-
Medical journals
- Career
Reduction of Thymoglobuline from 7.5 mg/kg to 6 mg/kg in conditioning regimen extended time to the first cytomegalovirus detection after allogenic haematopoietic stem cell transplantation
Authors: E. Vejražková 1; P. Hubáček 2; L. Plíšková 3; M. Košťál 1; A. Zavřelová 1; V. Štěpánová 4; P. Žák 1
Authors‘ workplace: IV. interní hematologická klinika, Fakultní nemocnice a Lékařská fakulta v Hradci Králové 1; Ústav lékařské mikrobiologie a Klinika dětské hematologie a onkologie, Fakultní nemocnice Motol a 2. lékařská fakulta, Univerzita Karlova, Praha 2; Ústav klinické biochemie a diagnostiky, Fakultní nemocnice a Lékařská fakulta v Hradci Králové 3; Ústav klinické mikrobiologie, Fakultní nemocnice a Lékařská fakulta v Hradci Králové 4
Published in: Epidemiol. Mikrobiol. Imunol. 68, 2019, č. 2, s. 71-74
Category: Original Papers
Overview
Introduction: The optimal dosage of anti-thymocyte globulin (ATG) may influence the outcome of patients after allogenic haematopoietic stem cell transplantation (HSCT). The aim of our study was to analyse human cytomegalovirus (CMV) infection data, incidence of graft-versus-host disease and other clinical endpoints comparing two patients’ cohorts that were administered two different Thymoglobuline Genzyme doses as part of the HSCT conditioning regimen.
Materials and Methods: Total of 65 adult patients received ATG (7.5 mg/kg or 6 mg/kg) as a part of the fludarabine/busulfan/ATG conditioning regimen. CMV DNAemia was monitored after HSCT using quantitative real-time PCR and preemptive treatment was started for viral loads above 1000 cp/ml.
Results: The mild ATG dose reduction extended the time to the first CMV detection after transplantation (28 days for 7.5 mg/kg dose vs. 40 days for 6 mg/kg dose, p = 0.04). But it did not reduce the incidence or influence first anti-CMV treatment onset, the initial viral load, peak viral load in whole blood or the antiviral therapy parameters (all p ≥ 0.18). No impact of ATG dose reduction on incidence of graft-versus-host-disease, relapse of underlying disease or mortality within first year after transplantation (all p ≥ 0.32) were observed.
Conclusions: The reduced ATG dosages can allow lower toxicity of conditioning regimen while keeping the performance.
Keywords:
thymoglobuline – human cytomegalovirus – CMV – haematopoietic stem cell transplantation – GVHD
INTRODUCTION
Anti-thymocyte globulin (ATG) is used for T-cell depletion in vivo to prevent graft-versus-host-disease (GVHD) after allogenic haematopoietic stem cell transplantation (HSCT), but carries a risk of serious infection complication (including human cytomegalovirus (CMV) infection) or relapse of haematological malignancy [1, 2]. The optimal ATG dosage has not yet been established [1, 2] and its determination may influence the outcome of patients after HSCT.
The aim of our study was to analyse CMV infection data, GVHD, and other clinical endpoints in patients, comparing thymoglobuline doses of 7.5 mg/kg vs. 6 mg/kg as part of the conditioning regimen in adult HSCT recipients in our centre.
MATERIAL AND METHODS
Our prospective study included 65 patients after allogeneic HSCT transplanted due to haematological diseases at the Hradec Kralove University Hospital, Czech Republic between July 2012 and January 2015. The used conditioning regimen were ATG with fludarabine (30 mg/m2 daily for 5 consecutive days prior to the graft infusion with a total dose of 150 mg/m2 for myeloablative regimens; 6 consecutive days with total dose of 180 mg/m2 for reduced intensity regimens) combined with busulfan (myeloablative regimens: 3.2 mg/kg daily for 4 consecutive days with a total dose of 12.8 mg/kg; reduced intensity regimens: 2–3 consecutive days a with total dose of 6.4–9.6 mg/kg). The ATG used was Thymoglobuline Genzyme (Cambridge, MA, USA, rabbit ATG) administered in days -3 to -1 as a total dose of 7.5 mg/kg and days -2 to -1 as a total dose of 6 mg/kg. There were no significant differences between patients’ groups who were administered higher (n = 27 patients) and lower (n = 38 patients) ATG doses. For details see Table 1. Tacrolimus with mycophenolate mophetil was used as a posttransplant immunosuppression. The initial GVHD therapy was started using doses of methylprednisolone of 1–2 mg/kg, gradually reduced based on patients’ clinical statuses. We used low dose methotrexate, cyclosporine A, sirolimus and photopheresis in cases of steroid-resistant GVHD. All patients received prophylactic treatment according to the institutional practice; trimethoprim-sulfamethoxazole as Pneumocystis jirovecii pneumonia prophylaxis, aciclovir prophylaxis and fluconazole. The length of prophylactic treatment was influenced by presence of GVHD or immunosuppressive therapy. Specific CMV prophylaxis (VGCV, valganciclovir) was used only in one patient after their heart transplantation, 296 days after HSCT. The follow-up was at least one year.
1. Patients’ characteristics according to the ATG dosage 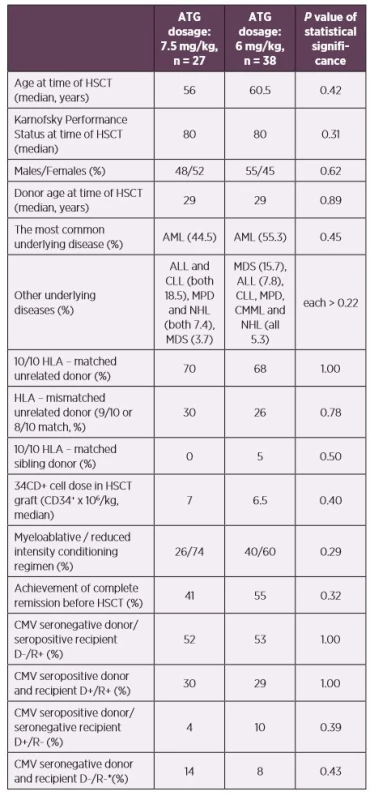
*No primoinfection was recorded in this patient group – excluded from the statistics
Abbreviations: ALL – acute lymphoblastic leukemia, AML – acute myeloid leukemia, ATG – anti-thymocyte globulin, CLL – chronic lymphocytic leukemia, CMML – chronic myelomonocytic leukemia, CMV – human cytomegalovirus, D – donor, HLA – human leukocyte antigen system, HSCT – hematopoietic stem cell transplantation, MDS – myelodysplastic syndrome, MPD – myeloproliferative disorders, NHL – non-Hodgkin lymphoma, R – recipientCMV DNAemia was determined by quantitative real-time PCR from whole blood as described previously [3]. The limit of detection was 100 copies of CMV DNA per millilitre (cp/ml, equivalent of 79.4 IU/ml) and the limit of quantification was 500 cp/ml of the assay. CMV DNAemia was monitored weekly until day 100 for patients with uncomplicated clinical statuses, surveillance then continued every 2–3 weeks.
CMV replication was considered if the viral load was above 100 cp/ml. Preemptive treatment using VGCV was started for viral loads above 1000 cp/ml. VGCV doses were 900 mg BID until viral load reduced to 50%. VGCV was then administered once a day for at least 2 weeks (maintenance therapy) until DNAemia was negative. Maintenance therapy was not initiated or was limited to one week if the initial viral load was lower than 104 cp/ml and DNAemia became negative during the initial therapy [3]. Ganciclovir (GCV, intravenous only) or foscarnet (FOS) treatment schema were used for CMV disease according to the NCCN Guidelines [4].
Categorical data was analysed using the Fisher exact test for 2 x 2 contingency table. Non-parametric Mann-Whitney test was used for comparing independent groups. P values ≤ 0.05 were considered statistically significant.
Study was reviewed and approved by University Hospital Hradec Kralove Ethics Committee.
RESULTS
The time to the first CMV DNA detection after HSCT was shorter in a cohort with higher ATG dosing (median of 28 vs. 40 days after HSCT, p = 0.043). No significant differences were found in CMV reactivation incidence after HSCT, the time of initiation or the length of antiviral therapy between these patients’ groups (p = 0.18–0.87). No significant differences were also found in the initial viral loads, the peak viral loads, or the CMV disease incidence (p = 0.22–0.82).
The mild reduction of ATG had neither significant impact on full chimerism achievement nor influenced the time to the engraftment after HSCT (p = 0.25–0.99). The incidence of GVHD, graft failure, relapse of underlying disease or mortality within first year after HSCT did not differ between the groups (p = 0.32–0.76). See Table 2 and Table 3 for details.
2. Incidence of CMV infection/reactivation and other CMV infection data during the first posttransplant year according to the ATG dosage 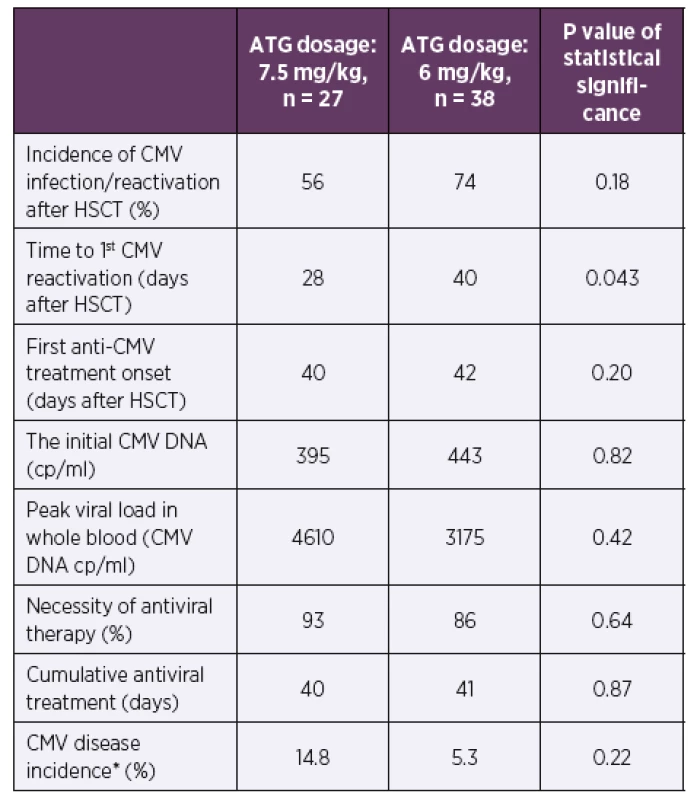
*Defined in Ljungman et al., 2002 [11]
Abbreviations: ATG – anti-thymocyte globulin, CMV – human cytomegalovirus, cp/ ml – copies of CMV DNA per millilitre of whole blood, HSCT – hematopoietic stem cell transplantation3. Clinical data according to ATG dosage during the first posttransplant year 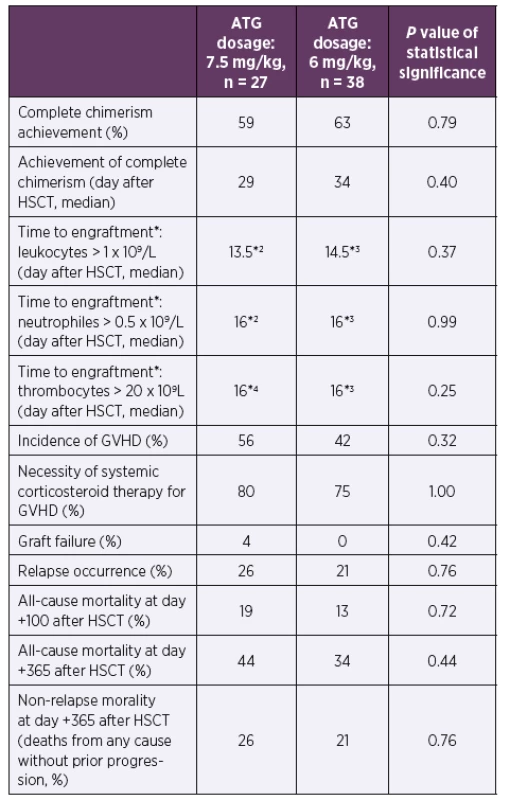
*According to the European Group for Blood and Marrow Transplantation classification [12]
*2One patient died before engraftment in the ATG 7.5 mg/kg group
*3One patient died before engraftment in the ATG 6 mg/kg group
*4Five patients died before engraftment in the ATG 7.5 mg/kg group
Abbreviations: ATG – anti-thymocyte globulin, GVHD – graft versus host disease, HSCT – hematopoietic stem cell transplantationDISCUSSION
The time to the first CMV DNA detection after HSCT was significantly shorter in a cohort with higher ATG dosing, probably due to more potent T cell depletion. George et al. [5] proved an earlier onset of CMV reactivation in patients using ATG or alemtuzumab in reduced intensity conditioning regimens. Liu et al. [1] described that higher ATG dosage (10 vs. 6 mg/kg in haploidentical HSCT) negatively influenced recovery of particular lymphocytes (CD4+, CD4+CD45RA+, CD4+45RO+, CD4-CD8 - and CD8+CD28+). ATG also impacts B cells, natural killer cells, macrophages and dendritic cells as well as HLA class 1 and HLA-DR [6, 7]. The flow cytometric analysis of lymphocyte subpopulation was not available at time of this work so detailed information of immune system recovery in HSCT recipients is missing.
We did not prove any difference in CMV incidence after HSCT using 7.5 vs. 6 mg ATG in conditioning regimens. This is in agreement with the large Beijing study [8]. There are two Ohio, USA studies [2, 9] with contradictory results for CMV infection. Both used different cut-offs for DNAemia significance levels as well as different post-HSCT monitoring periods; this can explain the contradictory results. The older study [9] which included all patients with any CMV detection (comparable to our work) showed a significant decrease in the risk of serious infections including CMV. Their patient cohorts were not fully comparable to ours as they were only including reduced intensity conditioning regimen recipients, a full third of patients’ cohort was CMV seronegative for both patients and donors, most of the patients were transplanted in HLA 8/8 match, etc.
Our results showed no significant effect on engraftment or chimerism after the ATG doses reduction, matching other studies’ results [8–10].
The mild reduction of ATG did not increase GVHD incidence and had no significant effect on mortality in our cohort, matching the published results [2, 8, 9]. A retrospective study by Ayuk et al. [6] showed a decreased 2-year transplant-related mortality with a lower dose of ATG-Fresenius (30 mg/kg) compared to a higher dose (60 mg/kg), mainly due to higher incidence of fatal infections in the ATG-60 group.
CONCLUSION
The reduction of ATG from 7.5 mg/kg to 6 mg/kg in conditioning regimen Fludarabine/Busulfan/ATG extended the time to the first CMV detection after HSCT but did not reduce incidence of CMV after HSCT. We did not observe any impact of ATG dose reduction on incidence of GVHD, relapse of underlying disease or mortality within first year after HSCT. We continue administering reduced ATG dosages to maintain lower toxicity while keeping the performance. Larger cohort of patients and longer follow up would be necessary to definitively confirm our results.
Acknowledgement
This research was supported by the Progress Q40/08 program and by The Ministry of Health of the Czech Republic grant MH CZ-DRO (UHHK, 00179906) as well as project for conceptual development of research organization 00064203 of Motol University Hospital.
Do redakce došlo dne 9. 12. 2018.
Adresa pro korespondenci:
MUDr. Eva Vejražková
IV. interní klinika FN
Sokolská 581
500 05 Hradec Králové
e-mail: vejrazkova.eva@fnhk.cz
Sources
1. Liu J, Xu JP, Bian Z, et al. Differential impact of two doses of antithymocyte globulin conditioning on lymphocyte recovery upon haploidentical hematopoietic stem cell transplantation. Journal of Translational Medicine [online], 2015;13 : 393 [cit. 2016-10-22]. ISSN 1479-5876. Dostupné na www: https://translational-medicine.biomedcentral.com/articles/10.1186/s12967-015-0748-x.
2. Salem G, Ruppert AS, Elder P, et al. Lower Dose of Antithymocyte Globulin (ATG) does not increase Graft-vs-Host Disease (GVHD) in Patients Undergoing Reduced-Intensity Conditioning (RIC) Allogeneic Hematopoietic Stem Cell Transplant (allo HSCT). Leukemia & lymphoma, 2015;56(4):1058–1065.
3. Vejrazkova E, Pliskova L, Hubacek P, et al. Clinical and genotypic CMV drug resistance in HSCT recipients: a single center epidemiological and clinical data. Bone Marrow Transplantation, 2019;54(1):146–149.
4. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Prevention and Treatment of Cancer-Related Infections. Version 2.2014. [online]. 2014-11-08 [cit. 2017-12-12]. Dostupné na www: http://file.trsgo.org/userfiles/file/NCCN%20Cancer-Related%20Infections%20Guideline%202015.pdf
5. George B, Kerridge I, Gilroy N, et al. Fludarabine-based reduced intensity conditioning transplants have a higher incidence of cytomegalovirus reactivation compared with myeloablative transplants. Bone Marrow Transplantation, 2010;45(5):849–855.
6. Ayuk F, Diyachenko G, Zalebina T, et al. Comparison of Two Doses of Antithymocyte Globulin in Patients Undergoing Matched Unrelated Donor Allogeneic Stem Cell Transplantation. Biology of Blood and Marrow Transplantation, 2008;14(8):913–919.
7. Baron F, Monthy M, Blaise D, et al. Anti-thymocyte globulin as graft-versus-host disease prevention in the setting of allogeneic peripheral blood stem cell transplantation: a review from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica, 2017;102(2):224–234.
8. Wang Y, Fu HX, Liu DH, et al. Influence of two different doses of antithymocyte globulin in patients with standard-risk disease following haploidentical transplantation: a randomized trial. Bone Marrow Transplantation, 2014;49(3):426–433.
9. Hamadani M, Blum W, Phillips G, et al. Improved Nonrelapse Mortality and Infection Rate with Lower Dose of Antithymocyte Globulin in Patients Undergoing Reduced-Intensity Conditioning Allogeneic Transplantation for Hematologic Malignancies. Biology of Blood and Marrow Transplantation, 2009;15(11):1422–1430.
10. Meijer E, Cornelissen JJ, Löwenberg B Bob, Verdonck LF. Antithymocyteglobulin as prophylaxis of graft failure and graft-versus-host disease in recipients of partially T-cell-depleted grafts from matched unrelated donors: a dose-finding study. Experimental Hematology, 2003;31(11):1026–1030.
11. Ljungman P, Griffiths P, Paya C. Definitions of Cytomegalovirus Infection and Disease in Transplant Recipients. Clinical Infectious Diseases, 2002;34(8):1094–1097.
12. Ruutu T. Engraftment. In: European Group for Blood and Marrow Transplantation 2011 [online]. Přednáška. Francie: Paříž. 2011-04-05 [cit. 2016-06-02]. Dostupné na www: https://portal.ebmt.org/Contents/Resources/Library/Slidebank/Documents/EBMT%202011%20SC%20Slide%20Bank/1439%20Ruutu.pdf.
Labels
Hygiene and epidemiology Medical virology Clinical microbiology
Article was published inEpidemiology, Microbiology, Immunology

2019 Issue 2-
All articles in this issue
- Determination of antimicrobial activity of Achatina reticulata slime
- Surveillance of antibiotic resistance of Streptococcus pneumoniae in the Czech Republic, respiratory study results, 2010–2017
- Tularemia – zoonosis carrying a potential risk of bioterrorism
- Actinomycosis – an umbrella review and three case reports of severe pelvic actinomycosis treated conservatively
- In-vivo interspecies transmission of carbapenemase KPC in a long-term treated female patient
- Diagnosis and treatment of Bartonella endocarditis
- Clostridium difficile infection and colonisation in children under 3 years of age: prospective comparative study
- Reduction of Thymoglobuline from 7.5 mg/kg to 6 mg/kg in conditioning regimen extended time to the first cytomegalovirus detection after allogenic haematopoietic stem cell transplantation
- Epidemiology, Microbiology, Immunology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Actinomycosis – an umbrella review and three case reports of severe pelvic actinomycosis treated conservatively
- In-vivo interspecies transmission of carbapenemase KPC in a long-term treated female patient
- Tularemia – zoonosis carrying a potential risk of bioterrorism
- Clostridium difficile infection and colonisation in children under 3 years of age: prospective comparative study
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

