-
Medical journals
- Career
Cystatin C – Implementation in clinical laboratory practice
Authors: T. Šálek 1,2,3; B. Friedecký 1; M. Budina 1
Authors‘ workplace: SEKK, spol. s. r. o., Za Pasáží 1609, 530 02 Pardubice 1; Department of Clinical Biochemistry and pharmacology, Tomas Bata Hospital in Zlín a. s., Havlíčkovo nábřeží 600, 762 75 Zlín, Czech Republic 2; Medical Faculty of the University of Ostrava, Department of Biomedical sciences, Syllabova 19, 703 00 Ostrava – Zábřeh 3
Published in: Klin. Biochem. Metab., 27, 2019, No. 1, p. 16-18
Overview
Objectives: Kidney Disease Improving Global Outcomes (KDIGO) guidelines recommend reporting the cystatin C concentration result together with estimated glomerular filtration rate (eGFRcys). The extent to which the recommendation is implemented to practice is not known. The aim of this study is to determine the implementation extent and to show how laboratories that have not yet implemented the measure can be guided to adopting it to their routines.
Design: Cross – sectional study.
Settings: SEKK, spol. s.r.o., Za Pasáží 1609, 530 02 Pardubice.
Material and methods: Evaluation of cystatin C post-analytical phase was performed by an online electronic questionnaire which was added to routine cystatin C External Quality Assessment (EQA) scheme. A total of 70 participants (59 from the Czech Republic and 11 from Slovakia) were given the questionnaire. They reported traceability of their method calibration to international standard ERM DA471/IFCC, and equations for eGFRcys. Answers were analysed.
Results: In the end, 63 participants responded to the questionnaire. Traceability of calibration to ERM DA471/IFCC was declared by 53 responders. A total of 53 laboratories stated reporting eGFR in all adult patients and 4 participants stated reporting eGFR only on direct request. Six laboratories did not report eGFR. The Chronic Kidney Disease Epidemiology Collaboration (CKD - EPI) equation was used by 57 laboratories. Of them, 22 laboratories also used combined equation with creatinine and three laboratories also calculated Caucasian, Asian, Pediatric and Adult (CAPA) equation.
Conclusion: Majority of laboratories follow the KDIGO guidelines. Further education on calibration traceability and eGFR accompanying all cystatin C concentration results is still needed.
Keywords:
Cystatin C – Glomerular filtration – Estimated glomerular filtration rate – Post-analytical phase – external quality assessment
Introduction
EQA is a key tool for improvement of analytical performance characteristics of laboratory tests. Total tes-ting process (TTP) includes pre-analytical, analytical, and post-analytical phases. A problem in any of these phases can compromise patient care. Medical laboratories are responsible for all three phases. That is why EQA organisations created schemes also for pre-analytical and post-analytical phases [1].
Glomerular filtration rate (GFR) is a key parameter for the diagnosis of chronic kidney disease (CKD) and for dosing of drugs excreted by kidneys. Cystatin C is an alternative marker of GFR in patients who lost their muscle mass and creatinine is not suitable. KDIGO guidelines recommend eGFRcys or gold standard method in situations where creatinine is an unreliable method for estimation of GFR. Each result of serum concentration of cystatin C should be accompanied by eGFRcys [2]. Cystatin C is also included as the re-commended marker of GFR in Czech Republic national guidelines for the diagnosis of CKD [3]. Cystatin C is a stronger predictor of the risk of death and cardiovascular events in elderly persons than creatinine [4]. Cystatin C serum levels are also associated with non GFR factors such as C – reactive protein, white blood cell count, lower serum albumin, diabetes. All these factors should be considered when the GFR is estimated from serum levels of cystatin C in patients at high cardiometabolic risk [5].
Traceability of measurement to international re-ference material is the most important step to lower method bias, which leads to comparable results at different places and time. Standardisation of cystatin C measurement has been completed by traceability of calibration to international reference material ERM DA471/IFCC [6].
Standardisation of laboratory tests and mainly its implementation to routine laboratory practice are of key importance for patient safety [7]. Patient safety requires standardization of all phases of TTP [8]. EQA of post-analytical phase also has an important educational role for participants.
Patient safety is the reason why it was decided to perform the questionnaire study of implementation of standardized cystatin C measurement and its eGFR reporting.
Materials and methods
Subjects
A total of 70 laboratories (59 from the Czech Republic and 11 from Slovakia) participated in the April 2018 cycle of cystatin C EQA. Participation of medical laboratories in EQA is mandatory in the Czech Republic and Slovakia. The control samples for cystatin C scheme are distributed two times per year.
Methods
Laboratories were given online electronic questionnaires with three questions (see Table 1) about cystatin C measurement and post-analytical phase.
1. The text of the questions in the online questionnaire. 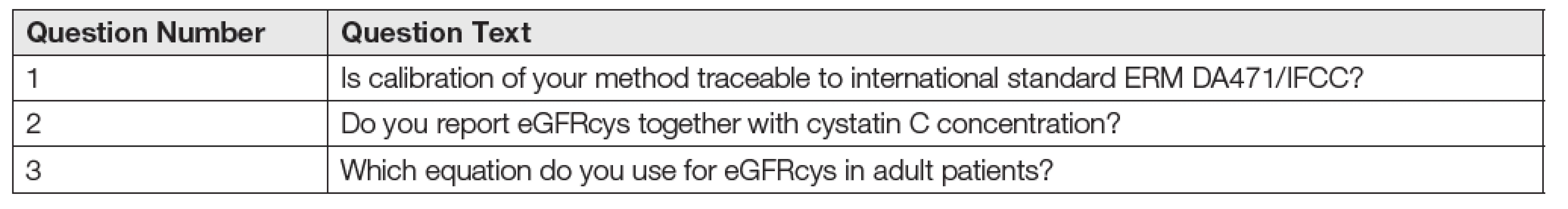
Results
A total of 70 laboratories were eligible for the online electronic questionnaire during cystatin C EQA in April 2018. Of them, 63 participants responded. Traceability of calibration to ERM DA471/IFCC was declared by 53 responders.
A total of 53 laboratories reported eGFR in all adult patients and 4 participants report eGFR only on direct request. Six laboratories did not report eGFR.
CKD - EPI equation was used by 57 laboratories. A total of 22 laboratories also used combined CKD – EPI equation with creatinine and the laboratories also calculated CAPA equation.
All participants received educational commentaries with intention to improve the current situation. Commentaries are in Table 2.
2. The text of the educational comments to questions in the questionnaire. 
Discussion
We analysed EQA results of cystatin C post-analytical phase. We found that 10 out of 63 laboratories did not know if their method was traceable to available international standard ERM DA471/IFCC and 10 out of 63 participants did not report eGFRcys together with cystatin C concentration result. It means that there is still opportunity to improve the situation.
EQA is a very important tool for assessment of all phases of a laboratory test [9]. It is consistent with our approach to assess not only the analytical phase.
Questionnaire is proposed as effective method for examination of post-analytical phase [10]. We have also adopted this practice.
Traceability of measurement is key to analytical method standardisation and common reference intervals [11]. It is consistent with our first comment which recommends performing cystatin C measurement with calibrator traceable to international standard ERM DA471/IFCC.
EQA has an important educational role, which may improve quality of TTP [12]. We also added educational comments to our survey results.
Harmonisation in laboratory medicine also includes reporting laboratory test results [13]. It is in accordance with our post-analytical EQA scheme that promotes reporting eGFR together with cystatin C concentration result.
The limitation of this study is a relatively small number of participants.
Conclusion
EQA was historically developed to improve analytical performance characteristics of laboratory tests. Nowadays extra-analytical phase schemes have been added to EQA to ensure quality of TTP and patient safety.
Střet zájmů: Autoři prohlašují že nejsou ve střetu zájmů
Do redakce došlo 20. 8. 2018
Adresa pro korespondenci:
MUDr. Tomáš Šálek, Ph.D.
Krajská nemocnice T. Bati, a. s.
Havlíčkovo nábřeží 600, 762 75 Zlín
email: tsalek@seznam.cz
Sources
1. Krleza, J. L., Dorotic, A., Grzunov, A. External qua-lity assessment of medical laboratories in Croatia: preliminary evaluation of post-analytical laboratory testing. Biochem Med (Zagreb) 2017, 27(1), p.144–52.
2. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease: Chapter 1: Definition and classification of CKD. Kidney Int Suppl 2013, 3(1), p. 1–150.
3. Zima, T., Racek, J., Tesař, V., Viklicky, O., Teplan, V., Schuck, O., Janda, J., Friedecky, B., Kubiček, Z., Kratochvila, J., Rajdl, D., Šalek, T., Kalousova, M., Granatova, J. Doporučení k diagnostice chronic-kého onemocnění ledvin (odhad glomerulární filtrace a vyšetřování proteinurie) České nefrologické společnosti ČLS JEP a České společnosti klinické biochemie ČLS JEP. Klin. Biochem Metab 2014, 22 (43), 3, p. 138-152.
4. Shlipak, M. G., Sarnak, M. J., Katz, R., Fried, L. F., Seliger, S. L., Newman, A. B.,et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. New England Journal of Medicine 2005, 352.20 : 2049-2060.
5. Stevens, L. A., Schmid, C. H., Greene, T., Li, L., Beck, G. J., Joffe, M. M., Levey, A. S. Factors other than glomerular filtration rate affect serum cystatin C le-vels. Kidney international 2009, 75(6), p. 652-660.
6. Grubb, A., Blirup-Jensen, S., Lindström, V., Schmidt, C., Althaus, H., Zegers, I. et al. First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clin Chem Lab Med 2010, 48(11), p.1619–21.
7. Bargnoux, A-S., Piéroni, L., Cristol., J-P., Kuster, N., Delanaye, P., Carlier, M-C., et al. Multicenter Evaluation of Cystatin C Measurement after Assay Standardization. Clin Chem 2017, 63(4), p.833–41.
8. Friedecký, B. Quality in clinical laboratory and patient safety. Klin Biochem Metab 2010,18(39), p. 136–43.
9. Badrick, T., Gay, S., McCaughey, E. J., Georgiou, A. External Quality Assessment beyond the analytical phase: an Australian perspective. Biochem Med (Zagreb) 2017, 27(1), p. 73–80.
10. Krleza, J. L., Celap, I., Tanaskovic, J. V. External Quality Assessment in Croatia: problems, challenges, and specific circumstances. Biochem Med (Zagreb) 2017, 27(1), p. 86–92.
11. Tate, J. R., Koerbin, G., Adeli, K. Opinion Paper: Deriving Harmonised Reference Intervals – Global Activities. EJIFCC 2016, 27(1), p. 48–65.
12. Parham, D. M., Coleman, D., Kodikara, S., Moss, S., Ellis, IO., Al-sam, S. et al. The NHS breast screening programme (pathology) EQA: experience in recent years relating to issues involved in individual performance appraisal. J Clin Pathol 2006, 59(2), p. 130–7.
13. Šálek, T., Franeková, J., Jabor, A., Friedecký, B. Postanalytická fáze a interpretace laboratorního testu (post-postanalytická faze). Klin Biochem Metab 2016, 24(45), p. 82–87.
Labels
Clinical biochemistry Nuclear medicine Nutritive therapist
Article was published inClinical Biochemistry and Metabolism

2019 Issue 1-
All articles in this issue
- Editorial
- Verification of the reference range for examination of thyroid parameters (TSH, fT3 a fT4) on the analyzer DxI 800 from Beckman Coulter
- Utilization of Prostate Health Index in prediction of prostate cancer aggressiveness
- Quality, clinical effectivity and harmonisation of POCT
- Measurement uncertainty and error in medical laboratories
- Diabetes mellitus – laboratorní diagnostika a sledování stavu pacientů
- Cystatin C – Implementation in clinical laboratory practice
- Clinical Biochemistry and Metabolism
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Measurement uncertainty and error in medical laboratories
- Diabetes mellitus – laboratorní diagnostika a sledování stavu pacientů
- Utilization of Prostate Health Index in prediction of prostate cancer aggressiveness
- Verification of the reference range for examination of thyroid parameters (TSH, fT3 a fT4) on the analyzer DxI 800 from Beckman Coulter
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

