-
Medical journals
- Career
Image Possibilities of the Peripheral Nerve Tumor Using Magnetic Resonance Imaging - Case Report
Authors: I. Humhej 1; I. Ibrahim 2; J. Lodin 1; M. Sameš 1; I. Čižmář 3
Authors‘ workplace: Department of Neurosurgery, J. E. Purkyně University, Masaryk Hospital, Ústí nad Labem, Czech Republic 1; MR Unit, Department of Radiodiagnostic and Interventional Radiology, Institute for Clinical and Experimental Medicine (IKEM), Prague, Czech Republic 2; Department of Traumatology, Faculty of Medicine, Palacky University, Faculty Hospital Olomouc, Czech Republic 3
Published in: ACTA CHIRURGIAE PLASTICAE, 60, 1, 2018, pp. 9-13
INTRODUCTION
Peripheral nerve tumours (PNTs) are relatively rare diseases, comprising less than 5% of all soft tissue tumours 1. They are predominantly benign affections, with schwannomas (neurinomas, neurilemmomas) and neurofibromas being the most common 2. PNTs are usually solitary lesions, however can present in multiples, such as in cases of neurofibromatosis type I. or schwannomatosis. Correct diagnosis performed using the patient’s history, physical examination and especially imaging techniques, is paramount in cases where PNTs are suspected. Electromyography (EMG) studies are less useful, as they commonly show normal nerve conduction 3. Imaging modalities include ultrasonography, most notably high-resolution techniques, and magnetic resonance imaging (MRI), a method with excellent soft tissue differentiation. Detailed anatomical analysis is key in preoperative planning, as the main surgical objective for most symptomatic growing PNTs is radical resection, with maximal preservation of neurological function 4-6. Continuing technological advancement has led to the development of novel MRI techniques and sequences, allowing complex 3D reconstructions of peripheral nerves (PNs). The following case report describes the use of these so-called advanced MRI techniques, in the diagnostic algorithm of a median nerve tumour.
CASE REPORT
The following case is a 64-year-old male, who presented with a growing mass distally on the medial border of the left arm. Palpation of the mass led to paraesthesia and spasms of the IIIrd and IVth finger. Upon clinical evaluation, the patient showed no signs of hypaesthesia or dysaesthesia in any neural dermatomes on the affected limb. Furthermore, there was no loss of muscle strength including fine motor skills when compared to the normal limb. The mass itself was a ball-like resistance of 3 cm in diameter, approximately 10 cm proximal to the medial epicondyle of the humerus. On palpation, it was tough and mobile perpendicularly to the humerus. Percussion of the tumour showed a positive Tinel’s sign, resulting in paraesthesias and shock-like sensations in the distribution of the median nerve (MN). Conduction studies as well as needle EMG of the affected nerve resulted in normal values. Bed-side ultrasonography was performed and showed a solid, well circumscribed, hypoechogenic mass, measuring 20 x 20 x 24 mm, in close proximity to the brachial vessels. MRI using T1, T2-STIR and T1 with gadolinium contrast sequences was performed and showed a contrast enhancing PNT. 3D T2-STIR (short tau inversion recovery) SPACE (sampling perfection with application optimized contrast using varying flip angle evaluation) sequences of the tumour are shown in Fig. 1a. Consequently, we performed a series of advanced MRI sequences, specifically MR neurography (MRN), diffusion tensor imaging (DTI) and MR tractography (MRT). Due to these novel techniques, we obtained a detailed anatomical image of the tumour originating from the MN – Fig. 1b (3D T2-STIR SPACE MIP), showing the MN course throughout the arm, including the tumour, using fraction anisotropy (FA) – Fig. 1c (FA-MIP) and visualization of neural fascicles adjacent to the tumour (MRT) – Fig. 1d, Fig. 2a and 2b.
1. Median nerve schwannoma showed using (a) conventional MRI sequence – 3D T2–STIR SPACE, (b) MRN–3D T2–STIR SPACE–MIP reconstruction, (c) DTI–FA–MIP reconstruction and (d) MRT DTI= diffusion tensor imaging; FA= fraction anisotropy; MIP= maximum intensity projection; MRN= MR neurography; MRT= MR tractography; SPACE= sampling perfection with application optimized contrast using varying flip angle evaluation; STIR= short tau inversion recovery; T2= T2 weighted images 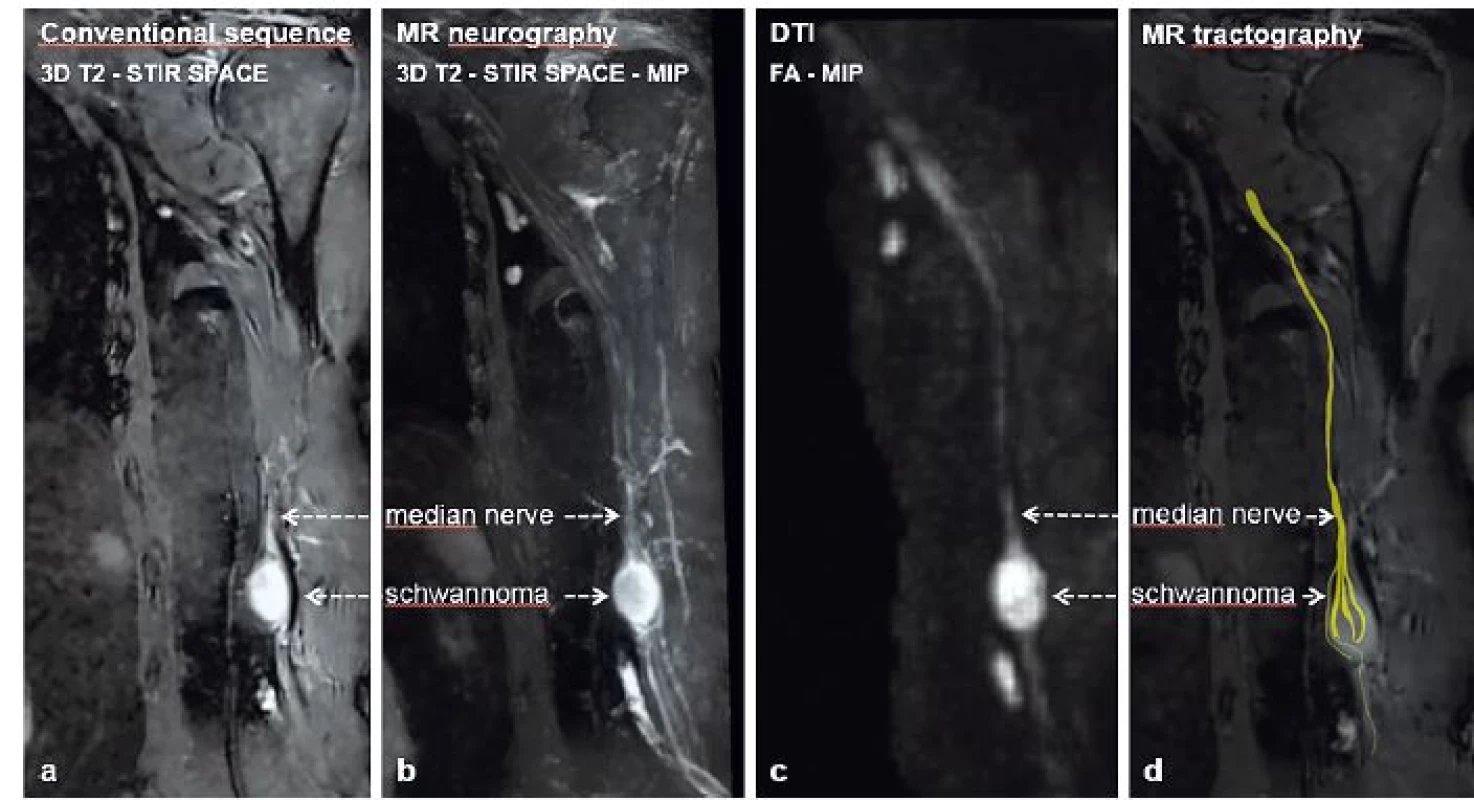
2. 3D MR tractography (MRT) of schwannoma of the median nerve – (a) neural fascicle projection into basic structural images of conventional MRI and (b) isolated MRT reconstruction 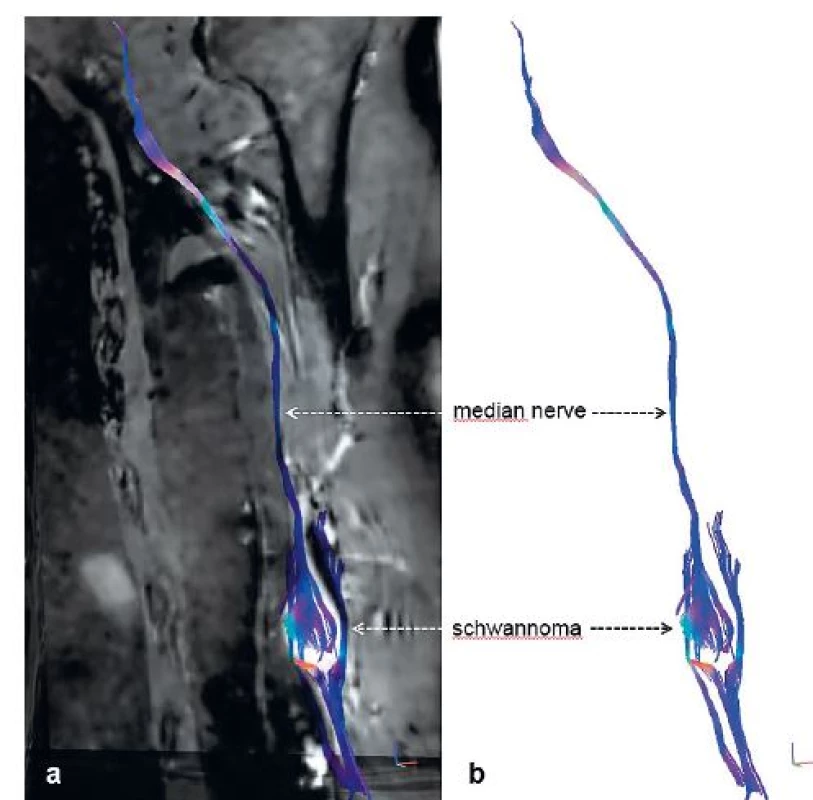
Following the diagnosis, a surgical procedure was planned and later performed, under regional anaesthesia using an axillary block at the outpatient clinic. Radical resection of the tumour, resembling a schwannoma, was performed using microsurgical techniques with the aid of peroperative electrostimulation and neurography – Fig. 3a-c. The procedure was accomplished without surgical complications; the originating fascicle of the tumour did not show an action potential or peripheral response under stimulation, which allowed its safe transection without the need of reconstruction. The tumour was then resected en bloc and preserved for histological examination. The patient was then shortly observed and later discharged with a follow-up plan, consisting of dates for changes of wound dressing, suture removal and a plan for rehabilitation. The postoperative period was without any complications, with the patient’s painful IIIrd and IVth finger, spasms and paraesthesias subsiding shortly following the surgery. For a short period, the patient experienced hypoesthesia as well as flexion weakness of the IInd and IIIrd fingers (4+/5 based on the British Medical Research Council grading system), however these deficits resolved completely within three months after the surgery. The patient’s wound on the medial aspect of the arm healed in a painless fashion per primam intentionem, with only minor signs of hypertrophy and no sensory deficits. Histological examination of the tumour verified the presence of a well-circumscribed, non-invasive neurinoma derived from Schwann cells (Antoni A type). Postoperative EMG has shown a minor lesion of the MN in the distal arm, with reinnervation activity in the abductor pollicis brevis muscle.
3. Peroperative image of the median nerve schwannoma resection – (a) fascicle course on the surface of the tumour preceding tumour resection, (b) microsurgical tumour resection, and (c) preserved neural structures following radical tumour resection 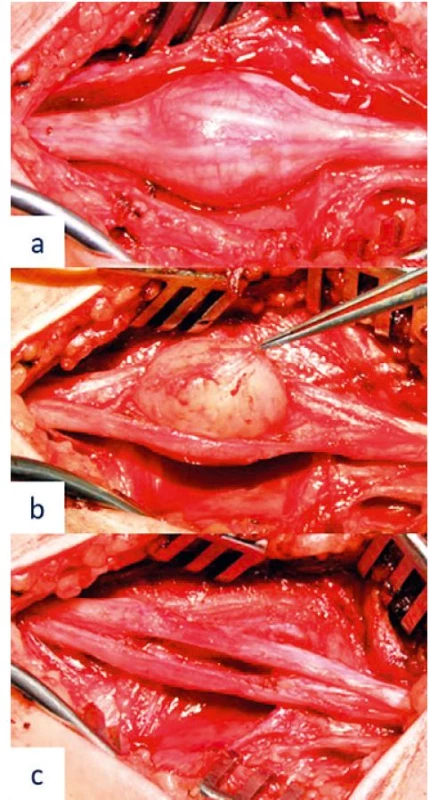
DISCUSSION
In general, PNT resections are accompanied with the risks of persisting neurological deficits. Successful surgeries, completely preserving PN function, make up 90% of schwannoma, 80% of solitary neurofibroma and 66% of neurofibromatosis type I. associated neurofibroma cases 7,8. In order to decrease the risk of surgical morbidity, it is crucial that PNT patients are referred to the centres experienced in microsurgery, equipped with tools for peroperative electrophysiological monitoring and adequately prepared for unpredictable surgical complications (for example reconstruction of damaged neural structures). Electrophysiological monitoring plays an important role in identifying the course of neural fascicles, which are often hidden within the tumour capsule and at risk of damage when initially entering the tumour 9. In order to gain better understanding of PNT anatomy, as well as their relationship to neural fascicles, advanced MRI techniques can be performed. These techniques allow detailed analysis of anatomical structures as well as identifying possible surgical risks preceding the actual procedure. Possible applications of these techniques are illustrated in the presented clinical case.
Advanced MRI techniques, especially DTI and MRT, are not used routinely in diagnostic algorithms of PNT; they are mostly considered experimental tools. However, increasing technological advancements and availability of these methods, has led to their gradual integration into clinical practice, which will most likely result in their routine use within MRI diagnostic protocols in the near future. Our department has adopted the use of advanced MRI techniques in selected patients in 2013, when we designed and technically modified our own diagnostic sequences. These were tested on a control group of healthy patients as well as patients with PN disorders, after signing informed consent. All scans are performed via a 3T MRI (Siemens Magnetom Trio, Erlangen, Germany). Advanced MRI techniques differ from conventional MRI techniques in that they do not only allow detailed depiction of the affected PN as well as its disorder (MRN), but also show and quantify the functional integrity of neural fibres on a microscopic level (DTI and MRT). Due to these features, advanced MRI techniques are well-established diagnostic tools of white matter tract lesions of the brain. They have only been used for PN examination since the early 21st century.
The MRN method is a specialized MRI technique used to demonstrate PN. It allows high resolution 3D demonstration of PN course, as well as detailed visualization of PN morphology and lesions 10. A 3T MRI is advantageous compared to 1,5T MRI, as it allows depiction of fine intraneural fascicles, which is useful for exact localization of PN pathologies 11. Other sequences, such as 3D T2-STIR SPACE with a spatial resolution of 1 mm3, can further complement MRN in order to visualize extremely fine neural structures. Data acquisition is followed by software processing, maximum intensity projection (MIP) reconstruction and 3D reconstruction. Individual sequences used to complement MRN as well as their setting vary, based on MRI manufacturers and strength of the magnetic field. It is necessary to tailor each diagnostic protocol based on the examined area 12.
Diffusion tensor imaging (DTI) is unique, compared to qualitative structural methods of MRN or conventional MRI, in that it provides quantitative microstructural information, which reflects functional integrity of neural fibres 13. This technique is based on principles similar to diffusion weighted imaging (DWI), which are based on visualization of water molecule diffusion (hydrogen proton bound in water molecules) in the examined tissue. Neural axons are a type of tissue, in which this process is heavily influenced by fibre microstructure. Water molecules diffuse most efficiently in a direction parallel to the long axis of neurons. Diffusion perpendicular to the neuron axis is limited due to the presence of myelin sheaths and neuronal membranes, which inhibit this process (anisotropic diffusion) 14. Damage or degradation of neuronal membranes or myelin sheaths, which can be caused by a variety of pathological processes, leads to a decrease in fraction anisotropy (FA), an increase of the apparent diffusion coefficient (ADC) and can be detected using DTI 15. FA and ADC are the most widely used scalar maps of the diffusion tensor. Consequent software processing of the obtained data allows 3D reconstruction of neural tracts – MR tractography (MRT). In order to obtain better anatomical context of the nerve, MRT allows projection of these reconstructed images into images of conventional MRI sequences 13. The disadvantage of DTI include, motion artefacts, which prevent the acquisition of usable images, or differentiation between neural and muscle fibres. These technical issues can be partially reduced with the use of adequate MR array coils, optimization of imaging protocols reducing their length or post-processing.
CONCLUSION
Advanced MRI techniques allow us to obtain detailed structural and microstructural information concerning PN disorders including PNTs. They expand the range of diagnostic tools available for the diagnosis of PNTs and allow optimal surgical planning, potentially decreasing the risks of patient morbidity. Technological advancements will most likely result in further improvement of these methods and their gradual integration into MRI diagnostic protocols used in clinical practice.
This study was supported by a grant of AZV MZ CR No. 17-28587A.
Corresponding author:
Ivan Humhej, M. D.
Department of Neurosurgery,
J. E. Purkyně University, Masaryk Hospital
Sociální péče 12A
401 13 Ústí nad Labem
Czech Republic
E-mail: ivan.humhej@kzcr.eu
Sources
1. Midha R, Zager EL. Surgery of peripheral nerves: a case-based approach. 1st ed. New York: Thieme Medical Publishers, Inc; 2008. Chapter 49, Schwannoma; p. 231-235.
2. Kim DH, Murovic JA, Tiel RL, Moes G, Kline DG. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg. 2005 Feb;102(2):246-55.
3. Russell SM. Preserve the nerve: microsurgical resection of peripheral nerve sheath tumors. Neurosurgery. 2007 Sep;61(3 Suppl):113-7.
4. Levi AD, Ross AL, Cuartas E, Qadir R, Temple HT. The surgical management of symptomatic peripheral nerve sheath tumors. Neurosurgery. 2010 Apr;66(4):833-40.
5. Matejcík V, Benetín J, Danis D. Our experience with surgical treatment of the tumours of peripheral nerves in extremities and brachial plexus. Acta Chir Plast. 2003;45(2):40-5.
6. Ball JR, Biggs MT. Operative steps in management of benign nerve sheath tumors. Neurosurg Focus. 2007 Jun 15;22(6):E7.
7. Mackinnon SE. Nerve Surgery. 1st ed. New York: Thieme Medical Publishers, Inc; 2015. Chapter 18, Tumours of the peripheral nervous system: p. 530-571.
8. Kim DH, Midha R, Murovic JA, Spinner RJ, Teil R. Kline and Hudson’s Nerve Injuries: Operative Results for Major Nerve Injuries, Entrapments and Tumours. 2nd ed. Philadelphia: Saunders Elsevier; 2008. Chapter 23, Tumours involving nerve: p. 433-472.
9. Sova M, Neuman E, Vybíhal V, Fadrus P, Šprláková A, Křen L. Naše zkušenosti s chirurgickou léčbou tumorů periferních nervů. Cesk Slov Neurol N. 2015; 78/111(6): 694-698.
10. Chhabra A, Flammang A, Padua A Jr, Carrino JA, Andreisek G. Magnetic resonance neurography: technical considerations. Neuroimaging Clin N Am. 2014 Feb;24(1):67-78. doi: 10.1016/j.nic.2013.03.032.
11. Chhabra A, Madhuranthakam AJ, Andreisek G. Magnetic resonance neurography: current perspectives and literature review. Eur Radiol. 2018 Feb;28(2):698-707. doi: 10.1007/s00330-017-4976-8.
12. Ibrahim I, Tintěra J, Škoch A, Jírů F. MR traktografie mozku a MR neurografie periferních nervů. Ces Radiol. 2017;71(4): 345-352.
13. Jeon T, Fung MM, Koch KM, Tan ET, Sneag DB. Peripheral nerve diffusion tensor imaging: Overview, pitfalls, and future directions. J Magn Reson Imaging. 2018;47(5):1171-89.
14. Keřkovský M, Šprláková-Puková A, Kašpárek T, Fadrus P, Mechl M, Válek V. Diffusion tensor imaging – současné možnosti MR zobrazení bílé hmoty mozku. Cesk Slov Neurol N., 2010;73/106(2):136–42.
15. brahim I, Tintěra J. Teoretické základy pokročilých metod magnetické rezonance na poli neurověd. Ces Radiol. 2013;67(1):9-19.
Labels
Plastic surgery Orthopaedics Burns medicine Traumatology
Article was published inActa chirurgiae plasticae

2018 Issue 1-
All articles in this issue
- Pedicled pectoralis major flap in head and neck reconstruction - technique and overview
- Securing intraoral skin grafts to the floor of the mouth: case report and technique desription
- Intraosseous haemangioma od the zygoma - case report and literature review
- Editorial
- Pedicled pectoralis major flap in head and neck reconstruction - our experience
- In memoriam: Professor Rajko Doleček
- Commemoration of professor Miroslav Fára, M.D., DSc.
- Use of licap and ltap flaps for breast reconstruction
- Image Possibilities of the Peripheral Nerve Tumor Using Magnetic Resonance Imaging - Case Report
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Pedicled pectoralis major flap in head and neck reconstruction - technique and overview
- Use of licap and ltap flaps for breast reconstruction
- Editorial
- Intraosseous haemangioma od the zygoma - case report and literature review
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

