomonas vaginalis.
Do redakce došlo dne 11. 2. 2020.
Adresa pro korespondenci:
MUDr. Jan Pavlíček, Ph.D.
Katedra dětského lékařství a neonatologie Fakultní nemocnice
17. listopadu 1790
708 52 Ostrava-Poruba
e-mail: jan.pavlicek@fno.cz
Sources
1. Hosny AEDM, El-khayat W, Kashef MT, Fakhry MN. Association between preterm labor and genitourinary tract infections caused by Trichomonas vaginalis, Mycoplasma hominis, Gram-negative bacilli, and coryneforms. J Chin Med Assoc, 2017;80(9):575–581.
2. Cotch MF, Pastorek JG II, Nugent RP, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The vaginal infections and prematurity study group. Sex Transm Dis, 1997;4(6):353–360.
3. Danesh IS, Stephen JM, Gorbach J. Neonatal Trichomonas vaginalis infection. J Emerg Med, 1995;13(1):51–54.
4. Smith LM, Wang M, Zangwill K, Yeh S. Trichomonas vaginalis Infection in a Premature Newborn. J Perinatol, 2002;22(6):502–503.
5. Hoffman DJ, Brown GD, Wirth FH, et al. Urinary tract infection with Trichomonas vaginalis in a premature newborn infant and the development of chronic lung disease. J Perinatol, 2003;23(1):59–61.
6. Carter JE, Whithaus KC. Neonatal respiratory tract involvement by Trichomonas vaginalis: a case report and review of the literature. Am J Trop Med, 2008;78(1):17–19.
7. Hiemstra I, Van Bel F, Berger HM. Can Trichomonas vaginalis cause pneumonia in newborn babies? Br Med J (Clin Res Ed), 1984;289(6441):355–356.
8. Temesvari P, Kerekes A, Tege A, Szarka K. Demonstration of Trichomonas vaginalis in tracheal aspirates in infants with early respiration failure. J Matern Fetal Neo M, 2002;11(5):347–349.
9. Trintis J, Epie N, Boss R, Riedel S. Neonatal Trichomonas vaginalis infection: a case report and review of literature. Int J STD AIDS, 2010;21(8):606–607.
10. Bruins MJ, van Straaten ILM, Ruijs GJHM. Respiratory Disease and Trichomonas Vaginalis in Premature Newborn Twins. Pediatr Infect Dis J, 2013;32(9):1029–1030.
11. Garber GE. The laboratory diagnosis of Trichomonas vaginalis. Can J Infect Dis Med, 2005;16(1):35–38.
12. Petrin D, Delgaty K, Bhatt R, Garber G. Clinical and Microbiological Aspects of Trichomonas vaginalis. Clin Microbiol Rev, 1998;11(2):300–317.
Labels
Hygiene and epidemiology Medical virology Clinical microbiology
Article was published inEpidemiology, Microbiology, Immunology

2020 Issue 2-
All articles in this issue
- The significance of p16 protein expression in oral squamous cell carcinoma
- Barriers to treatment of infectious and other somatic comorbidity in drug users
- Q fever and prevention
- Smuteční oznámení: zemřel profesor MUDr. Miroslav Votava, CSc.
- Blahopřání RNDr. Marii Brůčkové, CSc.
- Intra-abdominal candidiasis in surgical intensive care unit – epidemiology characteristics and trends
- Vzácná invazivní fungální infekce Mucor circinelloides a Fusarium u imunokompetentního pacienta po devastačním poranění dolní končetiny s rekonstrukcí volným lalokem m. latissimus dorsi
- Neonatal pneumonia caused by Trichomonas vaginalis
- Epidemiology, Microbiology, Immunology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- The significance of p16 protein expression in oral squamous cell carcinoma
- Q fever and prevention
- Neonatal pneumonia caused by Trichomonas vaginalis
- Vzácná invazivní fungální infekce Mucor circinelloides a Fusarium u imunokompetentního pacienta po devastačním poranění dolní končetiny s rekonstrukcí volným lalokem m. latissimus dorsi
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

