-
Medical journals
- Career
The efficacy of treatment of local residual neoplasia under standardized conditions
Authors: Ondřej Urban 1,2; Barbora Pipek 1; Ivana Mikoviny Kajzrlíková 3; Přemysl Falt 1,2; Petr Fojtík 1; Petr Vítek 1
Authors‘ workplace: Digestive Diseases Center, Vítkovice Hospital, Ostrava 1; Faculty of Medicine, University of Ostrava, Ostrava 2; Beskydy Gastrocenter, Frýdek-Místek Hospital, Frýdek-Místek 3
Published in: Vnitř Lék 2016; 62(5): 365-369
Category: Original Contributions
Overview
Objectives:
Piecemeal endoscopic mucosal resection (EMR) is frequently used for the treatment of non-polypoid colorectal lesions larger than 20 mm. Nevertheless, local residual neoplasia occurs (LRN) in as much as 15 % of cases. The aim of our prospective interventional study was to evaluate the efficacy of treatment of LRN under standardized conditions.Methods:
In two high volume non-university endoscopy centers, LRN has been treated according to the newly proposed classification based on endoscopic appearance by argon plasma coagulation (APC), endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD). Primary outcome, efficacy of LRN treatment, was defined as both endoscopic and histological absence of neoplastic tissue in the post-EMR site 6 months after LRN treatment session.Results:
Twenty-five patients with 25 LRN lesions were enrolled. Among them, 12 (48 %), 8 (32 %) and 5 (20 %) were treated by APC, EMR and ESD, respectively, with efficacy in 10 (90.9 %), 7 (87.5 %) and 4 (100 %), respectively.Conclusions:
Using standardized approach based on therapy directed by LRN morphology, LRN may be eradicated in 91.3 % during one session.Key words:
colonoscopy – endoscopic mucosal resection – local residual neoplasiaIntroduction
Colorectal cancer (CRC) is the second leading cause of cancer death in the western world. Its prevention is feasible by endoscopic removal of precursor lesions [1]. However, after successful endoscopic treatment, the residual risk of CRC without surveillance remains higher than in general population [2,3]. For removal of polypoid lesions, endoscopic polypectomy is used. Nevertheless, a proportion of neoplastic lesions are flat or depressed. Until recently, nonpolypoid lesions have been treated surgically. Nowadays, endoscopic mucosal resection (EMR) has replaced surgery in most of the cases.
Compared to surgery, EMR is less invasive. However, as entire colon and rectum are preserved, local residual neoplasia (LRN) at the post EMR site may develop. Two recent meta-analysis have shown that LRN occurrence in 13.8 % and 15 % [4,5]. It has been shown that incomplete adenoma removal contributes to a higher subsequent incidence of CRC [6–9]. Therefore, surveillance colonoscopies are recommended for timely LRN diagnosis and treatment.
As for the LRN treatment, several endoscopic techniques including argon plasma coagulation (APC), re-EMR and endoscopic submucosal dissection (ESD) are available so that surgery is only rarely necessary. Nevertheless, meta-analysis has shown that successful eradication of residual neoplasia during one session can be expected in 79 % of cases only [5]. Such a suboptimal result may be influenced by several factors, including the absence of LRN treatment standardization in reviewed studies.
The aim of our study is to evaluate the efficacy of LRN treatment under standardized conditions in patients after EMR of laterally spreading tumors (LSTs). The standardization is based on newly proposed endoscopic classification of LRN.
Methods
This prospective interventional study was conducted at two high volume non-university endoscopy centers in the Czech Republic during the period from October 2013 to September 2014. The study has been approved by ethics committee at Vítkovice hospital and registered at ClinicalTrials.gov registry with protocol identifier NCT02386618.
For the purpose of the study, LST was defined as slightly elevated (type 0–IIa according to Paris classification) lesion with lateral diameter of at least 10 mm [10]. The LRN was defined as a presence of neoplastic tissue in the post-EMR site. Inclusion criteria were presence of LRN 3 months after EMR of LST, age ≥ 18 years and signed informed consent. Exclusion criteria were age < 18 years, incomplete therapy of original lesion as judged by endoscopist, previous LRN therapy attempt and failure to identify the post-EMR site. All follow-up colonoscopies were performed by certified colonoscopists. Only high-resolution endoscopes equipped with narrow band imaging (NBI) function (OLYMPUS CF-H180 and CF-H190, Hamburg, Germany) were used. Histological samples were examined by pathologists with robust experience in the field of EMR and ESD.
Patients underwent standard split-dose bowel preparation with polyethylene glycol solution (Fortrans, Beaufour Ipsen Pharma, Paris, France). Sedation with intravenous midazolam was administered when requested by the patient. Carbon dioxide was used for bowel insufflation. Post EMR site was recognized as a mucosal pallor with indistinct vascular pattern localized next to the tattooing performed during initial colonoscopy. To diagnose surface microstructure and delineate LRN margins, chromodiagnosis with 0.2% indigocarmine and/or narrow-band imaging (NBI) were used. Biopsy from post-EMR site and/or any surrounding mucosal irregularity was performed with standard biopsy forceps.
In order to guide the therapy, new classification of LRN based on endoscopic appearance was proposed. According to this classification, a total of 5 (A–E) types with corresponding treatment modality were differentiated as shown in tab. 1. Examples of individual LRN types are shown on fig. 1–5. APC (ERBE 200EA Elektomedizin GmbH, Tubingen, Germany) was used for the treatment of type A and B lesions, at settings 40 W, gas flow 1 l/min. Argon plasma flow was applied to the whole post EMR site or to visible adenoma remnants in type A and B, respectively. For type C lesions, re-EMR was performed using standard lift-and-cut technique. Normal saline with admixture of adrenalin and methylene blue dye was used for submucosal injection. In most of cases, standard oval snares with diameter 10 mm were used. In cases of type D lesion, ESD was performed using dual knife (OLYMPUS, KD 650U, Hamburg, Germany). For submucosal injection, 10% hydroxyethylstarch solution (VOLUVEN, Fressenius Kabi, Bad Homburg, Germany) was used. For small remnant lesions after re-EMR or ESD, additional APC treatment was allowed. Following LRN diagnosis, treatment was performed during the same session for types B and C, while another colonoscopy was scheduled for patients with type A and D lesions.
1. Classification of local residual neoplasia and corresponding treatment 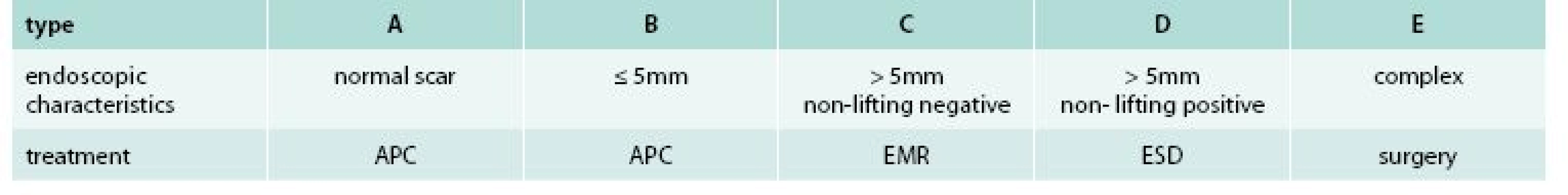
APC – argon plazma coagulation EMR – endoscopic mucosal resection ESD – endoscopic submucosal dissection Fig. 1. Type A of local residual neoplasia (LRN) 
Fig. 2. Type B of local residual neoplasia (LRN) 
Fig. 3. Type C of local residual neoplasia (LRN) 
Fig. 4. Type D of local residual neoplasia (LRN) 
Fig. 5. Type E of local residual neoplasia (LRN) 
Second follow-up colonoscopy was performed after 6 months from LRN treatment session. Local chromodiagnosis with 0.2% indigocarmine and/or NBI was used again. At least 3 forceps biopsy samples from post-EMR site were taken. The treatment was considered to be complete provided that post-EMR site was negative for the presence of neoplasia both endoscopically and histologically.
Results
Among 25 patients with 25 LRNs, 10 (40 %) were females and 15 (60 %) were males. Mean age (± SD) was 69.3 ± 13.8 years. The maximal diameter of original lesion as compared to polypectomy snares was 10–20 mm in one (4 %), 20–30 mm in 6 (24 %) and more than 30 mm in 18 (72 %) cases. LST subtype was LST-G in 18 (72 %) and LST-NG in 7 (28 %). The histology of original LST was LGIEN in 6 (24 %), HGIEN in 14 (56 %) and intramucosal carcinoma in 5 (20 %). The location of original LST was rectum in 16 (64 %), distal colon (distal to the splenic flexure) in 6 (24 %) and proximal colon in 3 (12 %). Types of LRN according to the proposed classification with corresponding treatment results are shown in tab. 2. The histology of LRN was LGIEN in 12 (48 %) and HGIEN in 13 (52 %) cases.
2. Results of local residual neoplasia treatment after 6 months (n = 25) 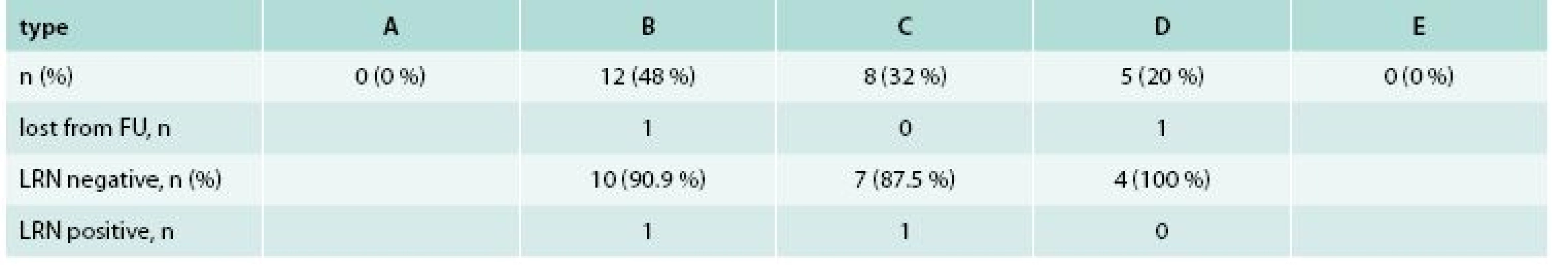
LRN– local residual neoplasia FU – follow up 23 (92 %) patients underwent second follow-up colonoscopy. Two (8 %) remaining patients with type B and type D lesions were excluded from final analysis due to loss to follow-up and anticoagulation treatment, respectively. The LRN treatment was complete in 21 (91.3 %) and incomplete in 2 (8.7 %) cases. Both persistent LRN present as small residual adenomas (type B) suitable for additional APC therapy. There was no mortality, perforation or delayed bleeding related to LRN treatment.
Discussion
Endoscopic mucosal resection (EMR) have become standard of treatment for colorectal neoplastic lesions invading up to the upper third (1 000 µm) of submucosa with virtually no risk of lymphatic spread. Compared to surgery, EMR provides the same long-term results but is less invasive with lower procedure-related morbidity and mortality. In one study, complications after surgical resection of non-malignant colorectal polyps occurred in 24 % and mortality was 0.5 % [11]. However, local residual neoplasia (LRN) after apparently complete EMR of original lesion remains an important issue. Residual neoplastic tissue may develop into the invasive cancer. The risk is 3.6 to 4.3 times higher than in the general population [2,14,12]. Endoscopic surveillance is therefore essential.
Concern about LRN exists mainly for non-pedunculated lesions larger than 20 mm. Among them, LSTs are most frequent, since they represent 4.5 % of all precancerous lesions and can be detected in 1 % of patients undergoing total colonoscopy [13]. In the western countries, LSTs are usually treated by EMR. As shown by many groups including ours, initial EMR is successful in 68–100 % of cases [14–19]. Despite the high efficacy in achieving complete resection, EMR is not always curative because of the LRN development. For instance, in recently published meta-analysis of 33 studies, the mean recurrence risk was 15 (4–54 %) during mean follow-up of 23 month [5]. Piecemeal resection was associated with significantly higher risk than en bloc resection (20 vs 3 %). Other studies, including ours, identified lesion size as another independent risk factor [5,20,21].
It has been suggested that LRN originates from residual adenomatous tissue capable of rapid regeneration [22,23]. Other studies have shown that incomplete resection contributes to higher incidence of subsequent colorectal cancer [6–9]. LRN should be considered an important limitation of EMR. To overcome these limits, the method of ESD has been developed by Japanese endoscopists. Due to its technical distinction, ESD has a potential of en bloc resection irrespective of the lesion size, resulting in very low (0–2 %) risk of LRN [24,25]. However, this advantage must be balanced with substantial risk of ESD complications, reported from western settings [26].
International guidelines recommend surveillance colonoscopy in 6 (2–12) month after piecemeal resection [5,6,27]. According to one of the studies, a single surveillance colonoscopy has a potential to decrease long-term cancer risk to the average population level [2]. On the other hand, surveillance is associated with additional patient discomfort, risk, and cost. Moreover, it presumes patient compliance, which is not always satisfactory. For example, in our previous study, one third (32.2 %) of patients were lost to follow-up despite phone-call invitation. This is in accordance with 41 % in other study [20,28].
In order to prevent subsequent development of invasive cancer, endoscopic eradication of LRN should be attempted. Reported efficacy is various. In systematic review including 351 recurrent lesions re-treated by APC or EMR, 75 (21 %) recurred again [5]. Importantly, in studies including histology for treatment evaluation, the efficacy is substantially lower (77.9 %) compared to studies without systematic post-EMR site biopsy (95.5 %) [21,28]. In our previous study, the histologically proven efficacy of LRN treatment was as low as 47.1 % (8/17) [20]. One of the reasons for such a suboptimal efficacy may be lack of standardization of LRN therapy. From endoscopy perspective, LRN manifests in several forms that may require different approaches. Therefore, in our present study, the therapy was standardized by using new classification based on endoscopic morphology. This approach resulted in successful LRN eradication in 91.2 % during one session as confirmed by advanced imaging colonoscopy and histology.
As for the treatment of LRN, several methods were used in our study, including APC, re - EMR and ESD. The method of APC has proved effective in 90.9 % of type B lesions. In several other studies, APC treatment has been shown effective either during initial or during follow-up examination [29,30]. However, in some studies, using of APC was identified as an independent risk factor for recurrence, rising concerns about reliability of APC for complete eradication of neoplastic tissue [15,21]. The method of EMR was used for type C lesions while ESD was reserved for type D lesions with severe fibrosis preventing lifting. For complex lesions (type E) surgery would have been considered but no such a case occurred in our study. In another large study, surgery was required in 5.5 % of LRN cases [21]. Finally, no type A lesion was diagnosed. Nevertheless, in other study, the occurrence LRN in endoscopically inconspicuous scar was 7 % [28].
Our study has several limitations. Firstly, number of included subjects is low. Secondly, multicentric rather than bicentric study design would be more reliable. Thirdly, to evaluate long-term efficacy of LRN treatment, longer follow up would be necessary. This would be especially needed to address the issue of recurrence occurring after at least one negative surveillance colonoscopy. This late recurrent neoplasia occurs in 4–11 % [20,21,28]. Lastly, since very low (1–3 %) risk of lymph node metastasis after EMR of intramucosal carcinoma has been shown in some studies, CT scan may be recommended during follow up but it was not addressed in our study.
Conclusion
In conclusion, LRN after EMR of colorectal LSTs remains an important issue. Using standardized approach based on therapy directed by LRN morphology, LRN can be eradicated in 91.3 % during one session. Further prospective studies with more patients and longer follow-up are necessary to validate this encouraging short-term results.
ClinicalTrials.gov ID: NCT02386618
MUDr. Přemysl Falt, Ph.D.
faltprem@centrum.cz
Centrum péče o zažívací trakt,
Vítkovická nemocnice,
Ostrava
www.nemocnicevitkovice.agel.cz
Doručeno do redakce 8. 2. 2016
Přijato po recenzi 26. 3. 2016
Sources
1. Winawer SJ, Zauber AG, Ho MN et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 1993; 329(27): 1977–1981.
2. Cottet V, Jooste V, Fournel I et al. Long term risk of colorectal cancer after adenoma removal: a population-based cohort study. Gut 2012; 61(8): 1180–1186.
3. Løberg M, Kalager M, Holme Ø et al. Long-term colorectal-cancer mortality after adenoma removal. N Engl J Med 2014; 371(9): 799–807.
4. Hassan C, Repici A, Sharma P et al. Efficacy and safety of endoscopic resection of large colorectal polys: a systematic review and meta-analysis. Gut 2015; 65(5): 806–820. Dostupné z DOI: <http://dx.doi.org/10.1136/gutjnl-2014–308481>.
5. Belderbos TD, Leenders M, Moons L et al. Local reccurence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy 2014; 46(5): 388–402.
6. Lieberman DA, Rex DK, Winaver SJ et al. United States Multi-Society Task Force on Colorectal Cancer. Guidelines for colonoscopy surveillance after screening polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012; 143(3): 844–857.
7. Robertson DJ, Greenber ER, Beach M et al. Colorectal cancer in patients under close colonoscopic surveillance. Gastroenterology 2005; 129(1): 34–41.
8. Farrar WD, Sawhney MS, Nelson DB et al. Colorectal cancers found after a complete colonoscopy. Clin Gastroenterol Hepatol 2006; 4(10): 1259–1264.
9. Loeve F, van Ballegooijen M, Boer R et al. Colorectal cancer risk in adenoma patients: a nation-wide study. Int J Cancer 2004; 111(1): 147–151.
10. Paris Workshop Participants. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon. Gastrointest Endosc 2002; 58(6 Suppl): S3-S43.
11. Le Roy F, Manfredi S, Hamonic S et al. Frequency of and risk factors for the surgical resection of non-malignant colorectal polyps: a population-based study. Endoscopy 2016; 48(3): 263–270.
12. Atkin WS, Morson BC, Cuzick J. Long term risk of colorectal cancer after excision or rectosigmoid adenomas. N Eng J Med 1992; 326(10): 658–662.
13. Rotondano G, Bianco MA, Buffoli F et al. The cooperative Italian FLIN study group: prevalence and clinicopathological features of colorectal laterally spreading tumors. Endoscopy 2011; 43(10): 856–861.
14. Saito Y, Fujii T, Kondo H et al. Endoscopic treatment for laterally spreading tumors in the colon. Endoscopy 2001; 33(8): 682–686.
15. Moss A, Bourke MJ, Williams SJ et al. Endoscopic mucosal resection outcomes and prediction of submucosal cancer from advanced colonic mucosal neoplasia. Gastroenterology 2011; 140(7): 1909–1918.
16. Tanaka S, Haruma K, Oka S et al. Clinicopathologic features and endoscopic treatment of superficially spreading colorectal neoplasms larger than 20 mm. Gastrointest Endosc 2001; 33(8): 682–686.
17. Hurlstone DP, Sanders DS, Cross SS et al. Colonoscopic resection of lateral spreading tumours: a prospective analysis of endoscopic mucosal resection. Gut 2004; 53(9): 1334–1339.
18. Kaltenbach T, Friedland S, Maheshwari A et al. Short - and long-term outcomes of standardized EMR of nonpolypoid (flat and depressed) colorectal lesions > or = 1 cm (with video). Gastrointest Endosc 2007; 65(6): 857–865.
19. Urban O, Vitek P, Fojtik P et al. Laterally spreading tumors-experience based on 138 consecutive cases. Hepatogastroenterology 2008; 55(82–83): 351–355.
20. Urban O, Kijonkova B, Kajzrlikova IM et al. Local residual neoplasia after endoscopic treatment of laterally spreading tumors during 15 months of follow-up. Eur J Gastroenterol Hepatol 2013; 25(6): 733–738.
21. Moss A, Williams SJ, Hourigan LF et al. Long term adenoma reccurence following wid-field endoscopic muscosal resection (WF-EMR) for advanced colonic mucosal neoplasia is infrequent: results and ris factors in 1000 cases from the Australian Colonic EMR (ACE) study. Gut 2015; 64(1): 57–65.
22. Matsuda K, Masaki T, Abo Y et al. Rapid growth of residual colonic tumor after incomplete mucosal resection. J Gastroenterol 1999; 34(2): 260–263.
23. Kunihiro M, Tanaka S, Haruma K et al. Electrocautery snare resection stimulates cellular proliferation of residual colorectal tumor: an increasing gene expression related to tumor growths. Dis Colon Rectum 2000; 43(8): 1107–1115.
24. Toyonaga T, Man-i M, Fujita T et al. Retrospective study of technical aspects and complications of endoscopic submucosal dissection of laterally spreading tumors of the colorectum. Endoscopy 2010; 42(9): 714–722.
25. Saito Y, Fukuzawa M, Matsuda T et al. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc 2010; 24(4): 345–352.
26. Farhat S, Chaussade S, Ponchon T et al. [SFED ESD study group]. Endoscopic submucosal dissection in a European setting. A multi-institutional report of a technique in development. Endoscopy 2011; 43(8): 664–670.
27. Cairns SR, Scholefield JH, Steele RJ et al. [British Society of Gastroenterology; Association of Coloproctology for Great Britain and Ireland]. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut 2010; 59(5): 666–689.
28. Knabe M, Pohl J, Gerges C et al. Standardized long-term follow-up after endoscopic resection of large non-pedunculated colorectal lesions: a prospective two-center study. Am J Gastroenterol 2013; 109(2): 183–189.
29. Zlatanic J, Waye JD, Kim PS et al. Large sessile colonic adenomas: use of argon plasma coagulator to supplement piecemeal snare polypectomy. Gastrointest Endosc 1999; 49(6): 731–735.
30. Regula J, Wronska E, Polkowski M et al. Argon plasma coagulation after piecemeal polypectomy of sessile colorectal adenomas: long term follow-up study. Endoscopy 2003; 35(3): 212–218.
Labels
Diabetology Endocrinology Internal medicine
Article was published inInternal Medicine

2016 Issue 5-
All articles in this issue
- Hepatic transit times and liver elasticity compared with meld in predicting a 1 year adverse clinical outcome of a clinically diagnosed cirrhosis
- Diagnostics of cystic fibrosis in adults
- Hypercalcemia, symptoms, differential diagnostics and treatment, or importance of calcium investigation
- Idiopathic inflammatory bowel disease as a prothrombotic state
- Clinical implications of polycystic ovary syndrome
- Treatment with rituximab as an opportunity for the prevention of infectious complications
- Can fish oil improve wound healing in surgery?
- Use of new drugs within primary therapy of multiple myeloma
- The efficacy of treatment of local residual neoplasia under standardized conditions
- Internal Medicine
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Hypercalcemia, symptoms, differential diagnostics and treatment, or importance of calcium investigation
- Diagnostics of cystic fibrosis in adults
- Can fish oil improve wound healing in surgery?
- Hepatic transit times and liver elasticity compared with meld in predicting a 1 year adverse clinical outcome of a clinically diagnosed cirrhosis
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

