-
Medical journals
- Career
Authors: K. Hirata; Y. Kimura; T. Mizuguchi; M. Mimamura; M. Meguro; Y. Nakamura; T. Ito; H. Yamaguchi; D. Kyuno
Authors‘ workplace: Sapporo Medical University School of Medicine ; Department of Surgical Oncology and Gastroenterological Surgery
Published in: Rozhl. Chir., 2012, roč. 91, č. 6, s. 340-345.
Category: Various Specialization
Introduction
Among non - inflammatory cystic lesions of pancreas, much opportunity on diagnosis of intraductal papillary-mucinous neoplasms tumor (IPMN) of the pancreas has internationally been reported with the progress of diagnostic imaging methods and widely spreading awareness of this disease-entity [1]. It is important that IPMN is sometimes accompanied by malignant diseases of other organs and would often develop to often invasive ductal adenocarcinoma [2]. Otherwise, IPMN has malignant potential and showed a broad spectrum in histology [3], ranging from adenoma to minimally invasive carcinoma, of which the frequency of lymph node metastasis has showed the lower incidence and a little more favourable prognosis, compared with those in so-called invasive adenocarcinoma. At surgical treatment, the important recognition for IPMN should be summarized in the following three points, i.e.:
- benign or malignant,
- multi-centric occurrence or not,
- the existence of atypical cells in surgical margin.
In this report, we would like to introduce the decision making of operation procedure, especially the justified surgical margin in IPMN.
The basic strategy in surgical treatment
The general indications of surgical treatment for IPMN are as follows [4, 5]:
- Significant findings as a carcinoma with multifocal stromal invasion,
- Strongly suspicious findings of characteristics in this entry as mixed-echo pattern, solid-echo pattern and so on,
- Repeated pancreatitis induced by mucous accumulation,
- Existence of an papillary lesion in main pancreatic duct(MPD) more than 4 - 7mm in height as the main-duct type group,
- Among the branched-duct type, the size of papillary cystic lesion (with papillary protrusion) more than 5 cm in diameter with large dense multilocular cysts.
General strategy on the selection of the surgical treatment for IPMNs is shown in Tab. 1. Pylorus preserving pancreaticoduodenectomy (PPPD) for intraductal papillary mucinous carcinoma IPMC in head of the pancreas and distal pancreatectomy for IPMC in body and tail of the pancreas should be indicated (Fig.1). Rarely, less-invasive surgery or tissue-preserving pancreatectomy might be selected, especially for weakly suspicious malignancy and smaller-sized IPMN (Fig. 2). When it is diagnosed as IPMC or intraductal papillary mucinous adenoma (IPMA) with partially IPMC, no guidelines and no consensus have defined the operation procedure (1), but, anyhow, the complete extirpation is strictly required. In such cases, non-typical modified resection method would have been introduced. The selection of surgical procedures might be introduced as follows;
1. Present strategy of the operation procedures for IPMN 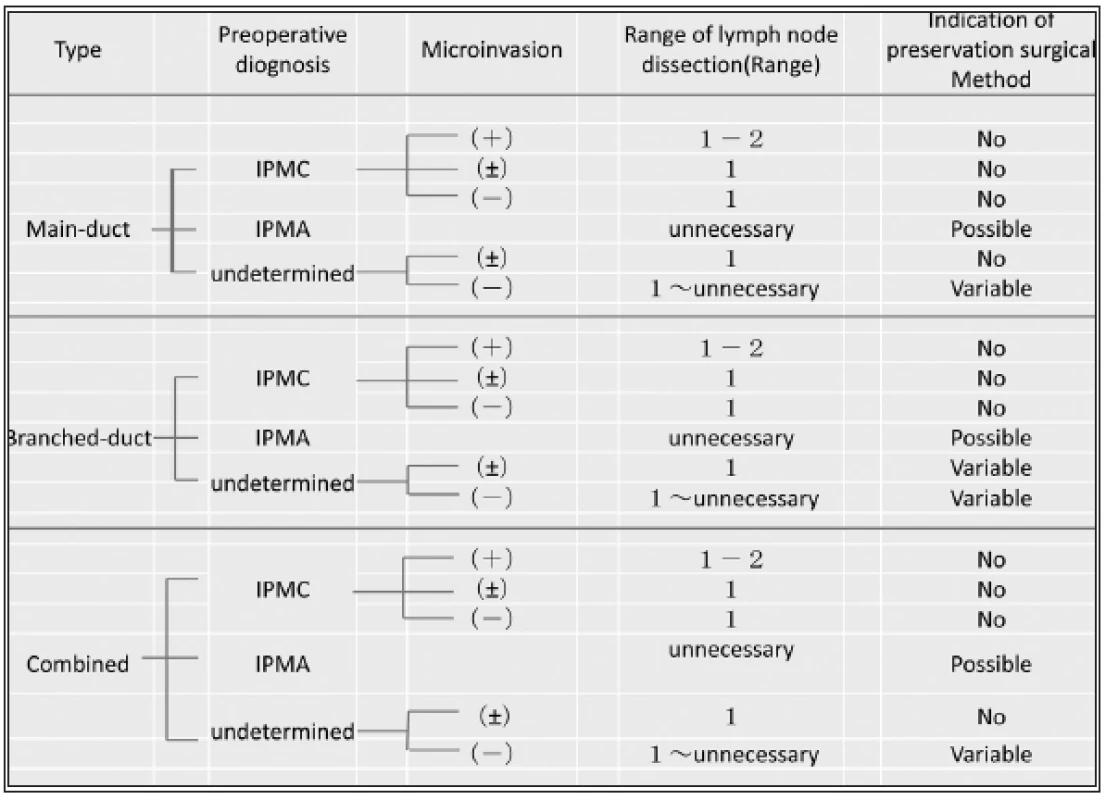
Fig. 1: Standard pancreatectomies by the reconstruction of Imanaga method: a. Pancreaticoduodenectomy (PD) with gastric resection, b. Pylorus - preservated PD(PPPD), c. PPPD by the reconstruction of Imanaga method,d.distal pancreatectomy. 
Fig. 2: Various kinds of pancreatectomies with pancreas - preservation method. Pro obrázek následující text minimal PD: pylorus - preserving minimum pancreaticoduodenectomy with paraduodenal - vessel preservation, DpPHR: duodenum preserving pancreatic head resection, UR: uncinate resection, SR: segmental resection, SpDp: spleen-preserving distal pancreatectomy, lapa-SpDp: laparoscopic spleen - preserving distal pancreatectomy 
Surgical treatment for IPMA
The conservative follow-up observation for the benign tumors among IPMNs is the first choice as the medical strategy. However, when the lesion shows the higher risks of malignancy with several findings as described in Tab. 1, typical pancreatectomy for radical resection should be indicated. Present strategy for the indication of less invasive surgery or tissue-preserving pancreatectomy (Fig. 2) is summarized in Tab. 2. As recognized, tissue-preserving treatment procedures should not be indicated for the cases with suspicious IPMC, even if such lesion was very small. First of all, as an essential principle, local resection of the pancreas would be indicated only for some of IPMA, because there have been reported about several limitations in the view of radicality.
2. Present limitations and problems on the selection of surgical treatments for IPMC 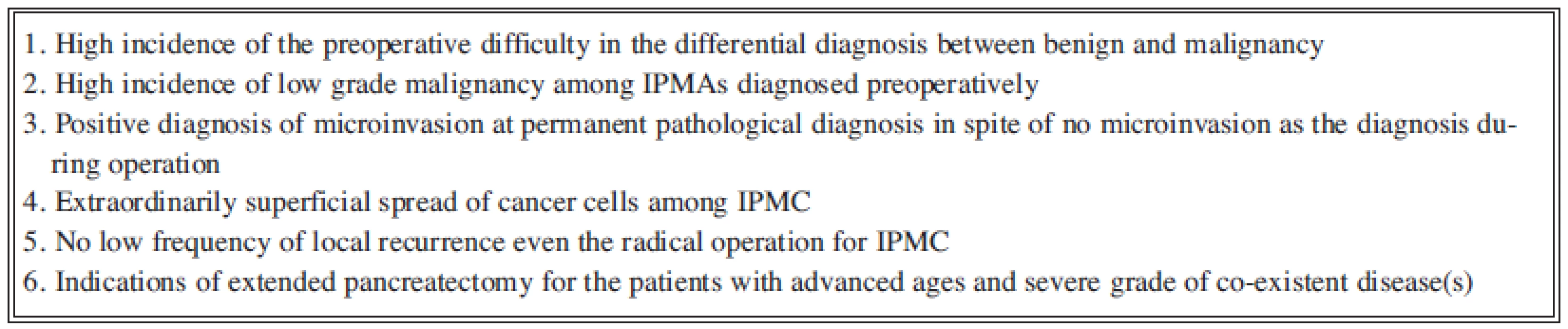
Fig. 3 introduces the characteristic distribution of atypical or cancer cells surrounding the main tumor in IPMN. Three types are classified on the basis of the localization of tumor, i.e. the main-duct type, the branched duct type and the combined type. And by the condition of main duct, both the main-duct and the branched duct types are divided in the dilated type and the non-dilated type. Moreover, the morphological pattern of main tumor among the dilated type shows the flat type or the polypoid type. International Consensus Guidelines for Management of IPMN and Mucinous Cystic Neoplasmo of the Pancreas6) defines each cystic neoplasm and also points the similar recognition of the atypical cells distribution. But no strict definitions were found in surgical procedures.
Fig. 3: Pathhistological findings and the schema among IPMN and the suspiciously related entities 
Surgical treatment for IPMC
The treatment procedure for IPMC should be required as the same manner with invasive ductal carcinoma. As known well, the prognosis of IPMC is significantly better than that of invasive ductal carcinoma. And the invasive manner of IPMC generally seems not to be aggressive. However, different kind of strategy in surgical treatment should be considered on the basis of the proliferated characteristics of IPMC in pancreas, especially in pancreatic duct.
As pointed out above, the preoperative decision for surgical dissection line should be based on the imaging diagnosis. Such decision would invite the smooth procedure in operation and the protection from the dissemination of cancer cell caused by mucous flow from the duct during operation. Moreover, the difficult judgement by the intraopenative pathohistological diagnosis for the surgical margin has well been known. Therefore, the responsibility for the surgical procedure should be required the decision just before operation [6]. Therefore, our present summary in the surgical treatment should be conclusive for the following principles (Fig.3). Namely, we have already recognized a limitation for the definitely preoperative diagnosis.
Group with limited distribution of atypical cells
Most in this group are characterized in the imaging diagnosis as the main-duct type with local dilatation of MPD and the branched-duct type without dilatation. Among them, the maximal distension of atypical cells is generally experienced within 20mm from the edge of the main tumor. However, unordinary cases have been observed, therefore, further studies would be expected to detect the character of imaging findings in such cases.
2. Group with superficial spread of atypical cells
This group is characterized in all cases with diffuse dilatation of MPD, wherever the location of the main tumor is. Therefore, it seems to difficult to determine the accurate surgical margin in such cases preoperatively and intraoperatively. But the result of positive margins in intraoperative or permanent histological diagnosis is not rare. Therefore, sometimes, total pancreatectomy would be performed and might have invited poor QOL. Namely, in the patients with old ages and/or severe co-existent disease(s), the worse prognosis and QOL has well been known even if the maximal best management in medicine would be undergone after operation. The present consensus has been that the positive atypical cells but no cancer cells in the surgical margin would permit partial pancreatectomy [7, 8]. Until now, the results in the cases with positive atypical cells reported the extremely low incidence of local recunence [9]. However, further studies among high volume of the cases would be required in near future. The determination of surgical procedure should depend on the patient’s self - decision under the objectively presented informed-consent.
On the situation of the clinical pathologists, highly frequent difficulties in intraoperative diagnosis for the resected margin have been discussed, therefore, as the general recognition, most of the surgeon have lower expectation and dependency for pathological confirmations during operation. Formerly, pancreatoscopy had been recommended for the determination of surgical margin during operation. However, recent consensus does not recommend the intraoperative endoscopy of the pancreatic duct as the first choice, because the dissemination of cancer cells during pancreatoscopy has been reported.
Therefore, the present strategy in the selection of surgical procedures for IPMC should indicates the same consensus for the patients with invasive ductal carcinoma of the pancreas. Namely, the accurate lymph node dissection and the securement of cancer-free surgical margin are required. Therefore, resection line much distant from the distinct edge of main tumor would be prepared, which is significantly different from that in patients with invasive ductal carcinoma of the pancreas.
3. Present limitation of the justification as the indication of the surgical treatment
In spite of the development on diagnostic procedures for pancreatic diseases, several limitations are now discussed (Fig. 3). Further clinical trials and basic researches may lead to the answer and then the accurate surgical procedure will be introduced for radicality and less-invasiveness.
4. Classification of modified-resection methods of the pancreas and its anatomical recognition
A segmental classification of the pancreas had been provided on the embryo-histogenesis and the anatomical findings [10]. Moreover, the pancreas head was divided in tow subsegments, i.e. ventral and dorsal sites (Fig. 4).
Fig. 4: New, consept of pancreatic segment: Takada’s proposal, (b) Ryu’s proposal. 
The ventral segment was defined as the superior area of the pancreas head which is distributed the Santorini duct and the distal portion of MPD. The dorsal subsegment was defined as the inferior area and the processus of the pancreas, which is distributed the Wirsung duct and intrahepatic bile duct. Fig. 5 introduces the summarized schema of each modified resection.
Fig. 5: Segmental resections of the pancreas head: a. resection of uncinate processus, b. resection of posterior segment, c. resection of the anterior segment. 
Next, the duodenum preserving pancreas head resection (DpPHR) had been proposed for the preservation of duodenal function and also the endocrine function of the pancreas. As indicated in Fig. 6, the procedures are different by the reservation procedures for vessels, intropancreatic bile duct, uncinate processus musculo-membrane, and the paraduodenal pancreas among them [11, 12].
Fig. 6: Duodenum preservated pancreatic head resection: a. Beger’s method, b. Imaizumi’s method, c. Takada’s method. 
Anyhow, in order to perform the complete resection in each method, the variations of the distributions of arteries around pancreas head (Fig. 7) should be recognized [3] (Fig. 6), because it is difficult to get the same result prevented from the complications among three kinds of procedures. And the distribution of drainage veins should influence on the circulation of the reserved tissues, but no detailed explanation and evidences has been reported. And also the modified pancreaticoduodenectomy was proposed to preserve the intestinal exocrine functions [13, 14]. Namely, the gastrointestinal hormone-producing cells distributed mainly in duodenum and upper jejunum may well be functional for the physiological condition after operation. But the significant data have not shown among different institutes.
Fig. 7: Different distributions around pancreaticoduodenal region. Pro obrázek následující text The distribution of arteries around (a) pancreas head and duodenal 1st. and 2nd. portion and (b) pancreas head and duodenal 3rd portion. A - 1 - Arcade distribution of pancreaticoduodenal artery in the head, 2 - Independent branching of posterior pancreaticoduodenal artery (PPDA) from superior mesenteric artery(SMA), 3 - Branching of PPDA between common hepatic artery and SMA, 4 - Branching of PPDA from posterior pancreatic artery. B - 1 - Ordinary branching of the inferior pancreaticoduodenal artery (IPDA) from SMA, 2 - Branching of the posterior IPDA from SMA, 3 - Distribution of the IPDA between the gastroduodenal artery and posterior pancreatic artery, 4 - Branching of the IPDA from the jejunal first branch, 5 - Branching of the inferior posterior and anterior PDAs from jejunal first and second branches, 6 - Branching of artery from SMA to right posterior hepatic artery. 
Surgical indication for modified resections of the pancreas have not been established and recognized not as the standard procedures. The incidences of the complications, i.e. pancreatic fistula, bile leakage, delayed gastric emptying, the stenosis of bile duct, partial necrosis of the duodenum and etc, seems to be still high. Further studies, would be required as the recommended procedures.
Conclusion
Order-made resection for each case may be ideal for the maintenance of the QOL. However, further efforts of operation procedures and early adequate diagnosis by specialists would be expected for the reliability and the feasibility. At present, typical procedures of pancreatectomy and of the dissection of lymph nodes for IPMC would be recommended as the standard consensus. And modified resections of the pancreas might sometimes be indicated for the typical smaller IPMA.
Prof. M.D. Koichi Hirat, PhD
Department of Surgical Oncology and Gastroenterological Surgery
Sapporo Medical University School of Medicine
South-1, West-16, Chuo-ku, Sapopro Japan 060-8543
e-mail: y.yamaya@sapmed.ac.jp
Sources
1. Tanaka M, Chari S, Adsay V, et al International Consensus Guidelines for Management of Intraductal Papilary Mucinous Neoplasms and Mucinous Cystic Neoplasms of the Pancreas. Pancreatology 2006;6 : 17–32.
2. Sugiyama M, Izumisato Y, Abe N, et al. Predictive factors for maligunacy in intraductal papillary-mucinous tumors of the pancreas. Br J Surg 2003;90 : 1244–1249.
3. Kloppel G, Socia E, Longnecker DS, et al. World Health Organization International Histological Typing of Tumors of the exocrine Pancreas. Berlin, Springer, 1996 : 1–61
4. Kobayashi G, Fujita N, Noda Y et al: Mode of prognoression of intraductal papillary-mucinous tumor of the pancreas: analysis of patients with follow-up by EUS. J Gastroentero 2005;40 : 744–751.
5. Wakabayashi T, Kawaura Y, Morimoto H, et al. Clinical management of intraductal papillary mucinous tumors of the pancreas based on imaging findings. Pancreas 2001;22 : 370–377.
6. Chari ST, Yadav D, Smyrk TC et al: Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology 2002;123 : 1500–7.
7. Terris B, Ponsot P, Paye F, et al. Intraductal papillary mucinous tumors of the pancreas confined to secondary ducts show less aggressive pathologic features as compared with those involving the main pancreatic duct. Am J Surg Pathol 2000;24 : 1372–1377.
8. Salvia R, Fernandez-del Castillo C, Bassi C, et al. Main duet initraductal papillary mucinous neoplasma of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg 2004;239 : 678–687.
9. Biankin AV, Kench JG, Biankin SA, et al. Pancreatic intraepithelial neoplasia in association with intraductal papillary mucinous neoplasms of the pancreas: implications for disease progression and recurrence. Am J Surg Pathol 2004;28 : 1184–1192.
10. Takada T, Yasuda H, Uchiyama K, et al. Duodenum-preserving pancreatoduodenectomy: a new technique for complete excision of the head of the pancreas with preservation of the biliary and alimentary integrity. Hepatogastroenterology 1993;40 : 356–359.
11. Beger H, Witte C, Krass E, et al. Erfahrung mit einer das duodenum erhaltender Pankreaskopf-resektion bei chronischer Pankreatitis Chirung 1980;51 : 303–309.
12. Imaizumi T, Hanyu F, Suzuki M, et al. Clinical experience with dusdenum-preserving total resection of the head of the pancreas with pancreatico-choledochoduodenectomy. J hepatobiliary Pancreat Surg 0995;2 : 38–44.
13. Hirata K, Mukaiya M, Kimura K, et al. The anatomy of the pancreaticoduodenal vessels and the introduction of a new pylorus-preserving pancreatoduodenectomy with increased vessel preservation. J Hepatoviliary Pancreat Surg 1994;4 : 335–341.
14. Nakao A: Pancreatic head resection with segmental duodenectomy and preservation of the gastroduodenal artery. Hepatogastroenterology 2004;145 : 533–535.
Labels
Surgery Orthopaedics Trauma surgery
Article was published inPerspectives in Surgery

2012 Issue 6-
All articles in this issue
- Meckel´s diverticulum in adults – our five-year experience
- Comparison of laparotomic and laparoscopic techniques for implantation of the peritoneal part of the shunt in the treatment of hydrocephalus
- Percutaneous interspinous dynamic stabilization (In-Space) in patients with degenerative disease of the lumbosacral spine – a prospective study
- Benign prostatic hyperplasia – awareness of the general public and quality of preventive care
- The significance of biological intracranial meningioma behaviour for their long-term management
- Laparoscopic appendectomy in pregnancy – a case report
- Treatment of an asymptomatic splenic cyst using percutaneous drainage
- Metastasis of breast cancer in gastointestinal tract – report of a case and review of the literature
- Perspectives in Surgery
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Treatment of an asymptomatic splenic cyst using percutaneous drainage
- Meckel´s diverticulum in adults – our five-year experience
- Laparoscopic appendectomy in pregnancy – a case report
- Percutaneous interspinous dynamic stabilization (In-Space) in patients with degenerative disease of the lumbosacral spine – a prospective study
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

