-
Medical journals
- Career
AUTOLOGOUS FAT TRANSFER, BREAST LIPOMODELLING AND FAT TRANSFER TO THE FACE: CURRENT GOLD STANDARDS AND EMERGING NEW DATA
Authors: Streit L. M. D. 1,2; R. Lhotsky 3; O. Mestak 4
Authors‘ workplace: Department of Plastic and Aesthetic Surgery, Faculty of Medicine, Masaryk University, Brno, Czech Republic 1; Centre for Plastic Surgery and Hand Surgery, University Hospital Ostrava, Ostrava, Czech Republic 2; Hand and Plastic Surgery Institute, Vysoké nad Jizerou, Czech Republic 3; Department of Plastic Surgery, 1st Faculty of Medicine, Charles University, Prague, Czech Republic 4
Published in: ACTA CHIRURGIAE PLASTICAE, 59, 2, 2017, pp. 97-108
INTRODUCTION
Autologous fat transfer is a surgical technique that is used to transfer adipose tissue in an injectable form from the donor area to the areas where adipose tissue is needed for tissue augmentation or for its regenerative properties. The method is often referred to as lipofilling, fat grafting, lipotransfer or autologous fat transplantation. In plastic and reconstructive breast surgery is the lipomodelling term probably the most accurate, because it reflects significant shaping potential of the technique on the breast. Whatever the method is called, however, the basic principles remain the same. Fat transfer may be used separately as a major surgical technique, or it may be an integral part of combined surgical procedures.
Fat transfer can be categorized according to the volume of transferred tissues to high - and low-volume fat grafting. High-volume fat grafting is performed primarily in order to supplement missing volume or to increase the size of the treated area. Typical examples of high volume fat grafting include breast lipomodelling in both reconstructive and aesthetic indications or correction of congenital chest deformities such as pectus excavatum 1-7.
In case of low-volume fat grafting, there is also the regenerative potential of fat utilized apart from its volume replenishment and contouring effects, especially in certain indications. Low-volume fat grafting is used in the treatment of radiation-damaged skin 8, in the treatment of scleroderma 9 or for the correction of the scars after burns 10,11. It was also recognized as an optimal surgical strategy in the correction of the progressive hemifacial atrophy 12. Finally, low-volume fat grafting is commonly used in aesthetic surgery for rejuvenation of the face 13-17, neck or hands 15. The regenerative effect is expressed as an improvement in skin elasticity, texture and colour and as improvement in tissue vascularity.
Fat transfer consists of three consecutive steps: 1) fat harvesting, 2) fat processing, and 3) fat graft injection. All these steps may differ significantly depending on what is the main goal of the surgery and whether it is a high or low-volume fat grafting. Also the preference of the surgeon plays a role.
FAT HARVESTING
Adipose tissue is harvested by liposuction from subcutaneous fat deposits in the areas where fat is in excess. During fat harvesting, the main goal of harvested areas infiltration is not tissue tumescence but only tissue saturation with epinephrine. Therefore, lower volumes of the solution are used compared with tumescent liposuction for cosmetic reasons. The amount of aqueous components in lipoaspirate is also rather a disadvantage because these aqueous components are to be eliminated during fat processing. Furthermore, when higher volume of infiltration solution is used, harvesting time is extended because of higher number of manipulations, which may also correspond with a higher consumption of material, e.g. sampling syringes. It is also recommended to use infiltration solution without local anaesthetics since they have been shown to have toxic effects on adipocytes and preadipocytes 18,19. For all these reasons, if the whole surgery is scheduled under general anaesthesia, we use low-volume infiltration with normal saline and higher concentration of epinephrine (2 : 1,000,000) and we recommend infiltration of harvesting areas by local anaesthetics when harvesting is finished.
Liposuction is performed most commonly manually using “luer-lock” syringes to connect the cannula or “Toomey” syringes with conical tip type of cannula. Vacuum is created by continuous movements of the syringe plunger or by using a holder for the plunger. Another option is usage of lipoaspirate collectors where output is connected to the aspirator and input to the cannula. The collectors are used more often, particularly when high volume fat grafting is carried out more frequently in the department. The advantage of using the collector is the fact that the vacuum can be defined more precisely. However, there is no study showing that higher values of negative pressure would significantly affect the viability of harvested adipose tissue 20-22.
Harvesting technique significantly affects the quality of fat graft. Cannulas differ in the number, size and spatial arrangement of openings in the tip and also in diameter and length. Cannulas for low-volume fat grafting are thinner and they usually have multiple openings with a diameter below 1 mm (Figure 1). Tonnard et al. have demonstrated significant differences in adipose tissue viability depending on the size of the openings in the tip and on the number of passages through the “luer-lock” syringes. Adipose tissue viability was significantly higher after fat harvesting using 3mm cannulas with three openings of 2x7mm in the tip then after using 3mm cannulas with multiple circular sharp holes of 1-mm diameter in the tip (microfat grafting technique). Furthermore, their study has shown that adipose tissue viability is further decreased by repeated passages between the syringes via “luer lock” connector (nanofat grafting technique). On the contrary, usage of a cannula with small openings in the tip or repeated passages through “luer lock” connector had no negative impact on the concentration and characteristics of stem cells in harvested adipose tissue that are associated with regenerative effect after fat grafting 23.
1. Harvesting cannulas and syringes. From the right to the left: 1) 60 ml “Toomey” syringe – part of PureGraft™ set, 2) 30 ml luer-lock syringe – used in our department for breast lipomodelling, 3) 10 ml luer-lock syringe – used in our department for harvesting for facial fat grafting or for application during breast lipomodelling, 4) harvesting cannula with a diameter of 3.5 mm and length 17cm with 6 openings on the apex (PLA187 model, Pouret Medical, France) – used for breast lipomodelling, 5) Tulip harvesting cannula for microfat grafting, Sorensen type – reusable, and 6) single use harvesting cannula from “St’rim” set from the Thiebaud company used for microfat grafting 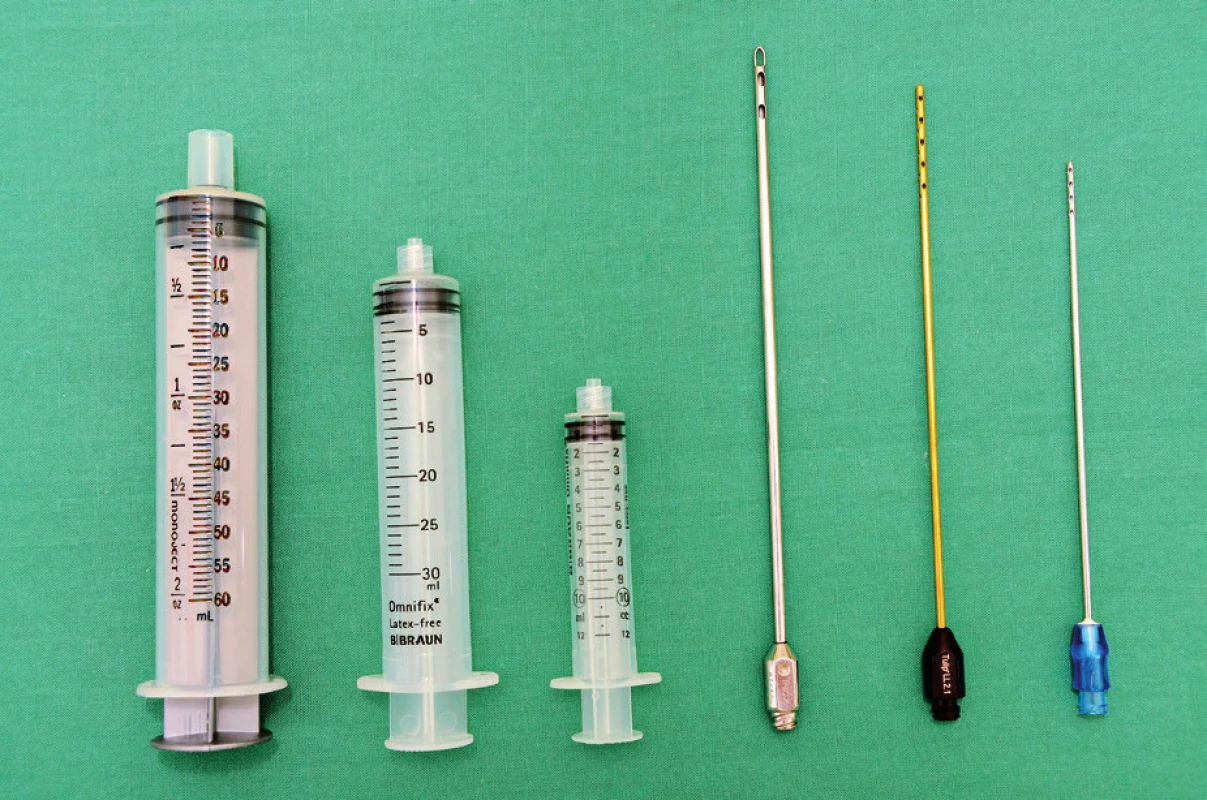
Liposuction system, which allows infiltration of harvesting area with tumescent solution by water jet and simultaneous harvest of adipose tissue has been recently developed (“water jet assisted liposuction”). An essential part of this system is a two-way cannula with an opening for the water jet in the tip with openings for lipoaspirate suction on its sides.
FAT PROCESSING
Harvested adipose tissue (lipoaspirate) is a mixture of adipose tissue fragments, lipid droplets, cellular debris, infiltration solution and blood. The quality and ratio of the individual lipoaspirate components is defined by harvesting technique. The aim of lipoaspirate processing is to obtain a purified cell mixture, which has a minimal extent of volume absorption and/or maximal regenerative effect.
For the surgeon, a large number of technical options exist for fat processing. The most commonly used techniques for lipoaspirate processing in clinical practice include 1) decantation; 2) centrifugation with different speeds, diameters, volumes, and tube shapes; and 3) filtering through a simple membrane (mesh) or filtering through a membrane combined with washing in more sophisticated devices such as PureGraftTM.
Decantation is the oldest method that is still used by some surgeons because it is simple and cheap, although time consuming. Lipoaspirate processing is based on decantation also in recent lipoaspirate collectors that seem to be more effective as they are directly connected to the aspirator and to the harvesting cannula.
Centrifugation is the most commonly used technique for processing of lipoaspirate. It was popularized by Coleman, who described in detail the processing techniques using 3-minute centrifugation with a relative centrifugal force of 1286 g. Lipoaspirate is divided by centrifugation into four clearly distinguished layers: 1) the upper oily layer, 2) the middle adipose layer, representing most of the adipose tissue, and 3) the lower aqueous layer, consisting mainly of tumescent solution, and 4) the pellet at the bottom of each tube (Figure 2). Centrifugation is carried out usually directly in the harvesting syringes with luer lock connector. The effect of centrifugation on the biological properties of adipose tissue has been described in many studies13,24-29. In our department, we centrifuge lipoaspirate at a relative centrifugal force of 1,200 g directly in the harvesting 10-ml syringes for low-volume fat grafting and in 30-ml syringes for high-volume fat grafting. We believe that usage of 30ml syringes reduces significantly the number of manipulations and thus operating time.
2. Fat processing by centrifugation in 30-ml syringes for high-volume fat grafting 
The third basic principle of lipospirate processing is filtration through a membrane. Perhaps the simplest option is filtration and washing with saline solution through a filter membrane positioned on top of the surgical bowl 30. The disadvantages are obvious, the tissue is prepared on open air and the number of manipulations is relatively high. Nevertheless, due to its simplicity and cost it is still used, especially for low-volume fat grafting procedures. More sophisticated devices that are based on the filtration principle are also available. These devices can be designed like canisters connected directly to the aspirator and harvesting cannulas. The outlet portion for the liquid components of lipoaspirate can be constructed as a single outlet at the base of the container, or it can be connected also with the aspirator. Another membrane-based tissue filtration system is PureGraft™, which is designed for manual sampling liposuction syringes with “Toomey” tip. The device consists of a plastic bag, which is divided inside by double filtration membrane into the inlet and outlet parts. Valves for the lipoaspirate installation orifice together with input for Ringer solution into the inlet part; outlet part is connected to the waste plastic bag (Figure 3). Advantages of these more sophisticated devices may be lower number of manual manipulations during fat preparation and the design as a closed system. On the other hand, their disadvantage is that it can eliminate undefined substances, such as cytokines and/or growth factors, that may augment the regenerative effect of lipoaspirate application 31.
3. Fat processing by washing and filtration through a membrane using PureGraftTM devices 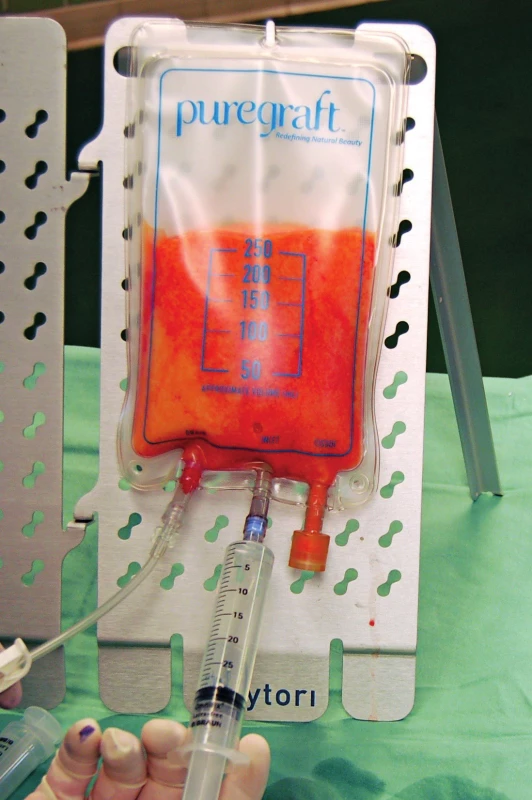
Streit et al. recently comprehensively compared these preparation techniques in vitro. Morphology of each preparation was assessed by electron microscopy and overall cell viability by live/dead assay. Number of adipose derived stem cells (ASC) was determined and their stem cell character was assessed by the presence of cell surface molecules and by their capacity to differentiate into adipogenic and osteogenic lineages. They have demonstrated that 1) decantation is a very gentle technique that best preserves components of the extracellular matrix, but the product of decantation is to some extent contaminated with oil droplets and residues of disrupted adipocyte membranes; 2) centrifugation isolated the highest concentration of ASC from the upper two-thirds of the adipose layer of centrifuged fat (low-density fraction). Centrifuged fat was almost completely devoid of contaminating oil droplets and debris, and it retained considerable amounts of the fibrillar components of the extracellular matrix; and 3) centrifuged fat compared with membrane-processed fat contained about the same concentration of ASC with similar viability. Although filtration also effectively removed oil droplets and cell debris, membrane-processed fat again retained a substantial part of the fibrillar components of the extracellular matrix. Finally, the watery component was minimal in membrane-processed fat 29. Because the differences were not dramatic, authors concluded that centrifugation and membrane-based tissue filtration are equivalent techniques and superior to decantation in clinical practice.
In the selection of processing technique for clinical practice, another criteria such as practicability for the individual surgical settings (close vs. open system, number of manipulations, price, required time, etc.) remain crucial for the surgeon 31.
Cell assisted lipotransfer is used to increase engraftment and decrease liponecrosis in the recipient tissue. This technique is based on enrichment of fat graft with stem cells or stromal vascular fraction cells 32. Core factor affecting absorption is cell apoptosis due to inflammatory condition after transplantation and the lack of angiogenesis. Stem cells may improve fat survival in three ways - they release growth factors and help the surrounding tissue to resist hypoxia and ischemia; they differentiate into adipocytes and regenerate the adipose tissue and they promote vasculogenesis. However, there is absolutely no statistical evidence that proves this assumption 33-36.
FAT TRANSFER
Purified adipose tissue is usually injected with syringes (10-ml syringes for high-volume fat grafting, 1-ml syringes for low-volume fat grafting) and special application cannulas (Figure 4). The cannula is introduced through multiple entry points created by a needle or scalpel in order to achieve a honeycomb structure of multiple microtunnels. Fat is injected in small quantities in the form of fine cylinders resembling spaghetti. Each microtunnel must be surrounded by well vascularized tissue (Figure 5). Transfer is done from a deep to a superficial plane. Good spatial visualization is necessary to form a sort of three-dimensional honeycomb, to avoid creating areas of fatty pools, which would lead to fat necrosis 4.
4. Application cannulas. On the left, 2mm cannulas with a length of 20 and 13 cm (PLA188 and 189 models, Pouret Medical, France). In the centre, 1.5mm cannulas with a blunt tip (Coleman™ Style 1, Mentor, USA) and “V” shaped tip to release scars (“V” Dissector Cannulae, Mentor, USA). These cannulas and similar styles are suitable for lipomodelling of the breast. On the right is an example of cannulas for fat grafting to the face, diameter 0.9 and 0.7 ml (Tulip, USA) 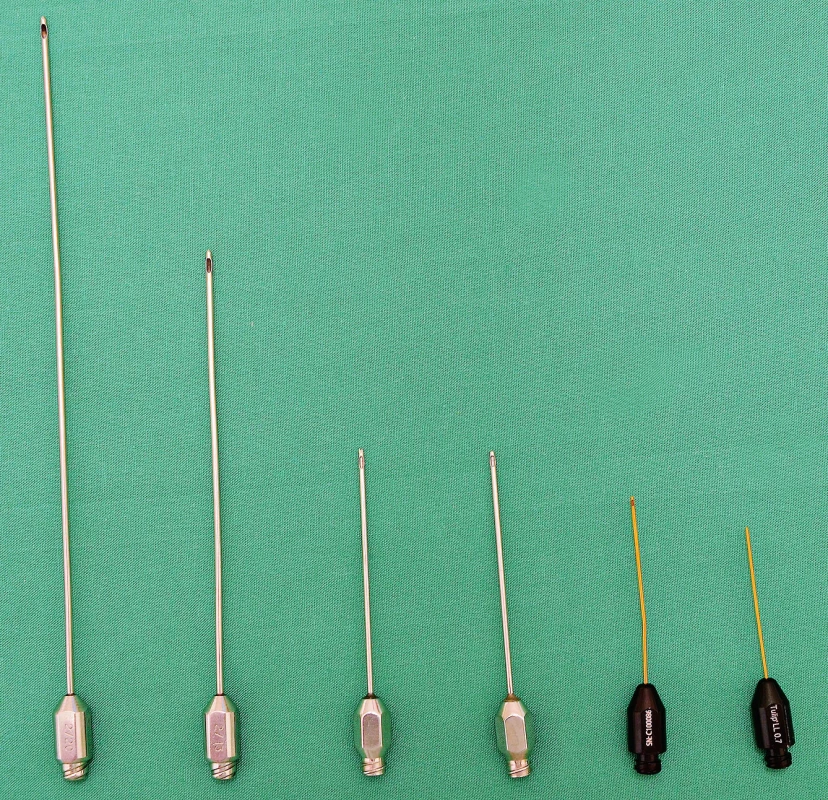
5. Principle of fat transfer into multiple layers of recipient subcutaneous tissue 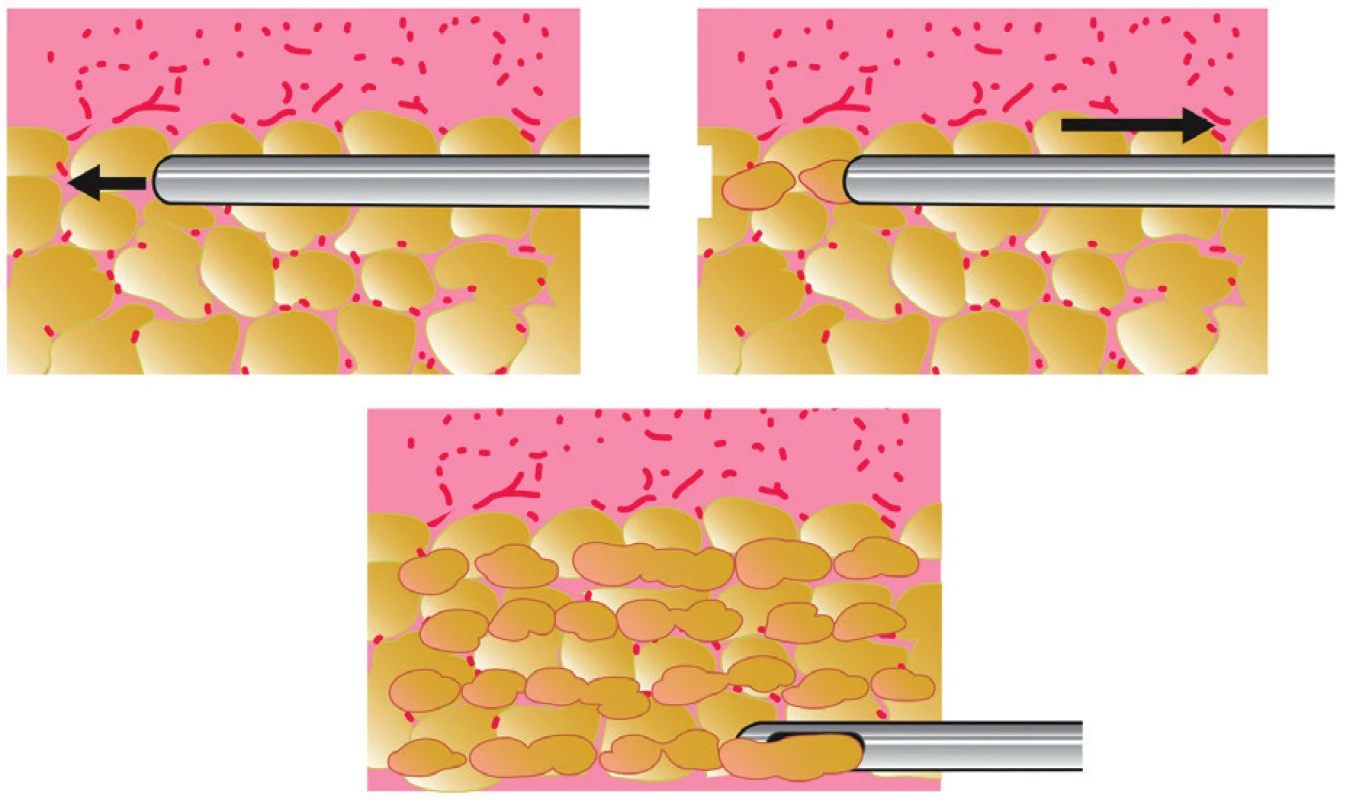
During high-volume fat grafting, it is necessary to know how to overcorrect the quantity of fat, if allowed by the recipient tissues, as absorption of about 30-40% volume may be expected. When the recipient tissues are saturated and cannot accept more fat, it is not advisable to continue, because of the risk to create areas of fat necrosis. It is better to plan an additional session than disregard saturation of the tissues. Incisions are sutured using very fine suture material.
Technique of multiple percutaneous fasciotomies performed with 17-gauge trocar or needle is one of the advancements of the lipomodelling technique. This technique is also called three dimensional ligamentous band release or “Rigottomy”. It is powerful technique for scar releasing, it allows us to move down the submammary fold and to sculpture bottom part of the breast if required (in tuberous breast) 4,37.
External breast tissue expander (BRAVA®) was introduced as a nonsurgical alternative to breast augmentation. The system consists of two domes with soft silicone edge and it is worn as a bra. Attached pump creates negative pressure within domes and consequently acts as an expander 38,39. Action of negative pressure leads to 1) the enlargement of breast tissue and its vascularity, and to expansion of skin, which is advantageous especially in breast reconstruction. This results in enlarged tissue bed for fat injection. The larger is the preoperative expansion, the more volume we are able to inject. We recommend wearing BRAVA 3 weeks/12 hours per day before fat grafting. It is advisable to over-expand the breast in order to gain sufficient skin envelope for fat application. With this system we can perform mega volume fat grafting, which means we can safely achieve volumes of around 300 ml per breast. While wearing BRAVA postoperatively we decrease pressure of surrounding tissue on the graft, which does not act as an internal expander anymore and we achieve better graft nutrition. The patients wear BRAVA for 12 hours per day postoperatively, for at least 4 weeks. Patient compliance is essential since it is necessary to wear the system pre - and postoperatively for a relatively long time.
BREAST LIPOMODELLING
Breast reconstruction by autologous fat transfer
In breast reconstruction, fat transfer may be used separately as a major surgical technique, or it may be an integral part of combined surgical procedures.
Breast reconstruction by repeated lipomodelling
Capacity of the donor site to accept fat graft is closely related to the initial volume of well-vascularized donor tissues. In secondary breast reconstruction, it is essential to reconstruct or expand missing skin, which is crucial in selection of reconstructive technique, especially in an irradiated patient. Furthermore, volume absorption after fat grafting is very high when applied into the irradiated chest wall. Thus, we recommend this reconstructive technique just for non-irradiated patients, despite the availability of BRAVA enlargement system. On the contrary, breast reconstruction by repeated lipomodelling is very promising in primary reconstruction after prophylactic mastectomy, or in hypoplastic breast malformations (e.g. Poland’s syndrome). Very important aspect of the consultation is to make the patient understand about the maximal possible breast size (cup size approximately B+) and about the need of higher number of lipomodelling sessions (usually 3-5)4.
Lipomodelling for the correction of sequelae of conservative treatment
Breast reconstruction after breast conserving therapy is problematic. Two factors must be considered. The first is the uneven breast shape resulting from partial mastectomy. The second is the preexisting irradiation of the breast 40. Therefore, breast reconstruction using fat grafting is an attractive tool for treatment of this deformity with possible local filling of the defect as well as regenerative properties of the graft 41.
We perform this procedure under general anesthesia. The injection is performed very slowly with a fan-shaped technique during the withdrawal of the cannula. We attempted (as much as possible) to prevent accumulation of the fatgraft and overfilling of the tissue to prevent ischemia, necrosis, colliquation and calcification, which is more likely in irradiated tissue.
Fat grafting to breast after breast cancer has been questioned regarding higher risk of cancer recurrence. This possible risk would be higher in patients after breast conserving treatment. However, this assumption has not been confirmed in clinical studies yet 42.
Lipomodelling as the complement of autologous breast reconstructions
The abdominal free flaps and the latissimus dorsi flap are the two most common autologous breast reconstruction techniques today.
The abdominal free flaps are often considered to be the gold standard for autologous breast reconstruction. Defects may however also develop after their use, in particular asymmetry of volume, lack of projection, defect in the décolleté or possibly partial flap loss. In these cases, we carry out intrapectoral lipomodelling and lipomodelling of the flap (Figure 6).
6. A 51-year-old patient with the history of secondary breast reconstruction using abdominal flap before and 5 months after one lipomodelling session with 180 ml of fat 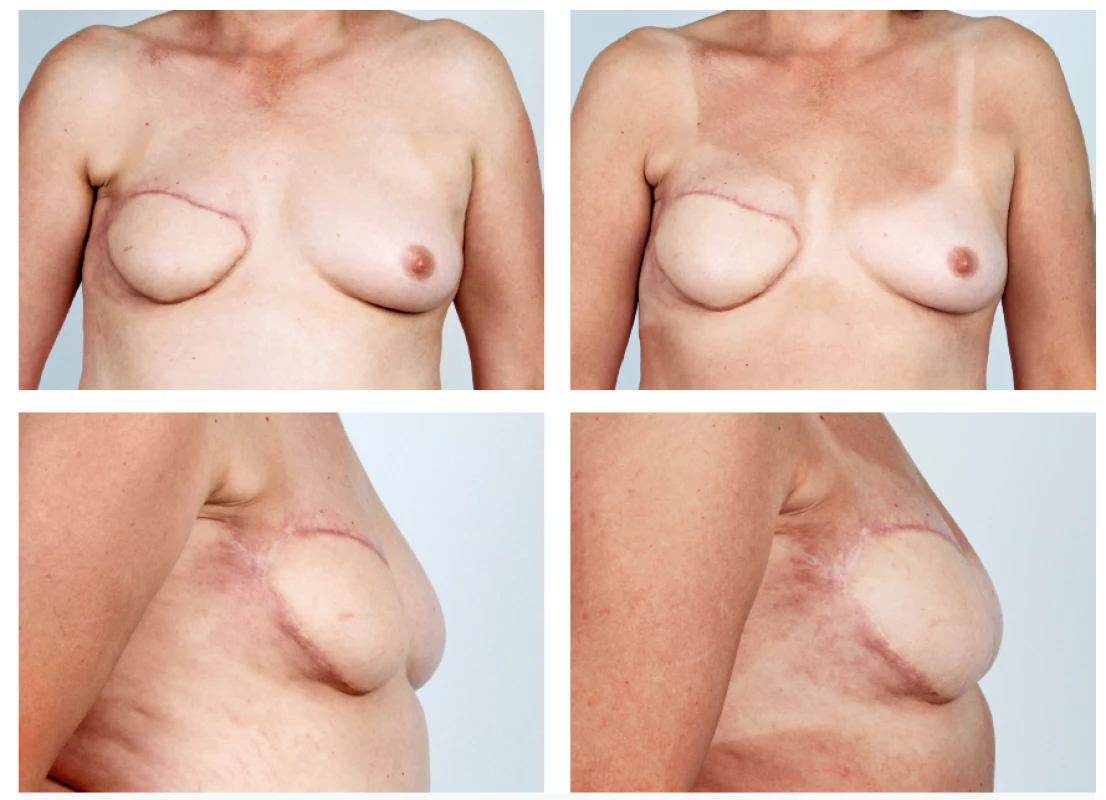
The autologous latissimus dorsi flap is an ideal tissue to receive fat transfer as it is very well-vascularized and very large quantities of fat can be injected into it. Volume of 200-450 ml per breast and per session may be injected with very good results 1,4,43. The autologous latissimus dorsi flap can now be considered as an auxiliary method that prepares the breast recipient site for future lipomodelling. We recommend performing lipomodelling quite early (after 3-4 months), before muscle atrophy is maximal, in order to take the advantage of the volume effect, which allows the area to accept sufficient amount of fat.
Breast augmentation by autologous fat transfer
There has been a boom in the demand for autologous fat breast augmentation in recent years. As our experience with this procedure extends, indications and limitations for this procedure clarify. Today, we know that autologous tissue transfer to the breast cannot replace breast augmentation using silicone implants 44. Indications for fat grafting are different then for breast augmentation with silicone implants. Also, contrary to fat grafting to the face, we are talking about high-volume, or sometimes “mega-volume fat grafting”, since we inject volumes of around 250-300 ml. This method is suitable for patients, who require rather smaller enlargement of the breast (at about one cup size), with sufficient breast skin envelope and sufficient adiposity in the lower part of the body. Breast augmentation by fat transfer is performed in sessions if more significant breast enlargement is requested by the patient.
First modern attempts for fat grafting to the breast in 1980’ launched a wave of negative attitude on the account of fear of calcifications, which could impair breast cancer detection. Subsequently, this method was banned for several upcoming years without any scientific data.
Radiologic follow-up of breasts treated with fat grafting is not complicated and should not prevent plastic surgeons from offering this procedure 45,46. Far more common are calcifications after different kinds of breast operations – for example reduction mammaplasty 47. Calcification after fat grafting, in case of the use of correct technique, are localized in different areas and reveal different radiological image then those in breast cancer. The best approach is to perform radiological examination ahead of the procedure to find out existing lesions. We advise performing follow-up examination one year after the procedure.
In addition, concerns about the oncologic safety remain a controversial topic among plastic surgeons and other physicians. In vitro studies have repeatedly shown an increased activation of breast cancer cells by adipose derived stem cells; however, clinical research, although limited, continually fails to show an increase in breast cancer recurrence after breast fat grafting not only in cosmetic breast augmentation patients but also in breast cancer patients 48-52.
We usually perform this procedure under general anaesthesia and we use methods described above for graft harvesting and processing. We infiltrate the breast from multiple entry points circularly around the breast base. We can infiltrate subcutaneous space, subglandular space and also the pectoral muscle. In order to ensure sufficient blood flow to the entire graft, we should be careful not to overfill the breast tissue. The maximal volume of graft is approximately 200–250 ml for B size breast, 300–350 ml for C size breast etc. We risk graft ischemia, absorption, necrosis and calcification with too large graft volumes. Based on authors’ experience, it is essential to overfill the size of the smaller breast in comparison with the contralateral side in cases of breast asymmetry because of the expected asymmetrical fat absorption. Relative volume resorption remains the same (30–40%), but the final volume absorption is different when asymmetrical volumes of processed fat is applied.
Preoperatively we educate the patient about the graft volume we plan to inject to the breast and about the possibility of 30–40% absorption. We do not use any compressive garment postoperatively, since it could impair blood supply to the graft. The patient should postoperatively keep stable weight, in order to prevent lowering of the volume of the augmented breast (Figure 7, 8).
7. Patient before and 6 months after one session of autologous fat breast augmentation 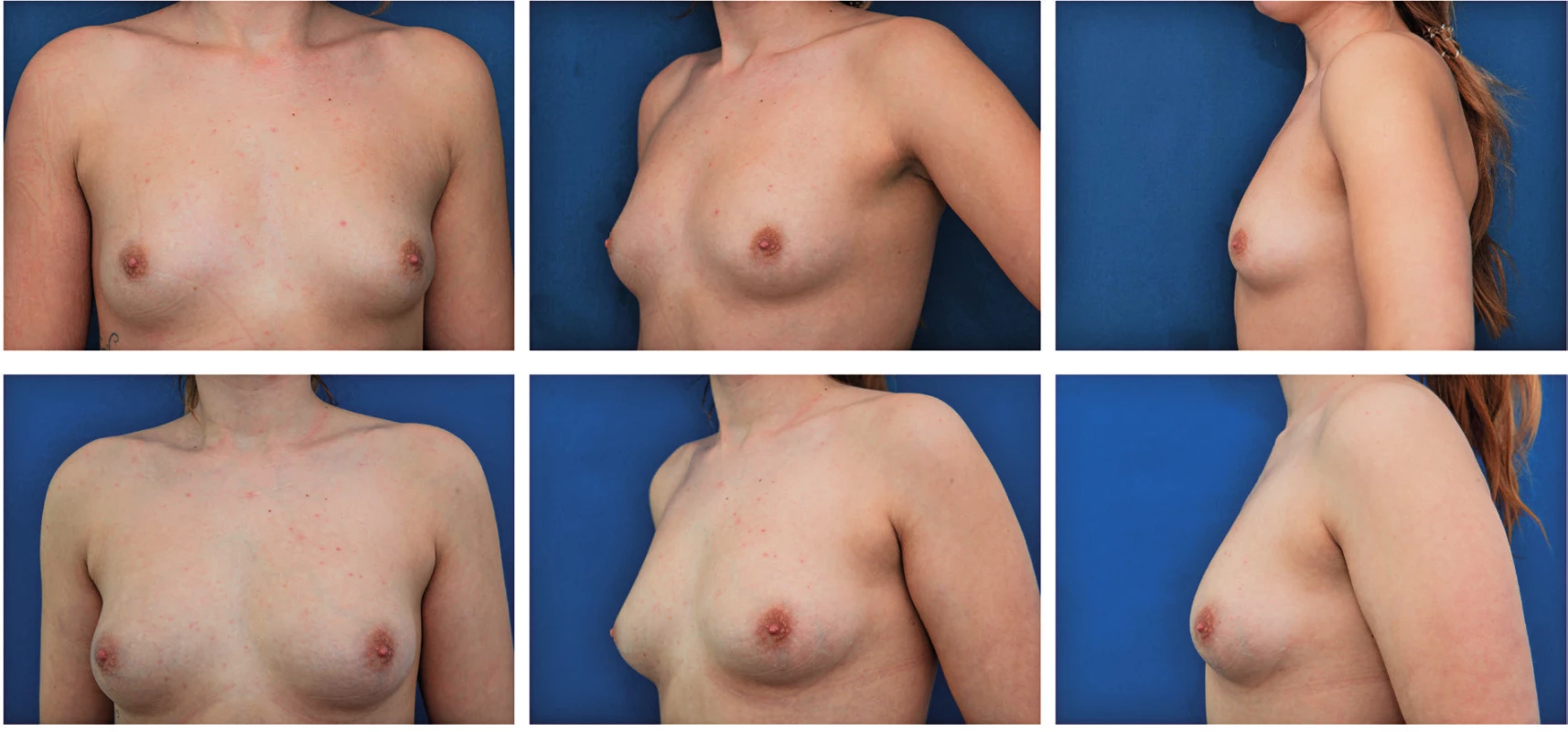
8. A 17-year-old patient with hypoplastic left breast and ptotic right breast before and after 2 sessions of lipomodelling on the left and contralateral vertical mastopexy with augmentation by fat grafting in décolleté area - 6 months follow-up 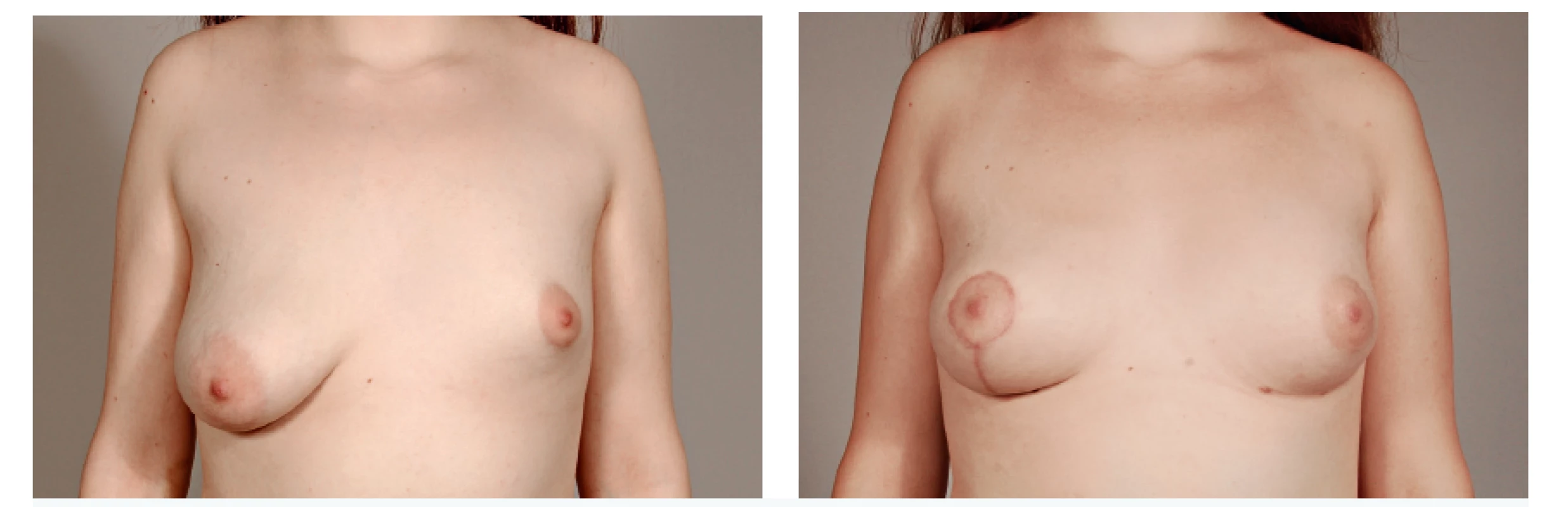
Simultaneous Breast Implant Exchange with Fat (SIEF)
Simultaneous Breast Implant Exchange with Fat is suitable for patients asking for breast implant removal while maintaining the volume 53,54. The procedure can be performed in two ways – fat is injected before or after implant removal. We inject fat into all possible planes, into subcutaneous and subpectoral after breast reconstruction and also into subglandular space after breast augmentation. We take care not to inject into the capsular space. In the case of implant removal before injection, we use finger guidance in this space to prevent penetration of capsule by the cannula. We also recommend using blunt cannula for injection.
Composite breast augmentation
As mentioned above, breast enlargement by fat grafting is limited and silicone implant augmentation continues to be a gold standard. However, we can use benefits of both techniques to achieve the best results – the volume created by the silicone implant, covered by the fat graft. With this technique, we cover the visible edges of the implants in thinner women. Fat can be injected before or after insertion of the implant. We prefer injection with implant already in place to achieve better overview of the final result. For this reason, we need to use blunt cannulas, which decrease the risk of implant penetration. Motiva (Establishment Labs) recently created a cannula with special tip intended to use for composite procedures. Fat is injected especially to the décolleté region and according to specific needs to any other regions of the breast.
AUTOLOGOUS FAT TRANSFER TO THE FACE
Autologous fat transfer to the face has become a common technique to treat volume and contour abnormalities in aesthetic and reconstructive facial plastic surgery 55. It has also been widely used for the management of facial aging signs 16,56.
Signs of aging with regards to fat grafting
Aging process affects all facial structures. The process includes changes in the skin and its appendages, subcutaneous tissues, and bony skeleton.
Skin and appendages are influenced by intrinsic (normal aging) and extrinsic (photoaging, smoking) factors and these are manifested by the changes of skin texture (wrinkling, atrophy and increased fragility, changes of colour, etc.).
Subcutaneous tissue includes fat compartments and the SMAS system with interconnections to skin that are essential for transfer of facial expression triggered by the facial muscles to the skin surface. Aging process in subcutaneous tissue is associated with atrophy of volume (deflation), migration of fat compartments and increased soft tissue laxity with ptosis causing changes of contour.
The changes in subcutaneous tissues are further enhanced by absorption of bony skeleton in the upper face and midface such as the orbital margins, prejowl mandible, pyriform aperture and nasal spine causing reduction of support for soft tissues.
Fat grafting may be used to address all of these changes in a certain extent, i.e. it can supplement volume and add support to tissues, replace missing bony structures and correct skin texture changes.
Technique for facial fat grafting
The technique of facial fat grafting differs from standard techniques used elsewhere in the body. It is necessary to use finer instruments to harvest smaller fat particles to be injected to the face. This prevents creation of bulges and irregularities that could develop after the standard fat grafting technique. Fat grafting to the face is referred to as microfat grafting or also low-volume fat grafting due to this difference.
Examples of such fine instruments include e.g. various Tulip cannulas with a small diameter for harvesting and various small diameter injectors for application of fat. (Figure 9, 10).
9. Examples of harvesters for facial fat grafting (Tonnard, Sorensen style) 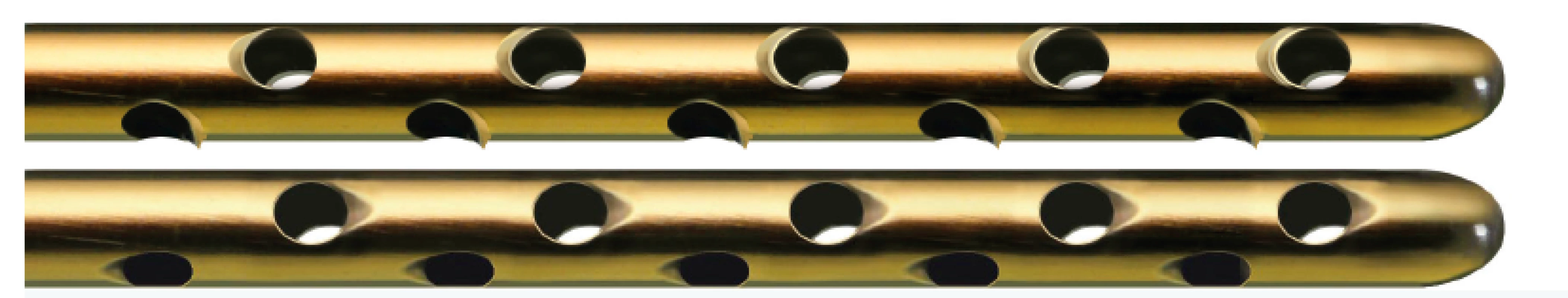
10. Examples of injectors for facial fat grafting. These may be curved or straight as per surgeon’s preference 
There are various application methods of microfat grafting and they include application of fat to the deep to act as a deep filler, superficially underneath the skin and intradermally into the dermis of the skin to treat fine and deep wrinkles and also a special form of fat for regenerative purposes. These various types provide different effects on target tissue.
Deep microfat grafting
Fat injected as a deep filler is used to supplement missing volume and for facial contouring. There are various areas in the face that loose volume with age and need filling (Figure 11). These include the temples, eyebrows and upper eyelids, infraorbital area, tear trough, malar area, nasolabial folds, lips, and the area of the Marionette lines.
11. Areas that usually need deep filling with fat during facial fat grafting 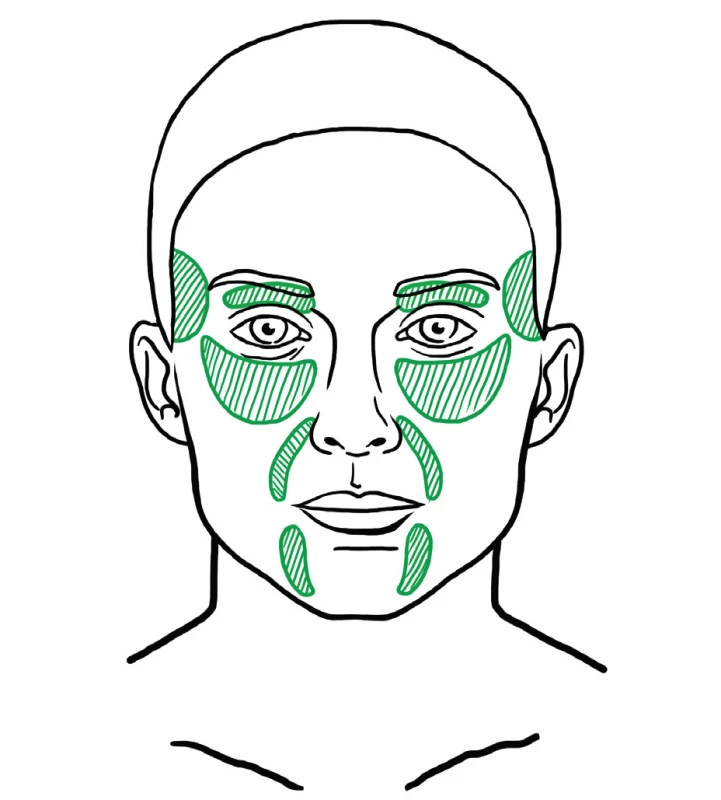
Filling effect of fat is also utilized in reconstructive procedures, such as augmentation rhinoplasty 57 to augment the dorsum of the nose or for correction of mandibular or maxillary hypoplasia that have traditionally been treated with orthognathic surgery 49,57-60.
Superficial fat grafting
Fat is injected to the superficial layer just underneath the skin. This technique addresses the aging induced atrophy that is characterized by disappearance of a continuous layer of fat underneath the skin. There are areas with more pronounced deflation to be treated with deep filling, but superficial fat grafting is beneficial in areas where fine wrinkling occurs that cannot be filled intradermally and there is no deep space to fill (Figure 12). This technique is more exactly referred to as superficial microfat grafting 61. Superficially injected fat has also regenerative potential on the skin. Study performed by Charles-de-Sá et al. demonstrated that treatment with fat and stromal vascular or expanded mesenchymal stem cells modified the pattern of the dermis, representing a skin rejuvenation effect. Effects of treatment are visible in the epidermis also, even though the injection takes place in the subcutaneous region 17.
12. Example of area that may benefit from superficial microfat grafting 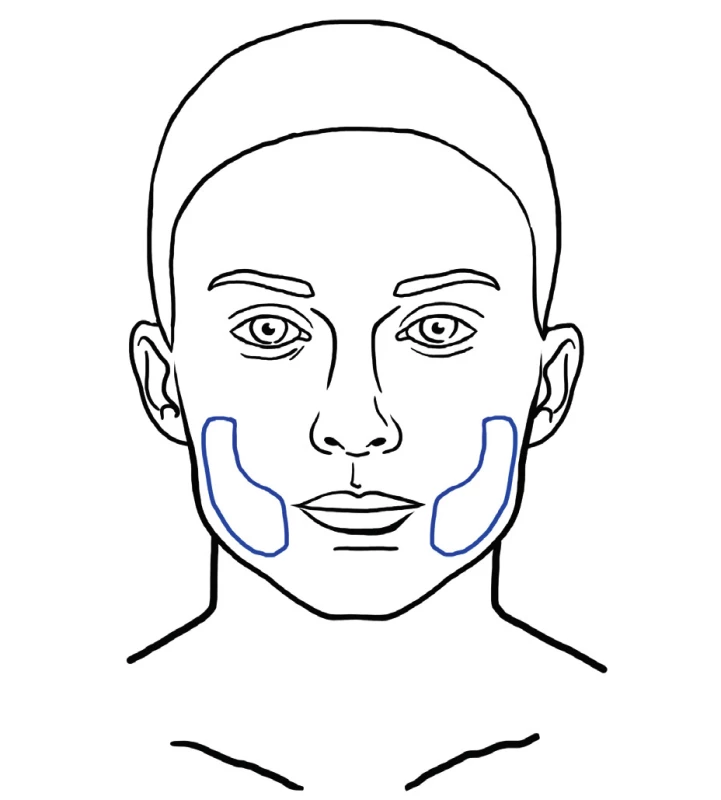
Various modifications exist, such as superficial enhanced fluid fat injection (SEFFI) where fat is combined with platelet rich plasma for enhancement of its effect 62,63.
Intradermal fat grafting
This technique was described by Zeltzer et. al. and popularized by Tonnard and Verpaele as SNIF (Sharp Needle Intradermal Fat grafting). Simple microfat is injected with a needle into the skin, into deep rhytides (Figure 13). This has an effect of a natural filler and smoothes wrinkles very effectively 30.
13. Areas for intradermal fat grafting usually involve the forehead, glabellar rhytides, nasolabial fold and perioral rhytides. SNIF technique can also be used for improvement of lip and philtrum contou 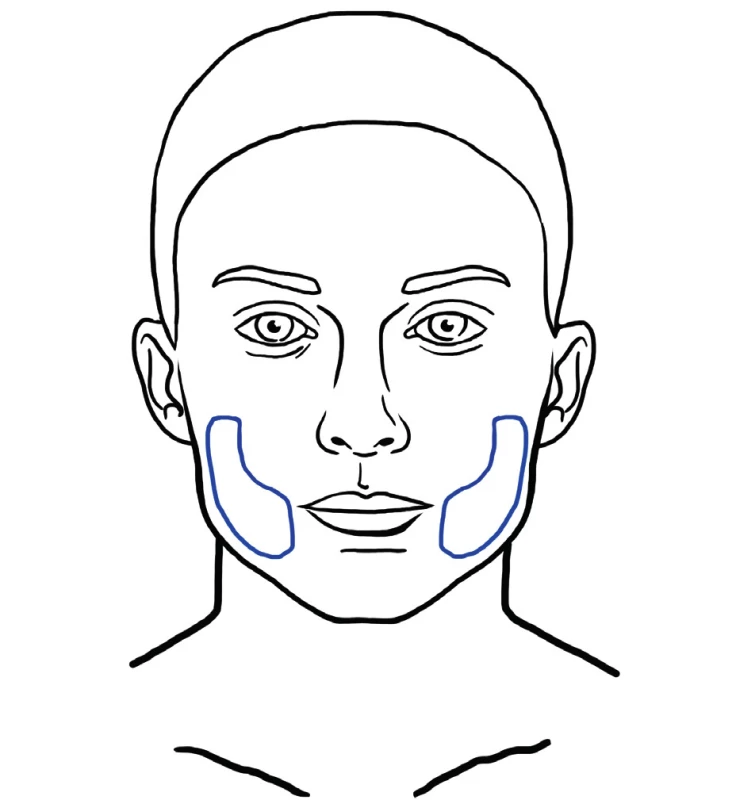
Nanofat and regenerative potential of fat
The therapeutic benefit of autologous fat is partly ascribed to the presence of precursors of adipose derived stem cells (ASCs), which are progenitor cells that reside in the stromal vascular fraction (SVF) of adipose tissue. ASCs are able to differentiate into various cell lineages and seem to be suitable for repair of damaged tissue in organs and aged skin 64. In cosmetic facial surgery, ASCs are reported to be beneficial for skin rejuvenation 65. Special fat processing techniques, which destroy mature adipose cells are able to prepare an injectable product, which contains a high concentration of ASCs and no viable mature adipose cells 64. The final injectable product is injected directly into the skin with a fine needle. Tonnard et. al. described the nanofat grafting technique, which is indicated for general skin rejuvenation, treatment of dark circles under the eyes, post-acne scaring, striae, etc. 66. Typical areas for nanofat grafting are show in Figure 14.
14. Red dotted areas represent examples of areas typically treated with nanofat for regenerative purposes 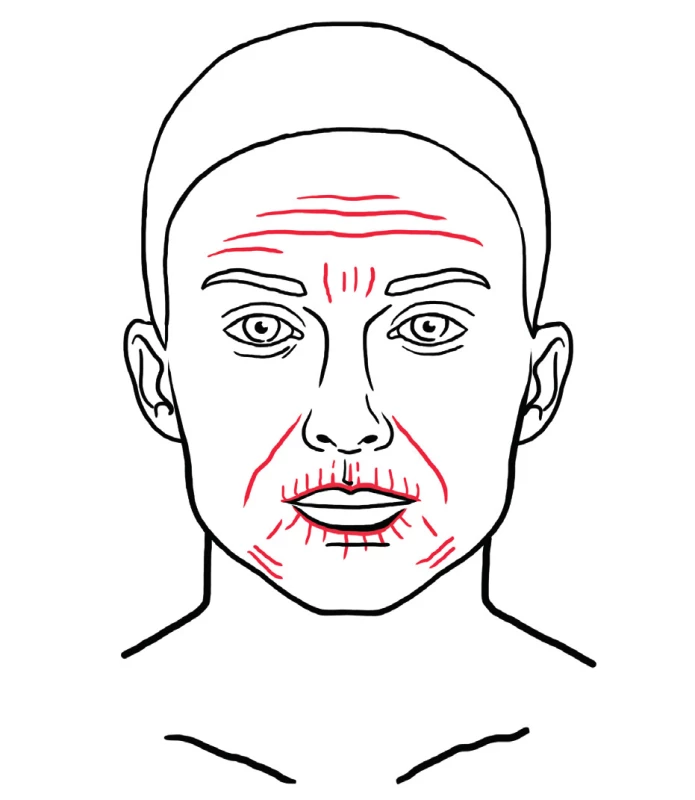
Survival of fat in the face
Survival of fat in the face varies and is influenced mainly by the mobility of the target area. Mobile areas such as glabella or lips are associated with lower retention of fat compared with less mobile areas, such as malar and lateral cheek 55. Stabilization of the graft may be considered after 4 months from the surgery.
Complications of facial fat grafting
Complications following fat grafting to the face are rare. The most common complications include irregularities in the donor area after fat harvesting, and asymmetry, irregularities, bulges, under-filling and overfilling in the target area. Rare complication is fat embolism to the ophthalmic artery and cerebral arteries with subsequent blindness and cerebrovascular accident that have been reported after fat injection mainly to the glabellar area 67,68.
Postoperative course and management
The postoperative course after facial fat grafting is associated with very low morbidity. There are signs and symptoms from the donor area, which is the most disturbing for the patient and it is similar to smaller liposuction. In the application area, there is usually swelling and bruising that subsides quickly after surgery, usually within 1–2 weeks. Pain is not a common symptom after facial fat grafting. In the postoperative management we usually do not use any special garments for the donor area, as the harvested volume is very low. It is advisable to provide antibiotic cover to the patient perioperatively.
Combination of facial fat grafting with other facial rejuvenation techniques
It is convenient to combine facial fat grafting with other facial plastic surgery techniques such as face-lifting, upper and lower blepharoplasty, chemical or laser resurfacing, etc. The effects multiply and the final cosmetic result of the combined surgery is potentiated 69,70.
Complex facial fat grafting
Complex facial fat grafting is a combination of several or all of the fat grafting techniques to treat various problems that are present in the face in the same time. These may be performed in one session. There is usually enough fat to be harvested to perform complex treatment, i.e. deep filling, superficial microfat grafting, intradermal fat grafting and possibly take the advantage of regenerative capacity of fat on facial skin (Figure 15). This is a great difference compared with artificial fillers based on hyaluronic acid, which are usually very costly, the quantity is limited mainly by price and it lacks any regenerative potential. The usual quantity of fat that is needed for complex fat grafting is 20–30 ml of processed fat or more.
15. A case of a 45-year-old female patient with profound deflation of the face seeking rejuvenation. Originally she requested filler injection but was offered facial fat grafting. The figure shows pre- and postoperative photographs. There was a total of 24 ml of microfat injected and included deep filling of malar, tear trough area and nasolabial folds, superficial fat grafting to the cheeks. Note mainly the change of contour of the malar area on the 45° view 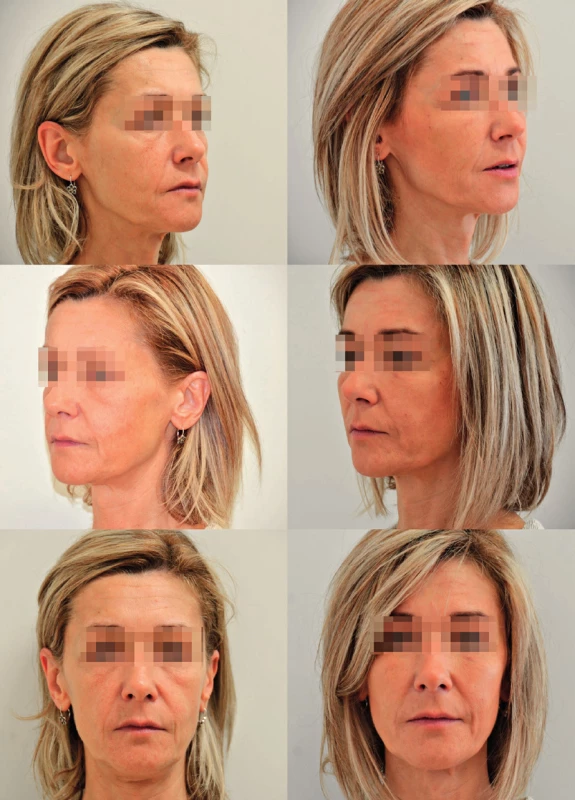
CONCLUSION
Autologous fat transfer techniques have experienced tremendous boom in the recent years. High-volume fat grafting is performed primarily in order to supplement missing volume, low-volume fat grafting has also a regenerative potential apart from its volume replenishment and contouring effects, especially in certain indications.
Lipomodelling is a major development in plastic, reconstructive and aesthetic surgery of the breast, and we consider it as one of the major advances of the last 20 years. The technique is now well codified and the complication rate is very low. Because of the very good results obtained and the excellent acceptance of the technique by the patients, lipomodelling has considerably modified our indications in plastic, reconstructive and aesthetic surgery of the breast.
Facial fat grafting is an effective and rather mini-invasive procedure that is able to provide very interesting effects in the treatment of overall facial aging signs. It has a filling as well as regenerative potential and may be combined with other facial procedures to achieve enhanced results. It may be performed under local anaesthesia with mild sedation and the postoperative course is very smooth.
Corresponding author:
Libor Streit, M.D., Ph.D.
Department of Plastic and Aesthetic Surgery
St. Anne’s University Hospital
Berkova 34
612 00 Brno, Czech Republic
E-mail: liborstreit@gmail.cz
Sources
1. Delay E, Garson S, Tousson G, Sinna R. Fat Injection to the Breast: Technique, Results, and Indications Based on 880 Procedures Over 10 Years. Aesthet. Surg. J. 2009;29(5):360-376.
2. Delay E, Sinna R, Chekaroua K, Delaporte T, Garson S, Toussoun G. Lipomodeling of Poland’s syndrome: a new treatment of the thoracic deformity. Aesthetic Plast Surg. Apr 2010;34(2):218-225.
3. 3Ho Quoc C, Delaporte T, Meruta A, La Marca S, Toussoun G, Delay E. Breast asymmetry and pectus excavatum improvement with fat grafting. Aesthet Surg J. Aug 2013;33(6):822-829.
4. Delay E, Streit L, Toussoun G, La Marca S, Ho Quoc C. Lipomodelling: An important advance in breast surgery. Acta Chirurgiae Plasticae. 2013;55(2):34-43.
5. Delay E, Sinna R, Ho Quoc C. Tuberous breast correction by fat grafting. Aesthet Surg J. May 2013;33(4):522-528.
6. Khouri RK, Rigotti G, Cardoso E, Biggs TM. Megavolume autologous fat transfer: Part I. Theory and principles. Plast. Reconstr. Surg. 2014;133(3):550-557.
7. Khouri RK, Rigotti G, Cardoso E, Biggs TM. Megavolume autologous fat transfer: Part II. Practice and techniques. Plast. Reconstr. Surg. 2014;133(6):1369-1377.
8. Rigotti G, Marchi A, Galiè M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: A healing process mediated by adipose-derived adult stem cells. Plast. Reconstr. Surg. 2007;119(5):1409-1422.
9. Roh MR, Jung JY, Chung KY. Autologous fat transplantation for depressed linear scleroderma-induced facial atrophic scars. Dermatol Surg. Dec 2008;34(12):1659-1665.
10. Pallua N, Baroncini A, Alharbi Z, Stromps JP. Improvement of facial scar appearance and microcirculation by autologous lipofilling. J Plast Reconstr Aesthet Surg. Aug 2014;67(8):1033-1037.
11. Brongo S, Nicoletti GF, La Padula S, Mele CM, D’Andrea F. Use of lipofilling for the treatment of severe burn outcomes. Plast Reconstr Surg. Aug 2012;130(2):374e-376e.
12. Hunstad JP, Shifrin DA, Kortesis BG. Successful treatment of Parry-Romberg syndrome with autologous fat grafting: 14-year follow-up and review. Ann Plast Surg. Oct 2011;67(4):423-425.
13. Coleman SR. Structural fat grafting: More than a permanent filler. Plastic and Reconstructive Surgery. Sep 1 2006;118(3):108S-120S.
14. Coleman SR. Facial recountouring with lipostructure. Clin. Plast. Surg. 1997;24(2):347-367.
15. SR C, RF M. Fat Injection: from Filling to Regeneration. St. Louis: Quality Medical Publishing; 2009.
16. Marten TJ, Elyassnia D. Fat Grafting in Facial Rejuvenation. Clin Plast Surg. Apr 2015;42(2):219-252.
17. Charles-de-Sá L, Gontijo-de-Amorim NF, Maeda Takiya C, et al. Antiaging treatment of the facial skin by fat graft and adipose-derived stem cells. Plast Reconstr Surg. Apr 2015;135(4):999-1009.
18. Moore JH, Kolaczynski JW, Morales LM, et al. Viability of fat obtained by syringe suction lipectomy: effects of local anesthesia with lidocaine. Aesthetic Plast Surg. 1995 Jul-Aug 1995;19(4):335-339.
19. Keck M, Zeyda M, Gollinger K, et al. Local anesthetics have a major impact on viability of preadipocytes and their differentiation into adipocytes. Plast Reconstr Surg. Nov 2010;126(5):1500-1505.
20. Keck M, Kober J, Riedl O, et al. Power assisted liposuction to obtain adipose-derived stem cells: impact on viability and differentiation to adipocytes in comparison to manual aspiration. J Plast Reconstr Aesthet Surg. Jan 2014;67(1):e1-8.
21. Lee JH, Kirkham JC, McCormack MC, Nicholls AM, Randolph MA, Austen WG, Jr. The Effect of Pressure and Shear on Autologous Fat Grafting. Plastic and Reconstructive Surgery. May 2013;131(5):1125-1136.
22. Leong DT, Hutmacher DW, Chew FT, Lim TC. Viability and adipogenic potential of human adipose tissue processed cell population obtained from pump-assisted and syringe-assisted liposuction. J Dermatol Sci. Mar 2005;37(3):169-176.
23. Tonnard P, Verpaele A, Peeters G, Hamdi M, Cornelissen M, Declercq H. Nanofat grafting: Basic research and clinical applications. Plastic and Reconstructive Surgery. 2013;132(4):1017-1026.
24. Kurita M, Matsumoto D, Shigeura T, et al. Influences of centrifugation on cells and tissues in liposuction aspirates: Optimized centrifugation for lipotransfer and cell isolation. Plastic and Reconstructive Surgery. Mar 2008;121(3):1033-1041.
25. Allen RJ, Jr., Canizares O, Jr., Scharf C, et al. Grading Lipoaspirate: Is There an Optimal Density for Fat Grafting? Plastic and Reconstructive Surgery. Jan 2013;131(1):38-45.
26. Conde-Green A, Baptista LS, Gontijo de Amorin NF, et al. Effects of Centrifugation on Cell Composition and Viability of Aspirated Adipose Tissue Processed for Transplantation. Aesthetic Surgery Journal. Mar 2010;30(2):249-255.
27. Conde-Green A, Gontijo de Amorim NF, Pitanguy I. Influence of decantation, washing and centrifugation on adipocyte and mesenchymal stem cell content of aspirated adipose tissue: A comparative study. Journal of Plastic Reconstructive and Aesthetic Surgery. Aug 2010;63(8):1375-1381.
28. Rohrich RJ, Sorokin ES, Brown SA. In search of improved fat transfer viability: A quantitative analysis of the role of centrifugation and harvest site. Plastic and Reconstructive Surgery. Jan 2004;113(1):391-395.
29. Streit L. A Comprehensive In Vitro Comparison of Preparation Techniques for Fat Grafting. Plast Reconstr Surg. 2017;Accepted for the publication.
30. Zeltzer AA, Tonnard PL, Verpaele AM. Sharp-needle intradermal fat grafting (SNIF). Aesthet Surg J. Jul 2012;32(5):554-561.
31. Mestak O, Sukop A, Hsueh YS, et al. Centrifugation versus PureGraft for fatgrafting to the breast after breast-conserving therapy. World Journal of Surgical Oncology. 2014;12(1).
32. Yoshimura K, Sato K, Aoi N, Kurita M, Hirohi T, Harii K. Cell-Assisted Lipotransfer for Cosmetic Breast Augmentation: Supportive Use of Adipose-Derived Stem/Stromal Cells. Aesth. Plast. Surg. Sep 01 2007;32(1):48-55.
33. Zhou Y, Wang J, Li H, et al. Efficacy and Safety of Cell-Assisted Lipotransfer. Plats. Reconstr. Surg. Jan 2016;137(1):44e-57e.
34. Wang L, Luo X, Lu Y, Fan Z-H, Hu X. Is the Resorption of Grafted Fat Reduced in Cell-Assisted Lipotransfer for Breast Augmentation? Ann Plast Surg. Aug 2015;75(2):128-134.
35. Jung HK, Kim CH, Song SY. Prospective 1-Year Follow-Up Study of Breast Augmentation by Cell-Assisted Lipotransfer. Aesthet Surg J. Feb 2016;36(2):179-190.
36. Peltoniemi HH, Salmi A, Miettinen S, et al. Stem cell enrichment does not warrant a higher graft survival in lipofilling of the breast: A prospective comparative study. Br J Plast Surg. Nov 01 2013;66(11):1494-1503.
37. Ho Quoc C, Sinna R, Gourari A, La Marca S, Toussoun G, Delay E. Percutaneous fasciotomies and fat grafting: indications for breast surgery. Aesthet Surg J. Sep 2013;33(7):995-1001.
38. Khouri RK, Eisenmann-Klein M, Cardoso E, et al. Brava and Autologous Fat Transfer Is a Safe and Effective Breast Augmentation Alternative. Plast. Reconstr. Surg. May 2012;129(5):1173-1187.
39. Khouri RK, Eisenmann-Klein M, Cardoso E, et al. Brava and autologous fat transfer is a safe and effective breast augmentation alternative: Results of a 6-year, 81-patient, prospective multicenter study. Plast. Reconstr. Surg. 2012;129(5):1173-1187.
40. Momoh AO, Ahmed R, Kelley BP, et al. A systematic review of complications of implant-based breast reconstruction with prereconstruction and postreconstruction radiotherapy. Ann Surg Oncol. Jan 2014;21(1):118-124.
41. Mestak O, Sukop A, Hsueh YS, et al. Centrifugation versus PureGraft for fatgrafting to the breast after breast-conserving therapy. World J Surg Oncol. Jun 05 2014;12 : 178.
42. Mestak O, Hromadkova V, Fajfrova M, Molitor M, Mestak J. Evaluation of Oncological Safety of Fat Grafting After Breast-Conserving Therapy: A Prospective Study. Ann Surg Oncol. Mar 2016;23(3):776-781.
43. Delay E, Gounot N, Bouillot A, Zlatoff P, Rivoire M. Autologous latissimus breast reconstruction: A 3-year clinical experience with 100 patients. Plast. Reconstr. Surg. 1998;102(5):1461-1478.
44. Del Vecchio DA. Breast augmentation by fat transfer. ASPS The Meeting. Los Angeles2016.
45. Veber M, Tourasse C, Toussoun G, Moutran M, Mojallal A, Delay E. Radiographic findings after breast augmentation by autologous fat transfer. Plast. Reconstr. Surg. 2011;127(3):1289-1299.
46. Groen J-W, Negenborn VL, Twisk JWR, Ket JCF, Mullender MG, Smit JM. Autologous Fat Grafting in Cosmetic Breast Augmentation: A Systematic Review on Radiological Safety, Complications, Volume Retention, and Patient/Surgeon Satisfaction. Aesthet Surg J. Oct 2016;36(9):993-1007.
47. Rubin JP, Coon D, Zuley M, et al. Mammographic changes after fat transfer to the breast compared with changes after breast reduction: A blinded study. Plast. Reconstr. Surg. 2012;129(5):1029-1038.
48. Charvet HJ, Orbay H, Wong MS, Sahar DE. The Oncologic Safety of Breast Fat Grafting and Contradictions Between Basic Science and Clinical Studies. Ann Plast Surg. Oct 2015;75(4):471-479.
49. Wang Q, Guo X, Wang J. Autogenous Fat Grafting for Chin Augmentation: A Preliminarily Clinical Study of Cosmetic Outcome. J Craniofac Surg. Oct 2015;26(7):e625-627.
50. Krumboeck A, Giovanoli P, Plock JA. Fat grafting and stem cell enhanced fat grafting to the breast under oncological aspects-recommendations for patient selection. Breast. Oct 2013;22(5):579-584.
51. Voglimacci M, Garrido I, Mojallal A, et al. Autologous fat grafting for cosmetic breast augmentation: a systematic review. Aesthet Surg J. May 2015;35(4):378-393.
52. Petit JY, Botteri E, Lohsiriwat V, et al. Locoregional recurrence risk after lipofilling in breast cancer patients. Ann Oncol. Mar 2012;23(3):582-588.
53. Del Vecchio DA. “SIEF”—Simultaneous Implant Exchange with Fat. Plast. Reconstr. Surg. Dec 2012;130(6):1187-1196.
54. Ohashi M, Yamakawa M, Chiba A, Nagano H, Nakai H. Our Experience with 131 Cases of Simultaneous Breast Implant Exhange with Fat (SIEF). Plastic and Reconstructive Surgery – Global Open. Apr 2016;4(4):e691-698.
55. Strong AL, Cederna PS, Rubin JP, Coleman SR, Levi B. The Current State of Fat Grafting: A Review of Harvesting, Processing, and Injection Techniques. Plast Reconstr Surg. Oct 2015;136(4):897-912.
56. Coleman SR, Katzel EB. Fat Grafting for Facial Filling and Regeneration. Clin Plast Surg. Jul 2015;42(3):289-300, vii.
57. Kao WP, Lin YN, Lin TY, et al. Microautologous Fat Transplantation for Primary Augmentation Rhinoplasty: Long-Term Monitoring of 198 Asian Patients. Aesthet Surg J. Jun 2016;36(6):648-656.
58. Lindenblatt N, van Hulle A, Verpaele AM, Tonnard PL. The Role of Microfat Grafting in Facial Contouring. Aesthet Surg J. Sep 2015;35(7):763-771.
59. Agrawal KS, Bachhav M, Naik CS, Tanwar H, Sankhe SS. Autologous Fat Transfer for Esthetic Contouring of Face in Posttraumatic Nonfunctional Maxillofacial Deformities. Craniomaxillofac Trauma Reconstr. Jun 2016;9(2):113-120.
60. Guerrerosantos J, Haidar F, Paillet JC. Aesthetic facial contour augmentation with microlipofilling. Aesthet Surg J. Jul-Aug 2003;23(4):239-247.
61. Tonnard P. Personal communication. 2016.
62. Bernardini FP, Gennai A, Izzo L, et al. Superficial Enhanced Fluid Fat Injection (SEFFI) to Correct Volume Defects and Skin Aging of the Face and Periocular Region. Aesthet Surg J. Jul 2015;35(5):504-515.
63. Gennai A, Zambelli A, Repaci E, et al. Skin Rejuvenation and Volume Enhancement with the Micro Superficial Enhanced Fluid Fat Injection (M-SEFFI) for Skin Aging of the Periocular and Perioral Regions. Aesthet Surg J. May 30 2016.
64. van Dongen JA, Stevens HP, Parvizi M, van der Lei B, Harmsen MC. The fractionation of adipose tissue procedure to obtain stromal vascular fractions for regenerative purposes. Wound Repair Regen. Sep 26 2016.
65. Charles-de-Sa L, Gontijo-de-Amorim NF, Maeda Takiya C, et al. Antiaging treatment of the facial skin by fat graft and adipose-derived stem cells. Plast Reconstr Surg. Apr 2015;135(4):999-1009.
66. Tonnard P, Verpaele A, Peeters G, Hamdi M, Cornelissen M, Declercq H. Nanofat grafting: basic research and clinical applications. Plast Reconstr Surg. Oct 2013;132(4):1017-1026.
67. Kang JH, Park KH, Park JS. Acute mental change and hemiplegia after autologous fat injection. J Cosmet Laser Ther. Nov 2016;18(7):413-416.
68. Teimourian B. Blindness following fat injections. Plast Reconstr Surg. Aug 1988;82(2):361.
69. Willemsen JC, Mulder KM, Stevens HP. Lipofilling with minimal access cranial suspension lifting for enhanced rejuvenation. Aesthet Surg J. Sep 2011;31(7):759-769.
70. Tonnard PL, Verpaele AM, Zeltzer AA. Augmentation blepharoplasty: a review of 500 consecutive patients. Aesthet Surg J. Mar 2013;33(3):341-352.
Labels
Plastic surgery Orthopaedics Burns medicine Traumatology
Article was published inActa chirurgiae plasticae

2017 Issue 2-
All articles in this issue
- Editorial
- COMPLICATIONS OF LOWER EXTREMITY HEMATOMAS IN PATIENTS WITH PRE-INJURY WARFARINE USE
- SURGICAL CORRECTION OF LABIA MINORA HYPERTROPHY, A PERSONAL TECHNIQUE
- SUTURE VERSUS FIBRIN GLUE MICRONEURAL ANASTOMOSIS OF THE FEMORAL NERVE IN SPRAGUE DEWLY RAT MODEL. A COMPARATIVE EXPERIMENTAL ASSESSMENT OF THE CLINICAL, HISTOLOGICAL AND STATISTICAL FEATURES
- INTRAOPERATIVE FAT GRAFTING INTO THE PECTORALIS AND LATISSIMUS DORSI MUSCLES-NOVEL MODIFICATION OF AUTOLOGOUS BREAST RECONSTRUCTION WITH EXTENDED LATISSIMUS DORSI FLAP
- HAS A GLOMUS TUMOR ALWAYS A QUICK DIAGNOSIS?
- CURRENT CONCEPTS IN PERIPHERAL NERVE INJURY REPAIR
- SUBACUTE ARTERIAL BLEEDING AFTER SIMULTANEOUS MASTOPEXY AND BREAST AUGMENTATION WITH IMPLANTS: CASE REPORT
- AUTOLOGOUS FAT TRANSFER, BREAST LIPOMODELLING AND FAT TRANSFER TO THE FACE: CURRENT GOLD STANDARDS AND EMERGING NEW DATA
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- SURGICAL CORRECTION OF LABIA MINORA HYPERTROPHY, A PERSONAL TECHNIQUE
- INTRAOPERATIVE FAT GRAFTING INTO THE PECTORALIS AND LATISSIMUS DORSI MUSCLES-NOVEL MODIFICATION OF AUTOLOGOUS BREAST RECONSTRUCTION WITH EXTENDED LATISSIMUS DORSI FLAP
- COMPLICATIONS OF LOWER EXTREMITY HEMATOMAS IN PATIENTS WITH PRE-INJURY WARFARINE USE
- HAS A GLOMUS TUMOR ALWAYS A QUICK DIAGNOSIS?
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

